Abstract
CD96, previously named T cell activation increased late expression (Tactile), is a transmembrane molecule that functions as an activated receptor on natural killer cells. It is well known that many transmembrane molecules have soluble forms, which were either shed from the cell surface or spliced at mRNA level. In many cases, the levels of soluble forms in the circulation could be used as biomarkers of lymphocyte activation in bacterial or virus infection, tumour, transplantation and autoimmue disease. To investigate whether CD96 could be released into the sera and the possible biological fuction of soluble hCD96 (sCD96), we generated and characterized five clones of anti-hCD96 mouse monoclonal antibodies (mAb) and developed a sandwich enzyme-linked immunosorbent assay (ELISA) system based on two anti-hCD96 mAbs with different epitope specificities. Using this ELISA system, sCD96 in serum samples from 99 healthy individuals could be detected. Furthermore, we found that the level of sCD96 in serum samples from patients with chronic viral hepatitis B or classes B and C of hepatic cirrhosis classified using the Child–Pugh score was much higher (P < 0·001 versus healthy individuals; P = 0·006 versus healthy individuals respectively) than that from healthy individuals (0·98 ng/ml). Our study demonstrates for the first time that sCD96 existed in sera, and suggestes that sCD96 may be used as a serous marker for some diseases such as chronic viral hepatitis B infection or hepatic cirrhosis in classes B and C. The level of sCD96 in patients’ serum may have some relationship with a chronic inflammatory reaction.
Keywords: CD96, ELISA, hepatic cirrhosis, viral hepatitis B
Introduction
Viral hepatitis B is an infective disease caused by hepatitis B virus (HBV) infection and develops potentially into hepatic cirrhosis. In many cases it will develop into chronic hepatitis 6 months later after initial infection. It has been shown that the immune response plays a key role in these chronic liver diseases, besides the direct effect of the virus. In viral hepatitis B and hepatic cirrhosis patients, Küpffers cells, hepatic satellated cells and lymphocytes display activated phenotypes, and the levels of inflammatory cytokines in circulation are much higher than those in healthy individuals [1]. It has been accepted that some cytokines are involved in liver inflammation, necrosis of liver cells and fibrosis, such as tumour necrosis factor (TNF)-α and transforming growth factor-β[2–4]. Meanwhile, soluble forms of many adhesion molecules also have been reported, showing higher levels in these chronic inflammatory diseases such as intercellular adhesion molecule-1 and leucocyte function antigen-3 [5,6].
CD96 is a member of the immunoglobulin (Ig) superfamily (IgSF) which is homologous to CD226. Both of them are receptors for CD155. Previous investigations indicated that CD96 and CD226 were adhesion molecules [7]. Furthermore, it has been reported that the level of soluble CD226 in sera of human immunodeficiency virus-infected patients is higher than that in the sera of healthy individuals [8]. However, there is as yet no report of whether soluble CD96 exists in circulation and whether the serum levels of sCD96 are increased in some patients.
In this study, we cloned and characterized five clones of mouse monoclonal antibody (mAb) anti-hCD96 and developed a sandwich enzyme-linked immunosorbent assay (ELISA) system based on two anti-hCD96 mAbs with different epitope specificities. Using this ELISA kit, we determined the serum levels of sCD96 in the sera of viral hepatitis B and hepatic cirrhosis patients, and demonstrated that the level of sCD96 in serum samples from patients with chronic viral hepatitis B or hepatic cirrhosis in classes B and C was much higher than that from healthy individuals. These findings suggest that sCD96 may be used as a predictor in chronic infectious diseases.
Material and methods
Construction of hCD96 domain 1 expressed vector
Because HSB-2 cells is a human T cell acute lymphoblastic leukaemia cell line which has been reported as highly expressing CD96, its cDNA was cloned from phytohaemagglutinin-stimulated HSB-2 cells successfully by reverse transcription–polymerase chain reaction (PCR). The first extracellular Ig V-like domain of CD96 (CD96-D1) was amplified from HSB-2 cDNA with primers 5′-GGGGTACCGGGCTCTGATGTCAACCTGACCT-3′ and 5′-CGGGATCCTCCTCTGGATACAGAACAAGCATACAC-3′ by PCR and inserted into pMD18-T vector (Takara, Otsu, Japan). The sequence of CD96-D1 cDNA was ascertained by DNA sequencing. The confirmed cDNA of CD96-D1 was digested from pCD96-D1-T vector by two restriction endonucleases, KpnI and BamHI, and inserted into pCDM7 vectors which contains the human Ig Fc fragment-encoding sequence (a gift from Dr Xiaoning Xu, Immunology Unit, MRC, University of Oxford, Oxford, UK) to construct the eukaryotic expression vector pCDM7-CD96D1.
Establishment of stable transfected Chinese hamster ovary cells and preparation of hCD96D1-Fc fusion protein
The pCDM7-CD96D1 vector was transfected into Chinese hamster ovary (CHO) cells by Lipofectamine2000 (Invitrogen, Carlsbad, CA, USA). Stable transfectants were selected in the presence of 800 µg/ml G418 and cloned subsequently by the limiting dilution method. Expression levels of the hCD96D1-Fc fusion protein were assessed by a sandwich ELISA kit, which was used for the detection of human Ig Fc [9]. The supernatant containing soluble hCD96D1-Fc fusion protein was collected for protein purification by protein A affinity column (Pharmacia, Uppsala, Sweden). The purified hCD96D1-Fc fusion protein was identified by Western blot. Blots were detected using horseradish peroxidase (HRP)-conjugated mouse anti-human IgG mAb (Thermo, Rockford, IL, USA) and visualized using the Western blot kit (Thermo).
Production of mouse mAb to hCD96D1
CD96D1-Fc antigen (20 µg), purified by affinity chromatography, was mixed with complete Freund's adjuvant. Female BALB/c mice (10 weeks old) were immunized at day 0 by subcutaneous (s.c.) injection. The mice were subsequently immunized again 3 weeks later with 20 µg protein in incomplete Freund's adjuvant by s.c. and the third immunization with 20 µg protein by intraperitoneal (i.p.) injection, respectively, at 3-week intervals. Seven days after the last immunization, mice were bled from the caudal vein and the serum titres were determined by indirect ELISA. The immunized mice with high serum titres were boosted with 50 µg antigen by i.p. injection. Three days later, the mice were killed and the spleens were taken out aseptically. Then the splenocytes and Sp2/0 murine myeloma cells were fused in the presence of polyethylene glycol (MW4000; Merck, Darmstadt, Germany). The positive hybrids were selected by ELISA and subcloned five times using the limiting dilution method. Mouse mAbs were produced from female BALB/c mice in which hybridoma cells had been injected i.p. Immunoglobulin class and subclass determination of the mAbs was performed using a mouse mAb isotyping kit (Sigma, St Louis, MO, USA), following the manufacturer's recommendations.
Indirect ELISA to identify anti-CD96D1 mAbs
The 96-well flat-bottomed plates (Nunc, Roskilde, Denmark) were coated with hCD96D1-Fc fusion protein or human IgG (Sigma) (5 µg/Ml) diluted in coating buffer [0·05 M carbonate/bicarbonate buffer (pH 9·5)] and incubated overnight at 4 °C. After washing three times with phosphate buffered saline (PBS) containing 0·05% Tween-20, the supernatants from hybridoma cultures were added. Plates were incubated at 37 °C for 1 h and washed three times before incubation at 37 °C for 1 h with 100 µl of the working dilution of HRP-conjugated goat anti-mouse IgG (Sigma). After incubation at 37 °C for 1 h and three more washes, the substrate [2,2′-azinobis (3-ethyl benzthizazline sulphonic acid) (ABTS); Sigma] was added to each well. The plates were incubated at 37 °C for 15 min, and the optical density (OD) 410 nm absorbance was detected on a microplate reader (Bio-Rad, Hercules, CA, USA).
Flow cytometry
The binding of mAbs to CD96 molecule on cell surface was determined by flow cytometry analysis. HSB-2 cells were incubated with FMU-CD96·1–5 mAbs generated after blocking with normal rabbit serum (10%). Mouse mAb anti-staphylo-enterotoxin D which we prepared and identified previously was used as negative control. After washing twice in Dulbecco's PBS (DPBS), the cells were resuspended in DPBS containing a working dilution of fluorescein isothiocyanate-labelled rabbit anti-mouse IgG (Ancell, Bayport, MN, USA) and incubated at 4 °C for 30 min. The cells were washed, fixed and then analysed on a fluorescence activated cell sorter (FACScan) cytometer (Elite ESP, Miami, FL, USA).
Western blot analysis
The protein content of cell extracts from 2 × 107 HSB-2 cells was determined by the Broadford assay (Bio-Rad). A total of 20–30 µg of protein was electrophoresed on 8% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membranes (Amersham, Little Chalfont, UK). Membranes were blocked, incubated with FMU-CD96·1–5 at 4 °C overnight, and normal mouse IgG was used as negative control. After washing four times, the membranes were reacted with HRP-labelled anti-mouse IgG for 1 h at room temperature. After four washes, enhanced chemiluminescence reagent (Roche, Indianapolis, IN, USA) was applied to the membranes, which were then exposed to an X-ray film (Kodak, Rochester, NY, USA). Soluble CD96 in the sera of patients was also analysed by this protocol.
Immunohistochemistry
The paraffin sections of human normal colon were provided by the department of pathology, Fourth Military Medical University, with the approval of the patients. The slices were dewaxed, hydrated and incubated in peroxidase inhibitor for 30 min to remove endogenous peroxidase. They were then dipped into mouse FMMU-CD96·1∼5 mAbs (diluted to 1:1000) for over 2 h at room temperature after blocking with diluted goat serum (Sigma). Mouse IgG before immunization and antigen-absorbent immune serum were applied as negative controls. After three washes in PBS, the slices were dipped into HRP-conjugated goat anti-mouse IgG (Abcam, Cambridge, UK) for 30 min at room temperature. Antibody complexes were then visualized by incubation with diaminobenzidine chromogen. Sections were counterstained with Mayer's haematoxylin for 10 s, dehydrated through gradient ethanol, cleared in dimethyl benzene, mounted and examined using light microscopy (Olympus, Tokyo, Japan).
Sandwich ELISA to detect sera sCD96
One hundred µl of anti-hCD96D1 mAb (5 µg/ml in 0·05 M sodium carbonate buffer, pH 9·5) was added to each well of an ELISA plate (Nunc) and incubated overnight at 4 °C. After extensive washing with PBS containing 0·1% (v/v) Tween-20 (PBS/Tween), every well was blocked with 200 µl PBS containing 10% calf serum for 1 h at 37 °C. Then after washing, serum samples or standard CD96D1-Fc serially diluted with PBS containing 10% fetal calf serum were added to the wells and incubated for 1∼2 h at 37 °C. After three washes, the wells were reacted with another clone of anti-hCD96D1 mAb which was conjugated with HRP and diluted in PBS containing 3% calf serum for 1 h at 37 °C. Colour development was performed by adding 100 µl 0·1 mg/ml tetramethylbenzidine in 0·1 M citrate-phosphate buffer (pH 5·0) containing 0·01% H2O2. The absorbance at 450 nm was determined with a microplate reader (Bio-Rad).
Collection of patients’ and control sera and laboratory analysis
Serum samples were isolated from 116 healthy volunteers, 14 inactive infected HBV carriers, 56 viral hepatitis patients and 53 hepatic cirrhosis patients with complete clinical data in Xi'an Tangdu Hospital and Xijing hospital after approval by the patients and the hospitals. The sera were stored at 20 °C until use. The serum alanine transaminase (ALT) and aspartate transaminase (AST) concentrations were analysed by routine methods using the LX20 multiple automated analyser (Beckman Coulter, Fullerton, CA, USA). HBV DNA was extracted from 200 µl sera using the serum viral DNA purification kit (PG Biotechnology, Shenzhen, China). Serum viral load was measured in the LightCycler™ (Roche, Basel, Switzerland), using the quantitative HBV PCR Fluorogence Diagnostic Kit (PG Biotechnology, Shenzhen, China).
Statistical analysis
Non-parametric tests were used for group comparison (Mann–Whitney U-test for unpaired data). Pearson's correlation coefficient test was used to define the correlation between the levels of sCD96 and serum ALT, AST or HBV DNA load in sera of viral hepatitis B patients. All statistical analyses were performed using spss version 11·5.
Results
Recombinant protein expression
Recombinant hCD96D1-Fc fusion protein was obtained from the pCDM7-CD96-D1-transfected CHO cell culture. The eluted fraction from the protein A affinity column was consistent with the expected molecular weight (Mr) 41 kDa of the hCD96D1-Fc fusion protein, which contains domain 1 of hCD96 and the Ch2 and Ch3 domains of human Ig Fc, as determined by Western blot under reducing conditions. Normal human IgG was used as positive control (Fig. 1).
Fig. 1.
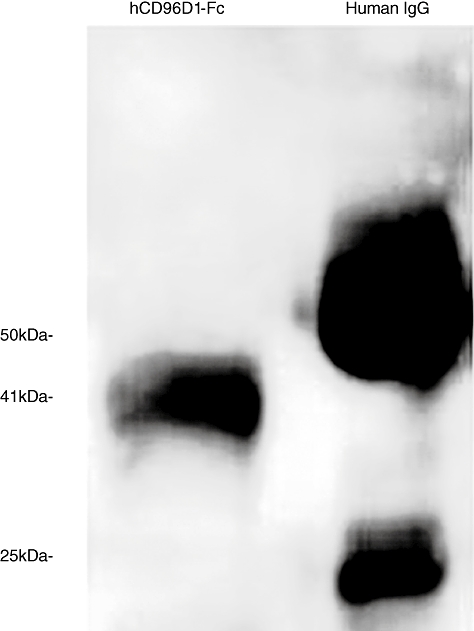
Western blot analysis for purified hCD96D1-Fc fusion protein. hCD96D1-Fc fusion protein was concentrated to 1 mg/ml after being eluted from the protein A affinity column. Fusion protein and human immunoglobulin G (IgG) (10 mg/ml) were analysed by Western blot on 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis. Lane 1, hCD96D1-Fc fusion protein (sample volume: 2 µl); lane 2, human IgG (sample volume: 1 µl).
Preparation of mAbs to hCD96D1
Five positive hybridoma clones secreting mouse mAbs against hCD96D1 were obtained and named FMU-CD96·1–5 respectively. The mouse Ig isotypes of most of these mAbs were IgG1, except FMU-CD96·1; two were IgG2b and IgG2a respectively. All the hybridoma clones could stably secrete specific mAbs, which reacted with hCD96D1-Fc but not human IgG in ELISA (Table 1).
Table 1.
Characterization of anti-CD96D1 monoclonal antibodies (mAbs).
| Clone number | Subclass/type | Titres | WB | FCM | IHC |
|---|---|---|---|---|---|
| FMU-CD96·1 | IgG2b/κ | 10−6 | + | ++ | − |
| FMU-CD96·2 | IgG2a/κ | 10−6 | − | ++ | + |
| FMU-CD96·3 | IgG1/κ | 10−6 | − | + | − |
| FMU-CD96·4 | IgG1/κ | 10−5 | − | − | + |
| FMU-CD96·5 | IgG1/κ | 10−6 | + | − | − |
FCM, flow cytometric analysis; IgG, immunoglobulin G; IHC, immunohistochemistry; WB, western blot; +, positive; ++, strongly positive; −, negative.
Flow cytometric analysis
The flow cytometric analysis (FCM) analysis showed that FMU-CD96·1,2,3 mAbs could recognize the natural membrane hCD96 molecule expressed on the human T cell line HSB-2 cells. The positive percentages were 90·2%, 97·2% and 61·7% respectively. Control antibody displayed negative reactivity (Fig. 2a).
Fig. 2.
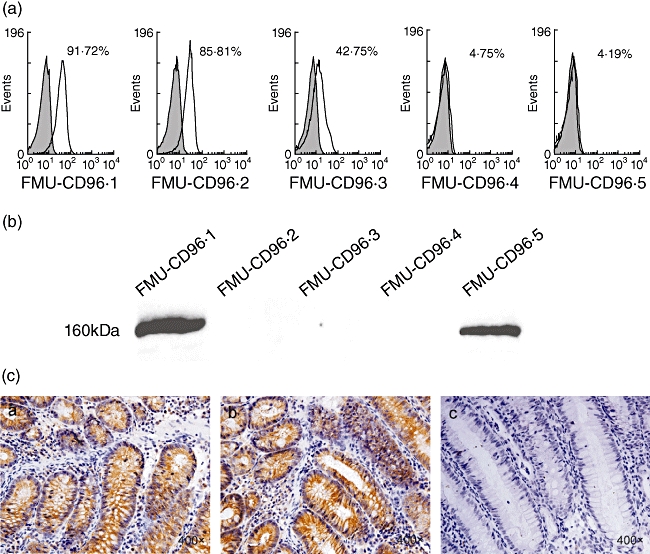
Identification and characterization of FMU-CD96·1–5 monoclonal antibodies (mAbs). (a) Reactivity of FMU-CD96·1–5 to membrane CD96 identified by flow cytometric analysis (FCM). HSB-2 cells were stained with the anti-staphylo-enterotoxin D (SED) mAb, FMU-CD96·1–5 respectively. (b) Western blot analysis of FMU-CD96·1–5. Lysate of HSB-2 cells was loaded onto 8% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (50 µg total protein each lane) and transferred to nitrocellulose membrane. FMU-CD96·1–5 were added to the membrane respectively. After reaction with horseradish peroxidase-labelled anti-mouse immunoglobulin G (IgG), the membrane was visualized using an enhanced chemiluminescence (ECL) reagent. (c) Identification of FMU-CD96·1–5 mAbs by immunohistochemistry. The paraffin sections of human normal colon were dewaxed, hydrated, incubated in FMU-CD96·1–5 and negative control respectively, and visualized by incubation with diaminobenzidine (DAB) chromogen. Among these mAbs, FMU-CD96·2 and FMU-CD96·4 could be used in immunohistochemistry. (c) a and (c) b showed that FMU-CD96·2 and FMU-CD96·4 were immunoreactive-positive. (c) c showed that FMU-CD96·1 was immunoreactive-negative. Both FMU-CD96·3 and FMU-CD96·5 were also immunoreactive negative (data not shown).
Western blot analysis
FMU-CD96·1,5 can probe the protein of Mr 41 kDa, which was the same molecular weight as that of hCD96D1-Fc fusion protein. They can also probe the natural hCD96 molecule, which is 160 kDa in the cell extracts of HSB-2 cells in Western blot analysis (Fig. 2b).
Immunohistochemistry
The immunohistochemistry results showed that FMU-CD96·2,4 could recognize CD96-reactive substances located in the mucosal epithelium of the colon, which was reported to express CD96 [10]. FMU-CD96·1,3,5 showed negative results (Fig. 2c). Mouse IgG before immunization and antigen-absorbent immune serum were used as negative controls.
Establishment of a sandwich ELISA system for detecting sCD96
Epitope mapping by competitive ELISA suggested that mAbs recognized three different epitopes. The first was recognized by FMU-CD96·1, FMU-CD96·3 and FMU-CD96·5; the second by FMU-CD96·2 and the third by FMU-CD96·4 (Fig. 3b). In order to determine the optimum combination, each of the five mAbs described here was labelled with HRP and then utilized as either the coating or detection antibody. The data shown in Fig. 3a indicate that the highest number of the OD 450 nm absorbence was obtained when mAb CD96D1·3 was used as coating antibody and HRP-labelled CD96D1·4 mAb was used as detection antibody. Significantly lower activity was observed using any of the other combinations of coating and detection antibody. As expected, low or no counts were observed when the same mAb was used as both the capture and detection antibody (Table 2). In Fig. 3c, using CD96D1·5 as coating mAb and CD96D1·4 as detection mAb, CD96D1·3 as coating mAb and CD96D1·2 as detection mAb, CD96D1·3 as coating mAb and CD96D1·4 as detection mAb respectively, when we diluted the CD96D1-Fc by end-point dilution to make standard curves, CD96D1·3 as coating mAb-CD96D1·4 as detection mAb was the most satisfactory combination. Figure 3c indicates typical standard curves of these three kinds of combination using CD96D1-Fc as standard. Following optimization of the working concentrations of coating and detection mAbs, a sensitive ELISA kit was developed with the detectable limitation of CD96D1-Fc 0·8 ng/ml. Using this kit, we found that sCD96 could be detected sensitively and specifically. At the same time, it did not cross-react with other soluble molecules, such as sCD226 and sCD155.
Fig. 3.
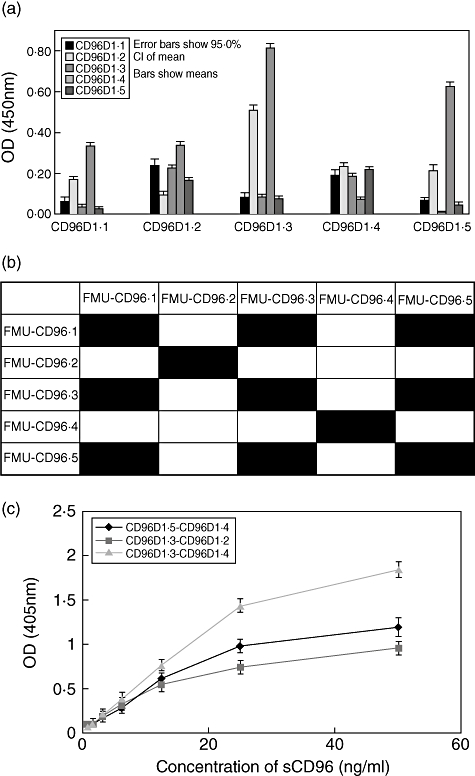
Establishment of a sandwich enzyme-linked immunosorbent assay (ELISA) system with FMU-CD96·3 as coating antibody and horseradish peroxidase (HRP) conjucted FMU-CD96·4 as detection antibody to detect sCD96. (a) Each of the five monoclonal antibodies (mAbs) was used as coating antibody or as horseradish peroxidase-labelled antibody. The optical density (OD) 450 nm absorbence was detected for each antibody combination using sCD96 concentration at 10 ng/ml. (b) Epitope mapping by competitive ELISA. The mAbs in the first column replaced detection antibodies and those in the first row replaced the competitive antibodies. Black box: the two clones of mAbs corresponded in the first row and column recognizing the same epitope. (c) Standard calibration curves of sandwich ELISA with three combinations of mAbs anti-sCD96. Plotted values were obtained with 0·8, 1·6, 3·2, 6·25, 12·5, 25 and 50 ng/ml CD96-D1-Fc by sandwich ELISA, as described in Materials and methods.
Table 2.
Different combinations of anti-sCD96 monoclonal antibodies (mAbs) detecting the same concentration of standard sCD96 (10 ng/ml).
| HRP-labelled mAb | |||||
|---|---|---|---|---|---|
| Coated mAb | CD96D1·1 | CD96D1·2 | CD96D1·3 | CD96D1·4 | CD96D1·5 |
| CD96D1·1 | 0·060 | 0·167 | 0·034 | 0·334 | 0·025 |
| CD96D1·2 | 0·238 | 0·090 | 0·227 | 0·339 | 0·167 |
| CD96D1·3 | 0·082 | 0·509 | 0·082 | 0·811 | 0·070 |
| CD96D1·4 | 0·190 | 0·232 | 0·182 | 0·067 | 0·219 |
| CD96D1·5 | 0·069 | 0·208 | 0·004 | 0·627 | 0·046 |
HRP, horseradish peroxidase.
Determination of sCD96 levels in sera of patients with viral hepatitis B and hepatic cirrhosis
Using the sCD96 ELISA kit, it was found that 98 individuals among 116 healthy volunteers showed detectable levels of serum sCD96, and the median of sCD96 level was 0·98 ng/ml. Meanwhile, serum sCD96 of inactive HBV carriers showed a similar level to that of healthy individuals (n = 14, P = 0·162, Fig. 4a). According to the clinical symptoms and time after initial infection, we classified the patients with viral hepatitis B into acute hepatitis B (n = 15, P = 0·433 versus healthy individuals) and chronic hepatitis B (n = 40, P < 0·001 versus healthy individuals). We found that the level of sCD96 in chronic viral hepatitis B patients was significantly higher than that in healthy individuals. However, there was no statistical difference between the level of sCD96 in acute viral hepatitis B patients and that in healthy individuals. At the same time, the patients with hepatic cirrhosis after HBV infection were classified with the Child–Pugh score into classes A, B and C. There was no statistical difference between the level of sCD96 in patients with hepatic cirrhosis after HBV infection who were class A (n = 24, P = 0·423) and that in healthy individuals. However, the level in patients with hepatic cirrhosis who were class B or class C (n = 29, P = 0·006) after HBV infection was apparently higher than that in healthy individuals (P = 0·014) (Table 3). We detected the sera aminopherase of patients with viral hepatitis B and found the level of sCD96 in patients sera was not correlated with the level of serum ALT (r = −0·113, P = 0·489), AST (r = 0·013, P = 0·907) or HBV DNA load (r = −0·181, P = 0·265).
Fig. 4.
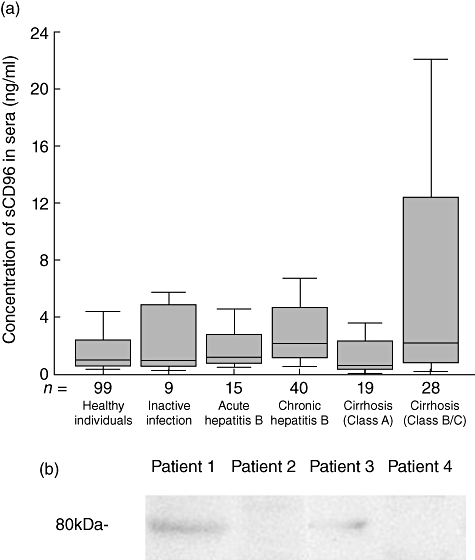
Detecting soluble CD96 in sera by the enzyme-linked immunosorbent assay (ELISA) kit and Western blot analysis for sCD96 in patients’ sera. (a) The level of soluble CD96 in sera from healthy individuals with or without inactive hepatitis B virus (HBV) infection, patients with acute and chronic viral hepatitis B and patients with hepatic cirrhosis at different stages (outliers and extremes were not displayed in the box plot but were involved in the statistical analysis). The bottom of each box represents the 25th percentile, the top is the 75th percentile, and the line in the middle represents the median. Upper adjacent represents maximum excluding outliers and extremes. Lower adjacent represents minimum. (b) Western blot analysis for sCD96 in patients’ sera. Lane 1, a patient with viral hepatitis B (sCD96, 396 ng/ml); lane 2, a patient with viral hepatitis B (sCD96, 3·65 ng/ml); lane 3, a patient with hepatic cirrhosis (sCD96, 181 ng/ml); lane 4, a patient with hepatic cirrhosis (sCD96, 3·95 ng/ml).
Table 3.
Serum sCD96 levels in healthy individuals and patients with viral hepatitis B and hepatic cirrhosis.
| Cases (n) |
|||||
|---|---|---|---|---|---|
| Diagnosis | Tested | Detectable | Median (ng/ml) | P-value (versus healthy indivaduals) | P-value (versus Inactive HBV-infected control) |
| Healthy indivaduals | 116 | 98 | 0·9758 | 0·162 | |
| Inactive HBV-infected control | 14 | 9 | 1·4533 | 0·162 | |
| Viral hepatitis B | 56 | 55 | 2·0354 | 0·001 | 0·003 |
| Acute viral hepatitis B | 16 | 15 | 1·2518 | 0·433 | 0·085 |
| Chronic viral hepatitis B | 40 | 40 | 2·1193 | < 0·001 | 0·001 |
| Hepatic cirrhosis | 53 | 45 | 2·3791 | 0·038 | 0·040 |
| Class A (Child–Pugh score) | 24 | 19 | 0·6749 | 0·423 | 0·461 |
| Class B/C (Child–Pugh score) | 29 | 28 | 3·6165 | 0·006 | 0·014 |
HBV, hepatitis B virus.
Determination of the molecular weight of sCD96 in sera
Using FMU-CD96.1, sera sCD96 was detected by Western blot. We detected sera from two viral hepatitis B patients and two hepatic cirrhosis patients with detectable sera sCD96. Among them, sCD96 could be detected in one viral hepatitis B patient with 396 ng/ml sCD96 in sera and one hepatic cirrhosis patient with 181 ng/ml sCD96 in sera, whereas sCD96 could not be detected in one viral hepatitis B patient with 3·65 ng/ml sCD96 in sera and the one hepatic cirrhosis patient with 3·95 ng/ml sCD96 in sera by Western blot. It was shown that the molecular weight of sCD96 was 80 kDa (Fig. 4b). Meanwhile, sCD96 in normal individuals’ sera could not be detected by Western blot (data not shown).
Discussion
In 1992, Wang and his colleagues identified and cloned cDNA of a novel cell-surface protein, named T cell activation increased late expression (Tactile) [10]. Subsequently, this molecule was designated as CD96 at the Human Leukocyte Differentiation Antigen workshop. Later in 2004, Fuchs and his colleagues showed that the receptor of CD96 was poliovirus receptor (PVR/CD155), which was also the CD226 receptor. Previous studies found that interaction between CD96 and CD155 could promote natural killer (NK) cell adhesion to target cells expressing PVR (CD155), stimulate cytotoxicity of activated NK cells and mediate NK cell acquisition of PVR from target cells [11].
CD96 is a member of the IgSF with three Ig domains highly N-glycosylated and a long serine/threonine/proline-rich motif in extracellular region. CD96 is expressed on normal T cell lines and clones and some transformed T cells [12]. The expression of sCD96 is at low level on peripheral T cells and strongly up-regulated after activation, peaking 6–9 days after stimulation. The expression level of CD96 could also be up-regulated on NK cells when NK cells were activated in mixed lymphocyte culture [10]. Recently, CD96 has been found to be an important marker of T cell acute lymphoblastic leukaemia and acute myeloid leukaemia–leukaemic stem cells [13,14]. Kaname and his colleagues found mutation in CD96 may cause a form of C syndrome, which comprises extensive anomalies [15]. It suggests that CD96 may be also expressed in non-haematopoietic tissues.
Using the bioinformatics method to analyse the sequence database of CD96, it has been shown that the first Ig domain of this protein in the N-tail is the potential binding site of CD96 to CD155, which may play an important role in immune responses. Until now, two types of CD96 mAbs have been prepared by immunizing mice with whole HSB-2 cells [10,13]. For the important function of CD96 domain 1, we prepared recombinant protein of hCD96 domain 1, generated mouse hybridoma clones secreting mouse mAbs against hCD96 domain 1 and identified the reactivity of these mAbs.
Among five mAbs, anti-CD96·1,2,3 mAbs could recognize the natural membrane hCD96 molecule expressed on the human T cell line HSB-2 cells by FCM. FMU-CD96·1,5 can probe either hCD96D1-Fc fusion protein at 41 kD or the natural hCD96 molecule at 160 kD by Western blot. With FMU-CD96·2 and FMU-CD96·4, the expression of CD96 in colon tissue was investigated by immunohistochemistry. These results showed that the CD96 mAbs we raised by domain 1 could recognize different kinds of CD96 molecules existing in different conditions.
It has been reported that some transmembrane molecules such as CD226 and CD155 have soluble forms which could be secreted into humor and regulate the function of transmembrane forms [16]. However, a soluble form of hCD96 has not yet been reported. In this report, we established a sensitive sandwich ELISA kit by two anti-hCD96D1 mAbs to detect sCD96. The kit's establishment has made a solid foundation for soluble CD96 research. Using this kit, we first detected sCD96 in sera of healthy individuals and found that 98 of 116 of serum sCD96 in healthy individuals could be detectable.
As CD96 is known as a T cell-activated late expressed molecule, we supposed the level of sCD96 might be elevated in sera of patients with chronic inflammation. Hepatitis B is recognized as endemic in China by the World Health Organization [17]. Roughly 400 million people are infected with HBV and 30 million people are infected chronically in China [18]. During a 5-year period, 10–20% of patients with chronic hepatitis developed to cirrhosis. The Child–Pugh score (sometimes referred to as the Child–Turcotte–Pugh score) is used to assess the prognosis of chronic liver disease, mainly cirrhosis, using bilirubin, albumin, international normalized ratio, the presence and severity of ascites and encephalopathy and to classify the prognosis of liver cirrhosis patients in classes A, B or C. Class A has a favourable prognosis, while class C is at high risk of death. By collecting sera of patients with viral hepatitis B or hepatic cirrhosis after HBV infection and analysing the levels of sCD96 in these sera with the ELISA system that we established, we found that the sera level of sCD96 in patients with chronic viral hepatitis B or hepatic cirrhosis of classes B and C was much higher than that in healthy individuals, but there was no significant difference between the level of sCD96 in sera of acute viral hepatitis B patients, class A hepatic cirrhosis and that of healthy individuals.
Immediately after the exponential phase of HBV expansion, a typical acute innate immune response was shown by the robust activation of interferon and TNF involved with NK and NK T cells [19,20]. In contrast to the innate immune response in the acute phase of hepatitis B, the adaptive immune response was involved in chronic hepatitis B by activation of specific CD8+ T cells and secretion of T cell-associated cytokines. The results showing that sera sCD96 levels were much higher in patients with chronic viral hepatitis B and hepatic cirrhosis in classes B and C than that in healthy individuals are in accord with the observation that CD96 is a T cell-activation increased late-expression molecule and suggest that sCD96 may be a serum marker in chronic inflammatory diseases.
Meanwhile, there was no apparent correlation between the level of sCD96 in sera of viral hepatitis B patients and the level of serum ALT (r = −0·113, P = 0·489), AST (r = 0·013, P = 0·907) or HBV DNA load in sera (r = −0·181, P = 0·265). To our knowledge, serum AST and ALT concentrations were used to evaluate the severity of hepatic injury, whereas sCD96 was involved in chronic inflammatory response, and sCD96 may be not involved in the function of hepatic cells or the direct effect of HBV virus. There was no apparent correlation between the level of sCD96 in sera of viral hepatitis B patients, and the level of serum ALT, AST or HBV DNA load in sera was acceptable.
Generally, secretion of the soluble form of membrane molecules is by two types of mechanism. One is by release of the extracellular domains of transmembrane proteins by ectodomain shedding. This shedding occurs in a variety of proteins, including cytokines and adhesion molecules, and is a known mechanism for the secretion of soluble molecules. The other mechanism involves splicing at mRNA level. Until now, the origin of sCD96 found in sera has remained unclear. Three subtypes of hCD96 at protein level have been found recently. Two of them, both having a transmembrane domain and constructed with 569 amino acids and 585 amino acids, respectively, may be transmembrane molecules. The other subtype, having no transmembrane domain with 402 amino acids, may be a soluble molecule. Comparing three subtypes of hCD96 at protein level, only two isoforms of the hCD96 mRNA have been cloned. Furthermore, using the blast method in National Center for Biotechnology Information, no corresponding mRNA isoform was found to match the soluble form of hCD96. Combining the molecular weight of sCD96 in sera we obtained by Western blot, we supposed that sCD96 detected in our study was released by shedding from the membrane molecule. However, the exact mechanism needs to be clarified by further studies.
In conclusion, in this study we first established a sandwich ELISA kit to detect sCD96 with mAbs anti-hCD96D1 and detected the level of sCD96 in sera of healthy individuals and patients with viral hepatitis B and hepatic cirrhosis. The level of sCD96 in patients with chronic viral hepatitis B or hepatic cirrhosis in classes B and C was much higher than that in healthy individuals. CD96 may be a clinical indicator in some chronic inflammatory diseases such as chronic viral hepatitis B and hepatic cirrhosis in classes B and C of hepatic cirrhosis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 30771964 and no. 30570268).We thank Dr Xuefan Bai and Ms Ye Zhang for help with collection of sera samples.
References
- 1.Velu V, Saravanan S, Nandakumar S, et al. Relationship between T-lymphocyte cytokine levels and sero-response to hepatitis B vaccines. World J Gastroenterol. 2008;14:3534–40. doi: 10.3748/wjg.14.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marek A, Brodzicki J, Liberek A, Korzon M. TGF-beta (transforming growth factor-beta) in chronic inflammatory conditions – a new diagnostic and prognostic marker? Med Sci Monit. 2002;8:145–51. [PubMed] [Google Scholar]
- 3.Kiki I, Yilmaz O, Erdem F, Gundogdu M, Demircan B, Bilici M. Tumour necrosis factor-alpha levels in hepatitis B virus-related chronic active hepatitis and liver cirrhosis and its relationship to Knodell and Child–Pugh scores. Int J Clin Pract. 2006;60:1075–9. doi: 10.1111/j.1742-1241.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 4.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno CM, Sciacca C, Cilio D, et al. Circulating adhesion molecules in patients with virus-related chronic diseases of the liver. World J Gastroenterol. 2005;11:4566–9. doi: 10.3748/wjg.v11.i29.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knolle PA, Eckardt AJ, Protzer-Knolle U, et al. ICAM-1 (sCD54) and LFA-3 (sCD58) in chronic hepatitis B – a longitudinal study in patients treated with interferon-alpha. Z Gastroenterol. 1997;35:459–67. [PubMed] [Google Scholar]
- 7.Fuchs A, Colonna M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16:359–66. doi: 10.1016/j.semcancer.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Ye X, Zhang Z, Jiang Y, et al. Expression of human CD226 on T cells and natural killer cells and of soluble CD226 in plasma of HIV-1-infected Chinese patients. Viral Immunol. 2006;19:576–81. doi: 10.1089/vim.2006.19.576. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Liu XS, Zhu Y, Liu Y, Xue XG, Jin BQ. Preparation, characterization and application of monoclonal antibodies to Fc fragment of Ig fusion protein. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003;19:170–1. [PubMed] [Google Scholar]
- 10.Wang PL, O'Farrell S, Clayberger C, Krensky AM. Identification and molecular cloning of tactile. A novel human T cell activation antigen that is a member of the Ig gene superfamily. J Immunol. 1992;148:2600–8. [PubMed] [Google Scholar]
- 11.Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172:3994–8. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 12.Burger R, Hansen-Hagge TE, Drexler HG, Gramatzki M. Heterogeneity of T-acute lymphoblastic leukemia (T-ALL) cell lines: suggestion for classification by immunophenotype and T-cell receptor studies. Leuk Res. 1999;23:19–21. doi: 10.1016/s0145-2126(98)00133-7. [DOI] [PubMed] [Google Scholar]
- 13.Gramatzki M, Ludwig WD, Burger R, et al. Antibody TC-12(‘unique’) and TH-111(CD96) characterize T-cell acute lymphoblastic leukemia and a subgroup of acute myeloid leukemia. Exp Hematol. 1998;26:1209–14. [PubMed] [Google Scholar]
- 14.Hosen N, Park CY, Tatsumi N, et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci USA. 2007;104:11008–13. doi: 10.1073/pnas.0704271104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaname T, Yanagi K, Chinen Y, et al. Mutations in CD96, a member of the immunoglobulin superfamily, cause a form of the C (opitz trigonocephaly) syndrome. Am J Hum Genet. 2007;81:835–41. doi: 10.1086/522014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baury B, Masson D, McDermott BM, Jr, et al. Identification of secreted CD155 isoforms. Biochem Biophys Res Commun. 2003;309:175–82. doi: 10.1016/s0006-291x(03)01560-2. [DOI] [PubMed] [Google Scholar]
- 17.Liang XF, Chen YS, Wang XJ, et al. A study on the sero-epidemiology of hepatitis B in Chinese population aged over 3-years old. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26:655–8. [PubMed] [Google Scholar]
- 18.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089–94. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 19.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–9. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 20.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:6669–74. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]


