Abstract
A number of immunological functions are dependent on circadian rhythms and regular sleep. This has impact on the type and magnitude of immune responses following antigenic challenge, for example in vaccination. Little is known about the underlying mechanisms. One possibility may be the circadian and sleep-dependent modulation of CD4+CD25− T cell responses by CD4+CD25+ natural regulatory T cells (nTreg). In a variety of studies, nTreg have been shown to regulate T cell responses negatively. Thus, we investigated the influence of sleep and circadian rhythm on the number and function of nTreg as well as on the function of CD4+CD25− T cells. Seven healthy young men were examined under defined conditions on two occasions, i.e. during sleep and sleep deprivation. Venous blood was drawn periodically; numbers of nTreg, suppressive activity of nTreg, interleukin-2 production and proliferation of CD4+CD25− T cells were explored in vitro. nTreg counts revealed a significant circadian rhythm with highest levels during the night (mean 95 nTreg/µl) and lowest levels during the day (mean 55 nTreg/µl). During normal sleep, the suppressive activity of nTreg was highest at 02.00 h and somewhat lower at 15.00 h. Surprisingly, almost no suppressive activity was present at 07.00 h. Deprivation of sleep abrogated this rhythm. CD4+CD25– T cell proliferation was dampened significantly by sleep deprivation. This is the first study in human cells to show that nTreg number and function follow a rhythm across the 24-h period. Furthermore, sleep deprivation severely disturbs the functional rhythm of nTreg and CD4+CD25– T cells.
Keywords: CD4+ T lymphocytes, CD4+CD25+, circadian rhythm, regulatory T cells, sleep
Introduction
A number of immunological functions are dependent on circadian rhythms and on regular sleep [1–5], including the type and magnitude of immune responses following antigenic challenge. For example, vaccination with hepatitis B vaccine in the afternoon resulted in a distinctly higher mean antibody titre when compared with morning vaccination [6]; circadian rhythm and sleep-dependent mechanisms also modulate immune responses. Lange and co-workers showed that sleep deprivation significantly dampens the immune response to hepatitis A vaccination [7]. The importance of sleep in the modulation of number and/or function of elements of the adaptive immune system has been demonstrated similarly by others [8]. However, the underlying mechanisms are unknown, although the importance of circadian and sleep-dependent rhythm of hormones with immunmodulatory function has been shown [9–12].
In this context, the natural regulatory T cells (nTreg) may be of utmost relevance as key regulators that shape adaptive immune responses. nTreg are defined as CD4+ T cells with a high cellular surface expression of CD25 molecules. CD25 is the alpha-chain of the interleukin (IL)-2 receptor and it has been shown that homeostasis and maintenance of nTreg are IL-2-dependent [13,14]. They are usually considered to attenuate excessive immune reactions which may otherwise result in tissue damage. In vitro studies have shown clearly that nTreg suppress the CD4+CD25− T cell proliferation and IL-2 production [13,15]. Experiments in animals and humans showed that nTreg control systemic homeostasis, total lymphocyte numbers [16] and immune responses to pathogens or self-antigens [17–21].
We hypothesized that both time and sleep play a major role in shaping immune responses by nTreg and CD4+CD25− T cells. Here, we analysed whether nTreg and CD4+CD25− T cell function follows a circadian rhythm and whether this rhythm is influenced by sleep or sleep deprivation.
Materials and methods
Experimental design, procedure and subjects
Experiments were performed with seven subjects (aged between 21–32 years) following a within-subject cross-over design with two conditions (sleep and sleep deprivation), as published previously [3]. Each subject participated in two experimental sessions, each covering 24 h and starting at 20.00 h. The protocol was approved by the local ethical committee and all subjects signed an informed consent. Subject inclusion criteria were as follows: subjects were male, mentally and physically healthy (examined by medical history, physical examination and routine laboratory testing), body mass index between 18–26 kg/m2, no sleep disturbances, were non-smokers and were not taking medication.
In brief, the experimental design was as follws: heparinized blood was sampled first at 20.00 h, then every 1·5–3 h for CD4+CD25+ nTreg counts were determined in the peripheral blood. At five time-points (20.00, 02.00, 07.00, 15.00 and 20.00 h) additional blood was sampled for isolation and functional analysis of nTreg and T cells. Each subject spent an adaptation night in the sleep laboratory; sleep was determined off-line from polysomnographic recordings following standard criteria [22]. All subjects received standardized meals and blood samples were always processed immediately.
Sleep quality
To ensure that the subjects slept well in the sleep condition, the sleep quality was monitored using polysomnographic recordings (electro-encephalogram: EEG). EEG measures were analysed according to previously published standards [22]. Mean time until sleep onset was 24·4 ± 5·5 min. Sleep time was 452·9 ± 6·0 min – time in stage 1 sleep 29·1 ± 4·9 min; stage 2 sleep 226 ± 23·7 min; slow wave sleep (SWS) 74·1 ± 10·4 min; and rapid eye movement (REM) sleep 72·3 ± 10·1 min. Latencies (with reference to sleep onset) were 34·8 ± 16·2 min for SWS and 188·1 ± 37·4 min for REM sleep. In all subjects, SWS predominated during the first half of the night (44·4 ± 7·3 min versus 29·8 ± 8·8 min during the first half and second half of the night respectively; P < 0·02), while REM sleep dominated during the second half of the night (6·8 ± 2·7 min versus 66·8 ± 8·7 min during the first half and second half of the night respectively; P < 0·0002).
CD4+CD25+ counts in peripheral blood mononuclear cells
Absolute counts of CD4+ T helper cells were determined by a ‘lyse no-wash’ flow cytometry procedure applying Truecount® tubes (BD Biosciences, Heidelberg, Germany) on the Calibur® using CellQuest® Software (BD Biosciences), as described previously [23]. For the detection of Treg whole blood was incubated with αCD4-monoclonal antibodies (mAb) labelled with fluorescein (Diatec, Oslo, Norway) and αCD25-mAb labelled with phycoerythrin (BD Biosciences), erythrocytes were lysed, washed and resuspended and at least 1 × 105 CD4+ cells were analysed. The absolute count of Treg was calculated on the basis of percentage of these cells from CD4+ cells.
Peripheral blood mononuclear cells and plasma isolation
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood using CPT® Vacutainer (BD Bioscience) following the manufacturer's instructions. Plasma was collected and inactivated for 30 min at 56°C and centrifuged at 4500 g. The supernatant was used as inactivated plasma.
Carboxyfluorescein diacetate staining
To detect cellular proliferation, cells were stained with carboxyfluorescein diacetate (CFDA) (Vybrant, Leiden, the Netherlands), according to the manufacturer's instructions (Cambrex, Verviers, Belgium).
Magnetic affinity cell sorter (MACS®) isolation of cellular subpopulations
For analysis of the suppressive activity of CD4+CD25+ nTreg on the proliferation CD4+CD25− T cells, two strategies were applied: the first was to purify nTreg and to add them to purified CD4+CD25− T cells. The second was a depletion strategy: here we analysed the CD4+ T cell proliferation in PBMC and nTreg-depleted PBMC.
T cell isolation
CD4+ T cells were isolated from PBMC and separated into CD4+CD25+ and CD4+CD25− populations by applying the autoMACS® Separator (Miltenyi Biotec, Bergisch-Gladbach, Germany) using the CD4+CD25+ Treg isolation kit® (Miltenyi Biotec), following the manufacturer's instructions. Purities were controlled using flow cytometry (Fig. 1a). The remaining cells were enriched for monocytes by plastic adherence for 2·5 h and, after harvesting, were irradiated with 60 Gy using a cobalt source. For proliferation assays, half the CD4+CD25− T cells were stained with CFDA. These cells served as reporter T cells. The other half were left unstained for control purposes.
Fig. 1.
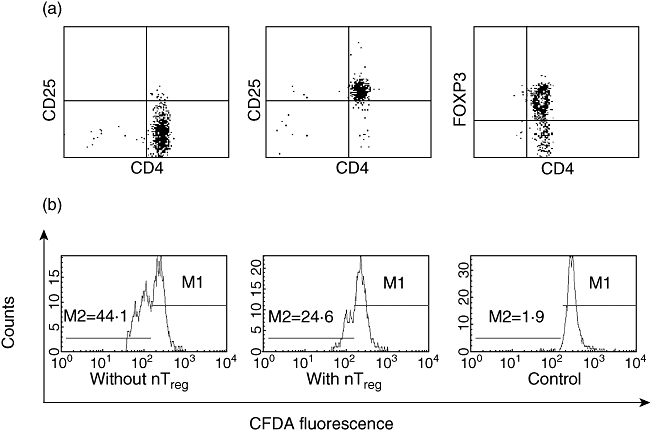
T cell and natural T regulatory (nTreg) cell purity and fluorescence activated cell sorter (FACS) analysis of CD4+CD25− T cell proliferation. (a) CD4+CD25− T cells (left panel) and CD4+CD25+ regulatory T cells (middle and right panel) were isolated from peripheral blood mononuclear cells (PBMC) applying magnetic affinity cell sorter (MACS®) technology. Purified T cells were stained with αCD4-monoclonal antibody (mAb) labelled with allophycocyanin and αCD25-mAb labelled with phycoerythrin or with αCD4-mAb labelled with fluorescein and αforkhead box P3 (FoxP3)-mAb labelled with allophycocyanin and were then analysed by flow cytometry. Mean purity of CD4+CD25− T cells was 96·8% ± 0·6% and mean purity of CD4+CD25+ nTreg was 79·4% ± 7·1%. (b) CD4+CD25−carboxyfluorescein diacetate (CFDA)+ T cells were cultured with nTreg (middle panel) or without nTreg (left panel) in the presence of irradiated adherent cells and αCD3-mAb. Proliferation of CD4+CD25−CFDA+ T cells was measured as the reduction in CFDA fluorescence. M2 represents the percentage of proliferated CD4+CD25− T cells. Control cultures were performed without αCD3-mAb (right panel). Data are from one representative experiment of 70.
Isolation of CD25− PBMC
The PBMC were stained with CFDA. Cells were split into two fractions. One was stored at 4°C until assayed and the other was incubated with 20 µl of CD25-microbeads per 1 × 104 PBMC (Miltenyi Biotec), following the manufacturer's instructions. The CD25-microbead incubated PBMC were then depleted from all CD25+ cells on the autoMACS® Separator (Miltenyi Biotec). These will be referred to as CD25− PBMC.
Functional assays
For functional analysis of nTreg suppressive activity on reporter T cell proliferation, we performed a widely used and previously published assay [24]. In brief, 4 × 104 CFDA-stained reporter T cells, 1 × 105 adherent cells and either 2 × 104 CD4+CD25− T cells or 2 × 104 CD4+CD25+ nTreg were co-cultured for 62 h in 200 µl X-Vivo 15 medium + 1% inactivated plasma and stimulated with 0·5 µg/ml αCD3-mAb (clone Okt3; eBioscience, San Diego, CA, USA). Negative controls were cultured without αCD3-mAb. T cell proliferation was analysed by flow cytometry [fluorescence activated cell sorter (FACS)Calibur® with CellQuest® Pro software; BD Biosciences] measuring the amount of incorporated CFDA. CFDA fluorescence intensity decreases with every cell division (Fig. 1b). The supernatant was then collected and frozen at −80°C for IL-2 analysis (see below).
For presentation the suppressive activity of CD4+CD25+ nTreg on the T cell proliferation the data were transformed by calculating the percentage of inhibition, as published previously [25], according to the following formula:
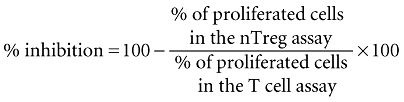 |
Secondly, 3 × 105 PBMC and 3 × 105 PBMC depleted for CD25+ cells (nTreg), both stained with CFDA, were stimulated with 0·5 µg/ml αCD3-mAb and cultured for 62 h in 200 µl X-Vivo 15 medium + 5% autologous, inactivated plasma. Subsequently, cells were stained with anti-human CD4-antibody labelled with allophycocyanin (APC) (Miltenyi Biotec) and analysed immediately by flow cytometry. The proliferation of CD4+ T cells was measured by gating on the CD4+ T lymphocytes and quantifying the decrease of CFDA fluorescence intensity.
Cytokine analysis
The collected supernatants from assays with and without nTreg were analysed for IL-2 using the Bio-Plex™ cytokine assay (BioRad, München, Germany) on the Bio-Plex™ protein array system (BioRad), following the manufacturer's instructions.
Forkhead box P3 expression in nTreg
Forkhead box P3 (FoxP3) expression was measured in isolated PBMC. PBMC were stained with αCD4-mAb labelled with fluorescein isothiocyanate and αCD25-mAb labelled with phycoerythrin (Miltenyi Biotec). FoxP3 expression was determined with the αFoxP3-mAb labelled with APC (clone PCH101, eBioscience) using the human Treg staining kit (eBioscience). FoxP3 expression was analysed by gating on CD4+CD25+ lymphocytes applying the geometric mean algorithm (average of the logarithm of axis channel number) on the FACSCalibur® (BD Biosciences) applying CellQuest® Pro software (BD Biosciences).
Statistics
Statistical analysis was based on repeated-measures analysis of variance (anova), including the factors ‘sleep/sleep deprivation’ (reflecting the condition) and time (reflecting the different time-points of measurement).
For post hoc statistical analysis between the same time-points under different conditions, Student's t-test for paired samples with a 95% confidence interval was performed. Because of the explorative character of the study no adjustment of the confidence interval was performed. To identify circadian rhythms in nTreg numbers in peripheral blood, cosinor analysis was performed separately for the sleep and sleep deprivation condition using Chronolab software [26]. Missing data were interpolated by taking the mean of the subject (from all time-points) plus the mean of the time-point from the remainder of all subjects divided by 2. anova was performed only if the amount of missing values were below 10% of all values.
Results
Numbers of nTreg in peripheral blood display a circadian rhythm
In a first set of experiments, we analysed the numbers of CD4+CD25high lymphocytes (nTreg) in the peripheral blood over a period of 24 h (Fig. 2a). As can be seen in Fig. 2b, there was a steady ascent from 20.00 h to 02.00 h during the night followed by a descent during the subsequent hours until 11.00 h. In the afternoon and evening, the numbers rose again. The same rhythm was observed when quantifying the percentage of nTreg of all CD4+ T cells (data not shown). There was no significant difference in the overall pattern between conditions of sleep and sleep deprivation. Cosinor analysis revealed a significant circadian rhythm (P < 0·001, Fig. 2b) with a peak (acrophase) of nTreg counts around 02.00 h.
Fig. 2.
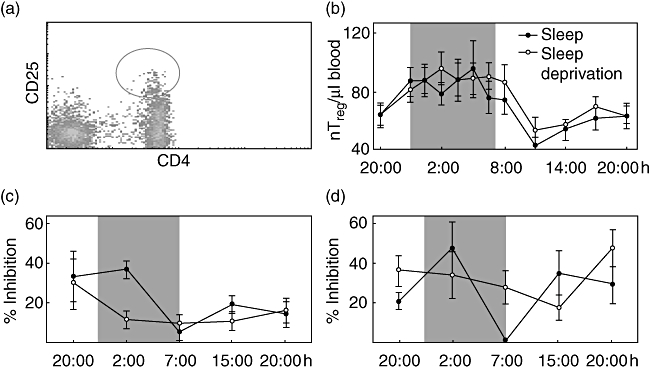
Absolute counts of natural T regulatory (nTreg) and suppression of CD4+ T cell proliferation through nTreg. (a) To analyse the number of CD4+CD25+ regulatory T cells in peripheral blood we quantified the CD4+CD25high cells indicated by the circle. The percentage of forkhead box P3+ (FoxP3+) cells within the CD4+CD25high population did not differ over the 24-h period or between the sleep and sleep deprivation condition; 91·5% ± 1% of the CD4+CD25high cells were positive for FoxP3 (data not shown). (b) CD4+CD25+ regulatory T cells were counted in peripheral blood from healthy men during sleep (closed circles) or sleep deprivation (open circles). (c) CD4+CD25− T cells and nTreg were isolated from peripheral blood of healthy young men with sleep (closed circles) and without nocturnal sleep (open circles). CD4+CD25− T cells were stimulated polyclonally either in the presence or absence of nTreg. The inhibition of CD4+CD25− T cell proliferation by nTreg was calculated (for detailed information see Material and methods). (d) Peripheral blood mononuclear cells (PBMC) and PBMC depleted of nTreg were stimulated polyclonally and the inhibition of CD4+ T cell proliferation through the presence of nTreg was quantified in cells isolated from healthy young men with sleep (closed circles) or sleep deprivation (open circles). Shaded area indicates bedtime. Mean values ± standard error of the mean (n = 7).
The nTreg suppressive activity has a rhythm and is sleep-dependent
In the sleep condition, the proliferation of CD4+CD25− T cells was suppressed significantly by addition of nTreg at all time-points (P < 0·05) except at 07.00 h (P = 0·78). nTreg suppressive activity [presented as % inhibition (see Material and methods) in Fig. 2c] showed a time-dependent rhythm, with a significant sleep-dependent peak at 02.00 h (P < 0·05) and a nadir at 07.00 h. In contrast, in the sleep deprivation condition nTreg suppressive activity showed no rhythm (Fig. 2c). nTreg were not able to suppress reporter T cell proliferation significantly at 02.00 h, 07.00 h and 15.00 h. From these data, we conclude that nTreg have their highest suppressive activity during the night and that expression of this activity requires normal sleep.
So far, fixed numbers of nTreg and reporter T cells were incubated. However, these experiments did not consider interindividual and circadian differences in the relative quote of nTreg. Therefore, we compared the suppressive activity of nTreg on the proliferation of CD4+ T cells in PBMC which were or were not depleted for CD25+ cells (nTreg). nTreg suppressive activity shown as % inhibition in Fig. 2d reveals a significant rhythm in the sleep condition (F(1,4) = 4, P < 0·01) but not in the sleep deprivation condition.
CD4+CD25− T cell proliferation
To analyse whether CD4+CD25− T cell proliferation is time- or sleep-dependent, we analysed pure populations of reporter T cells stimulated with αCD3-mAB and irradiated adherent cells. As can be seen in Fig. 3a, the proliferation of these cells follows a time-dependent rhythm (F(1,4) = 8·3, P < 0·01) with a peak (acrophase) at night (02.00 h). The proliferation of CD4+CD25− T cell was sleep-dependent (F(1,4) = 3·8, P < 0·05). Sleep deprivation significantly reduced the proliferative capacity of reporter T cells at 15.00 h (P < 0·05).
Fig. 3.
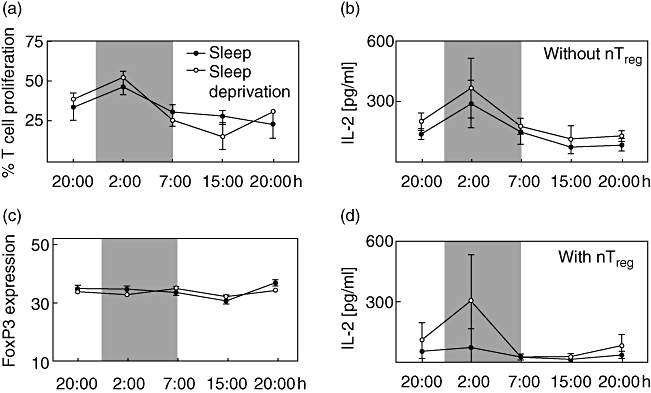
Proliferation and interleukin (IL)-2 production of CD4+CD25− T cells in vitro and forkhead box P3 (FoxP3) expression in natural T regulatory (nTreg) cells. (a) CD4+CD25− T cells were isolated from peripheral blood of healthy young men with sleep (closed circles) or sleep deprivation (open circles), labelled with carboxyfluorescein diacetate (CFDA) and polyclonally stimulated. The percentage of proliferated T cell was measured through the reduction in CFDA fluorescence. (b, d) IL-2 was measured in the supernatants of polyclonally stimulated CD4+CD25− T cells in the absence (b) or presence (d) of nTreg. (c) FoxP3 expression levels in nTreg (CD4+CD25high) were measured as ‘Geo Mean’ applying flow cytometry. Cells were isolated from peripheral blood of healthy young men with sleep or sleep deprivation. Shaded area indicates bedtime. Mean values ± standard error of the mean (n = 7).
The IL-2 levels in cell culture supernatants are time-dependent
Based on the results obtained so far, we were interested to determine whether IL-2 secretion and its suppression by nTreg was time- or sleep-dependent. In the assay with only purified CD4+CD25− T cells the IL-2 analysis in supernatant revealed a time-dependent rhythm (F(1,4) = 14·3, P < 0·001). The peak of IL-2 release was measured at 02.00 h. However, no sleep dependency was observed (Fig. 3b). In the presence of nTreg lower IL-2 amounts were secreted during sleep compared with sleep deprivation at 02.00 h, but this difference did not reach significance (Fig. 3d).
The FoxP3 expression in nTreg
To understand the possible mechanisms underlying the rhythm of nTreg suppressive function, we analysed the FoxP3 expression level in CD4+CD25+ T cells (nTreg). The FoxP3 expression in nTreg showed neither a time nor sleep dependency (Fig. 3c) and did not correlate with % inhibition.
Discussion
We could show that nTreg numbers in the peripheral blood of healthy donors follow a sleep-independent circadian rhythm, with highest numbers at night and lowest numbers in the morning. In contrast, nTreg function follows a sleep-dependent rhythm, with highest suppressive activity during sleep. Previous studies have analysed mainly polyclonally stimulated unseparated lymphocytes [27–29], whereas we analysed purified nTreg in their capacity to suppress the proliferation of polyclonally activated purified CD4+CD25− T cells (reporter T cells). It became apparent that the suppressive activity of nTreg required sleep. In only the sleep condition, nTreg suppressive activity followed a rhythm with a zenith at night and a subsequent nadir in the morning. In terms of immunological homeostasis it seems reasonable that at night, when reporter T cell proliferation and IL-2 production is highest, nTreg suppressive function also should be high, otherwise excessive immune responses could result. nTreg are considered to dampen detrimental immune reactions [13,18,20,30], therefore the data suggest that normal sleep might be essential for immune homeostasis. Further studies will address the question whether the rhythm of nTreg suppressive activity might be a factor in the aggravation of immune responses by sleep deprivation, as demonstrated in chronic inflammatory diseases and allergy [31,32].
Circadian rhythms and sleep dependency of immune functions often follow changes in cortisol, catecholamines, prolactin, growth hormone and melatonin, which are immune-modulating hormones that are regulated profoundly by the circadian pacemaker or sleep [33,34]. It has been shown in autoimmune syndromes as experimental autoimmune encephalomyelitis, systemic lupus erythematosus and multiple sclerosis that glucocorticoids and sex hormones promote nTreg function [35–38]. For other hormones an in vivo affect on nTreg function has not been shown.
After finding a sleep-dependent rhythm of the suppressive nTreg function we tried to identify the underlying molecular mechanism. FoxP3 is the best-described marker for nTreg[39,40]. Mutations in the FoxP3 gene are related to autoimmune diseases in humans [40] and FoxP3 expression in CD4+CD25+ nTreg correlates directly with the magnitude of the suppressive capacity of CD4+CD25+ nTreg[38]. We investigated whether FoxP3 expression is sleep-dependent and/or follows a circadian rhythm in nTreg. Surprisingly, we found no difference in FoxP3 expression, neither comparing sleep versus wake conditions, nor did we find a circadian rhythm. There might be other nuclear factors which are relevant to the activity of FoxP3, such as the nuclear factor of activated T cells [41]. Therefore, the detection of FoxP3 expression levels might not be sufficient for the analysis of FoxP3 activity.
In addition to the sleep-dependent suppressive activity of nTreg, we found the rhythm of reporter T cell proliferation and IL-2 production to be in line with previous reports on the circadian rhythms of adaptive immune responses with a proinflammatory activity peak at night [27,42–47]. In our study the nocturnal peak in IL-2 production and the reporter T cell proliferation was time-dependent but sleep-independent. Conflicting data are published on the role of sleep for IL-2 production. Some authors have shown that the production of IL-2 is increased in the sleep condition [27,29], whereas others have not [4]. The discrepancies are due probably to differences in the in-vitro assays used in those studies. Currently, it is impossible to decide which assay is most suitable to reflect the in vivo situation.
Interestingly, we found a sleep-dependent rhythm of reporter T cell proliferation, with lower levels in sleep deprivation condition during the day after sleep deprivation. This finding would be consistent with the wildly held view that sleep deprivation increases susceptibility to infectious diseases [2].
In conclusion, we demonstrated that nTreg numbers in the peripheral blood of healthy donors follow a circadian rhythm which is independent of sleep, whereas nTreg function follows a sleep-dependent rhythm, with the highest suppressive activity during sleep. We could also demonstrate that nocturnal sleep deprivation dampens reporter T cell proliferation the following day, which results probably in diminished adaptive immune responses. The mechanisms underlying these changes remain to be determined. Probable candidates that generate the necessary impulses are the clock genes, whose expression pattern follows in most cells an approximately 24-h rhythm [34,48]. We are currently addressing this question.
Acknowledgments
We are grateful to Susanne Diekelmann and Ines Wilhelm, Department of Neuroendocrinology, University of Luebeck for helping us with the planning of the study design and the sleep laboratory protocol and Monika Bajtus for laboratory work. None of the participating institutions and authors have conflicts of interest regarding the study. This study was funded by the DFG, SFB 654 and SFB/TR 22.
References
- 1.Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–5. doi: 10.1182/blood-2003-03-0779. [DOI] [PubMed] [Google Scholar]
- 2.Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–67. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 3.Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immun. 2004;18:341–8. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Dimitrov S, Lange T, Fehm HL, Born J. A regulatory role of prolactin, growth hormone, and corticosteroids for human T-cell production of cytokines. Brain Behav Immun. 2004;18:368–74. doi: 10.1016/j.bbi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- 6.Pöllmann L, Pöllmann B. Tagesrhythmische Unterschiede bei der Antikörperbildung nach Hepatitis-B-Schutzimpfung [Circadian differences of antibody response to hepatitis B vaccination] In: Hofmann F, Stößel U, editors. Arbeitsmedizin im Gesundheitsdienst. Stuttgart: Gentner Verlag [Occupational medicine in medical service]; 1988. pp. 83–7. [Google Scholar]
- 7.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831–5. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–2. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 9.Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–95. [PubMed] [Google Scholar]
- 10.Brand JM, Frohn C, Cziupka K, Brockmann C, Kirchner H, Luhm J. Prolactin triggers pro-inflammatory immune responses in peripheral immune cells. Eur Cytokine Netw. 2004;15:99–104. [PubMed] [Google Scholar]
- 11.Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians. 1996;108:374–81. [PubMed] [Google Scholar]
- 12.Petrovsky N. Towards a unified model of neuroendocrine–immune interaction. Immunol Cell Biol. 2001;79:350–7. doi: 10.1046/j.1440-1711.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 14.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sojka DK, Hughson A, Sukiennicki TL, Fowell DJ. Early kinetic window of target T cell susceptibility to CD25+ regulatory T cell activity. J Immunol. 2005;175:7274–80. doi: 10.4049/jimmunol.175.11.7274. [DOI] [PubMed] [Google Scholar]
- 16.Annacker O, Burlen-Defranoux O, Pimenta-Araujo R, Cumano A, Bandeira A. Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J Immunol. 2000;164:3573–80. doi: 10.4049/jimmunol.164.7.3573. [DOI] [PubMed] [Google Scholar]
- 17.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 19.Mills KH, McGuirk P. Antigen-specific regulatory T cells – their induction and role in infection. Semin Immunol. 2004;16:107–17. doi: 10.1016/j.smim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:389–400. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep of human subjects. Washington, DC: US Government Printing Office; 1992. [Google Scholar]
- 23.Ma L, Scheers W, Vandenberghe P. A flow cytometric method for determination of absolute counts of myeloid precursor dendritic cells in peripheral blood. J Immunol Methods. 2004;2004:215–21. doi: 10.1016/j.jim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk H. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Bäumel M, Männel D, et al. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. J Immunol. 2007;179:154–61. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 26.Mojon A, Fernandez JR, Hermida RC. Chronolab: an interactive software package for chronobiologic time series analysis written for Macintosh computer. Chronobiol Int. 1992;9:403–12. doi: 10.3109/07420529209064552. [DOI] [PubMed] [Google Scholar]
- 27.Born J, Lange T, Hansen K, Mölle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- 28.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–39. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–53. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 30.Yu A, Malek TR. Selective availability of IL-2 is a major determinant controlling the production of CD4+CD25+Foxp3+ T regulatory cells. J Immunol. pp. 5115–21. [DOI] [PubMed]
- 31.Kimata H. Enhancement of allergic skin responses by total sleep deprivation in patients with allergic rhinitis. Int Arch Allergy Immunol. 2002;128:351–2. doi: 10.1159/000063854. [DOI] [PubMed] [Google Scholar]
- 32.Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflamm Res. 2007;56:51–7. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- 33.Cutolo M, Sulli A, Pizzorni C, et al. Circadian rhythms: glucocorticoids and arthritis. Ann NY Acad Sci. 2006;1069:289–99. doi: 10.1196/annals.1351.027. [DOI] [PubMed] [Google Scholar]
- 34.Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu Rev Biochem. 2002;71:307–31. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur J Immunol. 2006;36:2139–49. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- 36.Azab N, Bassyouni IH, Emad Y, Abd El-Wahab GA, Hamdy G, Mashahit MA. CD4+CD25+ regulatory T cells (Treg) in systemic lupus erythematosus (SLE) patients: the possible influence of treatment with corticosteroids. Clin Immunol. 127:151–7. doi: 10.1016/j.clim.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Navaro J, Aristimuno C, Sanchez-Roman S, et al. Circulating dendritic cells subsets and regulatory T-cells at multiple sclerosis relapse: differential short-term changes on corticosteroids therapy. J Neuroimmunol. 176:153–61. doi: 10.1016/j.jneuroim.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Offner H, Vandenbark AA. Congruent effects of estrogens and T-cell receptor peptide therapy on regulatory T cells EAE and MS. Int Rev Immunol. 2005;24:447–77. doi: 10.1080/08830180500371462. [DOI] [PubMed] [Google Scholar]
- 39.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–7. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–62. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Borde M, Heissmeyer V, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 42.Carandente F, Angeli A, De Vecchi A, Dammacco F, Halberg F. Multifrequency rhythms of immunological functions. Chronobiologia. 1988;15:7–23. [PubMed] [Google Scholar]
- 43.Long AA. Findings from a 1000-patient internet-based survey assessing the impact of morning symptoms on individuals with allergic rhinitis. Clin Ther. 2007;29:342–51. doi: 10.1016/j.clinthera.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Miller MA, Cappuccio FP. Inflammation, sleep, obesity and cardiovascular disease. Curr Vasc Pharmacol. 2007;5:93–102. doi: 10.2174/157016107780368280. [DOI] [PubMed] [Google Scholar]
- 45.Petrovsky N, Harrison LC. The chronobiology of human cytokine production. Int Rev Immunol. 1998;16:635–49. doi: 10.3109/08830189809043012. [DOI] [PubMed] [Google Scholar]
- 46.Schenkel EJ. Effect of desloratadine on the control of morning symptoms in patients with seasonal and perennial allergic rhinitis. Allergy Asthma Proc. 2006;27:465–72. doi: 10.2500/aap.2006.27.2936. [DOI] [PubMed] [Google Scholar]
- 47.Spiegel K, Sheridan JF, Van Cuter E. Effect of sleep deprivation on response to immunization. JAMA. 2002;288:1471–2. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 48.King DP, Takahashi JS. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci. 2000;23:713–42. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]


