Abstract
Matrix metalloproteinases (MMPs) have been implicated in tissue damage associated with inflammatory bowel disease (IBD). As the role of the intestinal epithelium in this process is unknown, we determined MMP expression and enzyme activity in human colonic epithelial cells (CEC). MMP mRNA expression was assessed by reverse transcription–polymerase chain reaction in HT-29 and DLD-1 cells and in CEC isolated from biopsies from IBD and control patients. Total MMP activity in the cells was measured by a functional assay, based on degradation of a fluorescent synthetic peptide containing the specific bond for MMP cleavage. HT-29 and DLD-1 expressed several MMPs and levels of MMP-3, -10 and -13 mRNA expression were increased significantly by tumour necrosis factor (TNF)-α exposure. Transcripts of MMP-1, -3, -7, -9, -10 and -12 were detected in CECs and all, except MMP12, at significantly increased levels in cells from inflamed IBD mucosa. MMP-2 and -8 mRNA were expressed inconsistently and MMP-11, -13 and -14 mRNA undetectable. Proteolytic MMP activity was detected in CEC supernatants and the level was increased significantly in inflamed IBD epithelium. The enzyme activity was inhibited strongly by a specific MMP inhibitor (GM 6001). A significant TNF-α-mediated increase in MMP enzyme activity was also detected in HT-29 cells in vitro. In conclusion, the expression of several MMPs as well as the level of functional MMP activity is increased in CEC from patients with active IBD. The results suggest that MMPs released by the intestinal epithelium may be involved in the pathogenesis of IBD by promoting local mucosal damage.
Keywords: Crohn's disease, cytokines, intestinal epithelial cells, MMP, ulcerative colitis
Introduction
Matrix metalloproteinases (MMP) are a large family of zinc-dependent endopeptidases that mediate degradation of essentially all components of the extracellular matrix [1]. A disturbance of the tightly regulated balance between MMPs and their natural tissue inhibitors (TIMPs) has been implicated in the pathogenesis of inflammatory bowel disease (IBD), i.e. Crohn's disease (CD) and ulcerative colitis (UC) [2–8]. It is also apparent that certain MMPs actually act as final mediators of tissue damage associated with intestinal inflammation [9–13]. This has been demonstrated most clearly in an ex-vivo fetal gut model for human IBD in which addition of recombinant MMP-3 caused extensive tissue injury [12]. Moreover, a functional role of MMPs in intestinal inflammation has been suggested from animal experiments, showing that treatment with MMP inhibitors improves experimental colitis in rodents [14–17].
The MMP expression has been detected previously in several cell types in the intestinal mucosa, most notably myofibroblasts, smooth muscle cells, activated T cells and macrophages [6,18–21]. In contrast, little is known currently about the expression pattern, regulation and functional role of MMPs in intestinal epithelial cells. Previous studies using immunohistochemistry have shown only scattered and inconsistent expression of few MMPs localized to the epithelium [22,23]. Interestingly, studies in other areas, such as gingival or dermal cell models, have shown consistently that epithelial cells express strongly a range of MMPs known to be involved in wound repair, inflammation and chronic ulceration [19,24–26]. Finally, recent studies in murine IBD models suggest that MMPs expressed in the epithelium may play a role in the development of colitis through the release of proteolytic activity [16,27]. It is now well established that the human intestinal epithelium plays an active role in local immune responses through expression of proinflammatory cytokines, chemokines, adhesion molecules as well as NOD2/CARD15 and Toll-like receptors [18,28–31]. Hence, expression and release of MMPs from these cells may also be involved in the local inflammatory cascades and tissue remodelling processes involved in human IBD.
Here we show here that isolated human intestinal epithelial cells express mRNA for a wide range of MMPs and that transcript levels are increased substantially in epithelial cells obtained from colonic mucosa with active inflammation. Moreover, we provide evidence that functional MMP activity, as measured by a peptide cleavage assay, is present in colonic epithelial cells and that proteolytic activity is increased in patients with active IBD.
Materials and methods
Patients
Colonic biopsies were obtained from 11 patients with UC and eight patients with CD, diagnosed according to standardized criteria. They comprised eight females and 11 males, with a median age of 41·5 years (range 22–66). All patients with UC received oral 5-aminosalicylic acid at a daily dose of 1·6–3·2 g. One patient with CD received oral prednisolone (20 mg daily) and four received azathioprine (2–2·5 mg/kg daily). Biopsies were taken from endoscopically inflamed areas of the transverse, descending or sigmoid colon and, if present, in parallel from uninflamed mucosa from the same patient.
The control group comprised nine patients, three females and six males with a median age of 54 years (range 27–71), undergoing diagnostic colonoscopy showing no endoscopic sign of inflammatory or malignant disease. Biopsy specimens were taken with radial jaw biopsies (Microvasive, Watertown, MA, USA). The study was approved by the Regional Ethics Committee for Copenhagen County Hospitals (KA00077) and all participants gave informed and written consent.
Isolation of primary colonic epithelial cells
Colonic epithelial cells were isolated from eight endoscopical biopsies using short-time ethylenediamine tetraacetic acid (EDTA)/ethylene glycol-bis(β-aminoethyl ether)-N,N,N,N'-tetraacetic acid) (Sigma, St Louis, MO, USA) treatment, as described previously [32]. This method provides intact crypts with less than 5% contaminating cells and approximately 50% viable cells at 24 h as judged by 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) metabolism, vital staining and electron microscopy [32]. Total mRNA was extracted by the addition of Trizol LS Reagent (Invitrogen, Parsley, UK) to the cells immediately after the isolation procedure and reverse transcriptase–polymerase chain reaction (PCR), performed as mentioned below. For MMP activity assay, cells were seeded in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Paisley, UK) for 4 h and enzyme activity assessed in cell medium. Viability of the cell cultures were assessed by MTT assay, as described previously [32,33].
Transformed human intestinal cell line cultures
HT-29 and DLD-1 cells were passaged in 5% CO2 at 37°C in DMEM supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco). For experiment, 106 and 105 cells were seeded in 24- or 96-well culture plates (NUNC, Naperville, IL, USA) respectively, and cultured in DMEM containing FCS, 10 mM HEPES buffer (Gibco), 50 IU/ml penicillin, 50 µg/ml streptomycin and 0·5 mg/ml gentamycin (Gibco) and grown for 24 h to obtain semi-confluence prior to use. Cell cultures were stimulated with tumour necrosis factor (TNF)-α (10–9 M) alone or in combination with interleukin (IL)-1β and interferon (IFN)-γ (Sigma) (both 10–9 M) for 6–48 h and total mRNA was extracted by the addition of Trizol LS Reagent.
The MMP mRNA expression in intestinal epithelial cells
Complementary single-stranded DNA (cDNA) was synthesized using 1 µg of total RNA from HT-29, DLD-1 cells and primary colonic epithelial cells and amplified, as described previously [22]. The MMP primers, annealing temperatures and numbers of cycles used were optimized in preliminary experiments and are summarized in Table 1. As a control for genomic DNA in the samples, PCR was performed using total RNA without the reverse-transcription reaction. A placental cDNA library was used as positive control for the individual MMPs. PCR products were size-separated in 2% agarose gels in Tris–acetate–EDTA. The products were scanned using an Image Master (Amersham-Pharmacia Biotech, Hørsholm, Denmark). The MMP PCR products were adjusted to glyceraldehyde-3-phosphate dehydrogenase expression and mRNA-expression in cytokine-stimulated cultures are presented relative to the expression in parallel unstimulated cultures.
Table 1.
Primers used in reverse transcriptase-polymerase chain reaction
| Gene | Primer sequence | Length | Annealing temperature | Number of cycles |
|---|---|---|---|---|
| GAPDH | 5′-GAGAATTCGAGTCAACGGATTTGGTCGT-3′ | 175 bp | 55°C | 18 |
| 5′-GCGAATTCGGTGCCATGGAATTTGGCAT-3′ | ||||
| MMP-1 | 5′-CTGTTCTGGGGTGTGGTGTCTCA-3′ | 352 bp | 58°C | 37 |
| 5′-CTCTTGGCAAATCTGGCGTGTAAT-3′ | ||||
| MMP-2 | 5′-TGCGGCACCACTGAGGACTACGAC-3′ | 355 bp | 55°C | 37 |
| 5′-TCCGGGAGCTCAGGCCAGAATGT-3′ | ||||
| MMP-3 | 5′-CATTTTGGCCATCTCTTCCTTCAG-3′ | 347 bp | 55°C | 45 |
| 5′-GAACCCAAATTCTTCAAAAACAGC-3′ | ||||
| MMP-7 | 5′-GGTCACCTACAGGATCGTATCATAT-3′ | 373 bp | 58°C | 24 |
| 5′-CATCACTGCATTAGGATCAGAGGAA-3′ | ||||
| MMP-8 | 5′-ACCCCAGGAAACCCCAAG-3′ | 204 bp | 59°C | 37 |
| 5′-GTCACCGTGATCTCTTTGGTAAA-3′ | ||||
| MMP-9 | 5′-GGTCCCCCACTGCTGGCCCTTCTACGGCC-3′ | 640 bp | 60°C | 45 |
| 5′-GTCCTCAGGGCACTGCAGGATGTCATAGGT-3′ | ||||
| MMP-10 | 5′-CACTCTACAACTCATTCACAGAGCT-3′ | 408 bp | 60°C | 40 |
| 5′-CTTGGATAACCTGCTTGTTGTACCTCAT-3′ | ||||
| MMP-11 | 5′-GACTGCAGGGGCGTTCAA-3′ | 444 bp | 55°C | 37 |
| 5′-CTCGGGACCCCAGACCAA-3′ | ||||
| MMP-12 | 5′-CTCTTCCCCTGAACAGCTCTA-3′ | 192 bp | 59°C | 37 |
| 5′-CCAGTTGCCCGGTCACTTT -3′ | ||||
| MMP-13 | 5′-CTGGCTGCCTTCCTCTTCTTGA-3′ | 735 bp | 60°C | 40 |
| 5′-GCTTTTGCCGGTGTAGGTGTAG-3′ | ||||
| MMP-14 | 5′-GGGCCTGCCTGCGTCCATCAACAC-3′ | 517 bp | 58°C | 45 |
| 5′-GCCGCCCTCCTCGTCCACCTCAAT-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP, matrix metalloproteinase; bp, base pairs.
Functional MMP assay
HT-29 cells were incubated with TNF-α (10–9 M) for 24 h. Culture medium was collected and cells were then incubated for additional 4 h in the presence of MTT (2·5 mg/ml) to assess viability of the cultures. Isolated primary human colonic epithelial cells were cultured for 4 h prior to collection of the cell medium and addition of MTT, as above.
Total MMP activity in the cell cultures was measured as the ability to degrade a fluorescent peptide (Mca-Pro-Leu-Gly-Leu-Dap(Dnp)-Ala-Arg-NH2) (M1895, Bachem Biochemical, GMbH, Weil am Rhein, Germany) containing the known cleavage site (Gly-Leu) for MMPs. The cleavage of the Gly-Leu bond separates the highly fluorescent Mca group from the efficient 2,4-dinitrophenyl quencher, resulting in an increase in fluorescence. Cell supernatants were added to an assay buffer containing a cocktail of protease inhibitors (pepstatin (10 µM), leupeptin (10 µM), soyabean trypsin inhibitor (1 µg/ml), trasylol (100 units/ml), Ca2+ (0·2 mM) and Mg2+ (1 mM) (all from Sigma) before incubation with the peptide (25 µg/ml) for 2 h at 37°C. Incubation with a specific MMP inhibitor GM 6001 (1 µM) (Calbiochem, Darmstadt, Germany) was performed in parallel for all samples [34]. Enzyme activity was evaluated by assessment of changes in the fluorescence signal using a multi-channel fluorometer for enzyme-linked immunosorbent assay-type plates (96 wells) (Synergy HT, wavelength 320/387 nm) (Bio-Tek Instruments, Winooski, VT, USA). The reaction was stopped by addition of HCL (0·2 M). Peptide cleavage at the Gly-Leu bond was determined further by reverse-phase high-pressure liquid chromatography (HPLC) (Merck Hitachi) using Lichrocart 75 mm 4 µ c-18 column (Merck, Darmstadt, Germany).
Statistics
Results are presented as means ± standard error of the mean. Data were analysed by t-test for paired or unpaired variables where appropriate (GraphPad Prism version 4·0). Values of P < 0·05 (two-tailed) were considered significant.
Results
Spontaneous and cytokine-induced MMP mRNA expression in intestinal epithelial cell lines
As shown in Fig. 1, we found initially that HT-29 cells expressed mRNA for several MMPs, including MMP-1, -3, -7, -10, -11 and -13, whereas MMP-2, -8, -9, -12 and -14 were undetectable.
Fig. 1.
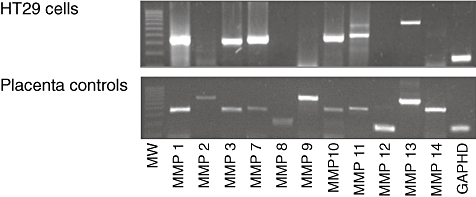
Constitutive matrix metalloproteinase (MMP) mRNA expression in HT-29 cells (upper panel) analysed by reverse transcriptase–polymerase chain reaction. A placental cDNA library was used as a positive control for the MMPs (lower panel). The figure shows data from one representative experiment out of six.
HT-29 cells are known to respond to cytokine exposure by secretion of immunological mediators [28,29,33], and we examined next if this influenced MMP expression. Among the MMPs detected in unstimulated cells, the expression levels of MMP-3, -10 and -13 mRNA increased significantly in HT-29 cell cultures after stimulation with TNF-α for 6 h (P < 0·01) compared with controls (Fig. 2). In contrast, no significant changes were observed in MMP-1, -7 and -11 mRNA expression levels. The maximal effect of TNF-α on MMP mRNA expression was already reached after 6 h of stimulation, and prolonged exposure for up to 48 h tended to decrease mRNA levels, as shown for MMP-3 in Fig. 2 (insert). Among the MMPs not detected in unstimulated HT-29, MMP-12 mRNA, was expressed clearly at a high level after TNF-α stimulation (Fig. 2). In contrast, MMP-2, -8, -9 and -14 mRNA remained undetectable in HT-29 cells even after cytokine stimulation. Exposure to a mixture of TNF-α/IL-1β/IFN-γ induced an increase in MMP mRNA similar to TNF-α exposure, but the effect was less pronounced (data not shown), and TNF-α was therefore used alone in subsequent experiments. Finally, very similar results were observed in another intestinal epithelial cell line, i.e. DLD-1 cells (data not shown).
Fig. 2.

Tumour necrosis factor (TNF)-α effect on matrix metalloproteinase (MMP) mRNA expression in HT-29 cells. After stimulation with TNF-α (10–9 M) for 6 h, MMP and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression were measured by reverse transcriptase–polymerase chain reaction. The MMP PCR products were adjusted to GAPDH expression and mRNA-expression in cytokine-stimulated cultures are presented relative to the expression in parallel unstimulated cultures. MMP-12 mRNA was undetectable in unstimulated cultures, and increase is therefore calculated relative to the arbitrary value 1. Insert shows time-dependency for TNF-α-mediated MMP-3 mRNA expression. The figure includes results from six independent experiments and bar represent means (standard error of the mean). #P < 0·01 compared with expression in unstimulated cultures.
Initial experiments showed that both HT-29 and DLD-1 cells express TIMP1, -2 and -3 mRNA, but (unlike the MMPs) the expression levels remained completely unchanged after cytokine stimulation and therefore this line of investigation was not pursued further (data not shown).
The MMP expression in human colonic epithelial cells from IBD and control patients
Having shown that transformed intestinal epithelial cell lines express mRNA for several MMPs and respond to a proinflammatory stimulus, we next examined whether these features were shared by primary epithelial cell cultures. To achieve this objective, epithelial cells were isolated from colonic biopsy specimens from control patients and patients with active and inactive IBD. As shown in Fig. 3, which displays a representative set of experiments, normal colonic epithelial cells expressed transcripts for MMP-1, -3, -7, -10 and -12 (lane 1). In contrast, MMP-2, -8 and -9 were weaker and expressed more inconsistently. MMP-11, -13 and -14 mRNA were not detected in colonic epithelial cells from control patients. A similar pattern of MMP expression was observed in non-inflamed epithelium from IBD patients (Fig. 3, lanes 2 and 4) and in cells from inflamed IBD areas (Fig. 3, lanes 3 and 5). However, some of the MMPs seemed to be expressed more strongly in the inflamed specimens and MMP-8 was detected clearly in some experiments (Fig. 3), but absent in other samples. These initial experiments showed similar MMP expression pattern and levels of MMP mRNA in cells from UC and CD patients, and the results from the two patient groups were combined in the following experiments.
Fig. 3.

Expression of matrix metalloproteinase (MMP) mRNA in primary human colonic epithelial cells. Reverse transcriptase– polymerase chain reaction was performed on extracted mRNA from colonic epithelial cells isolated from biopsies obtained in control subjects (C) (lane 1). In patients with Crohn's disease (lanes 2 and 3) and ulcerative colitis (lanes 4 and 5), cells were isolated in from biopsies taken in parallel from macroscopically non-inflamed (N) and inflamed (I) mucosa of the same patients. Expression of glyceraldehyde-3-phosphate dehydrogenase is included as a housekeeping gene control. Data from one representative experiment of five are presented.
To extend these qualitative data, we next measured MMP mRNA expression levels in primary cell cultures using semi-quantitative PCR analysis. As shown in Fig. 4, mRNA levels for MMP-1, -3, -7, -9 and -10 were found to be increased significantly in colonic epithelial cells from inflamed areas compared with macroscopic non-inflamed areas taken in parallel from the same patient. Although MMP-8 expression levels tended to be increased in cells from inflamed mucosa, this change did not reach significance (P > 0·05). No significant difference was observed in MMP-12 mRNA (data not shown).
Fig. 4.
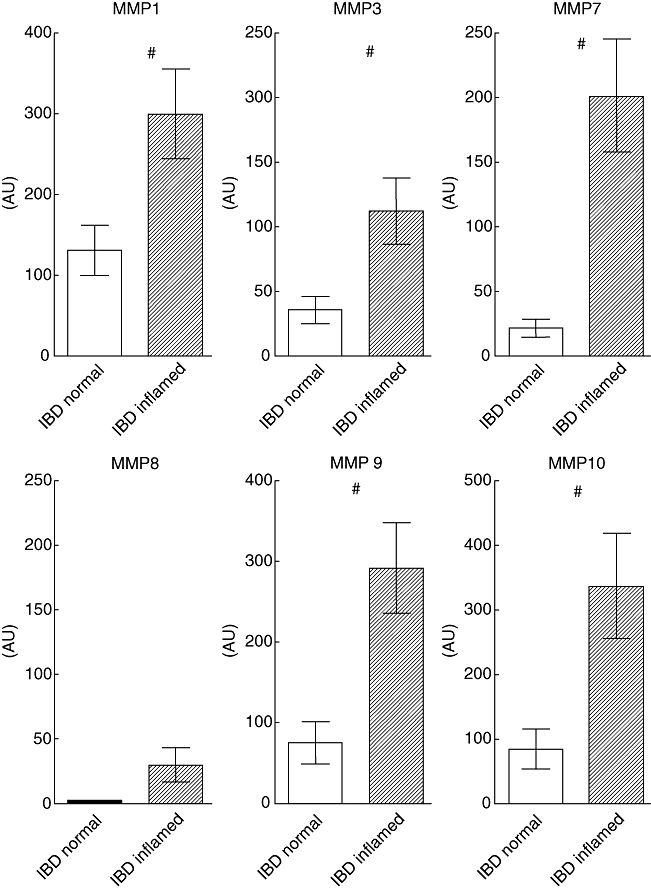
Expression of matrix metalloproteinase (MMP)-1, -3, -7, -8, -9 and -10 mRNA in colonic epithelial cells from inflamed and uninflamed colonic mucosa. Reverse transcriptase–polymerase chain reaction was performed on mRNA extracted from epithelial cells isolated from paired biopsies obtained from inflamed and non-inflamed colonic mucosa from five patients with inflammatory bowel disease. The PCR products were semi-quantified by densitometric scanning and expressed relative to glyceraldehyde-3-phosphate dehydrogenase expression (arbitrary unit, AU). #P < 0·05 compared with expression in epithelial cells from uninflamed mucosa.
Taken together, these results indicate clearly that differentiated colonic epithelial cells from human mucosa have the capacity to express a range of MMPs that is somewhat different from that observed in the cell lines. Furthermore, the presence of inflammation seems to increase expression of MMP-1, -3, -7, -9 and -10.
Functional MMP enzyme activity in human colonic epithelial cells from normal and inflamed mucosa
The MMPs are secreted as proenzymes, which must undergo proteolytic processing to become fully active. We examined first whether HT-29 cells secreted functionally active MMPs, as measured by an assay based on peptide cleavage. As shown in Fig. 5, MMP enzyme activity was present in unstimulated cell line supernatants, and stimulation with TNF-α for 24 h resulted in a significant increase in MMP activity (P < 0·01). Importantly, the levels of MMP activity were inhibited strongly by addition of the specific MMP inhibitor GM 6001. HPLC analysis confirmed that the peptide was cleaved at the MMP-specific bond (data not shown). Notably, we were able to demonstrate that functional MMP activity was also present in cell supernatants from epithelial cell cultures obtained from controls and IBD patients (Fig. 6). No significant difference in enzyme activity was observed between cultures obtained from controls and cells from non-inflamed IBD mucosa. In contrast, the level of functional MMP activity was increased significantly in cell supernatants from inflamed IBD mucosa compared with activity in cells from non-inflamed IBD mucosa from the same patient (P < 0·02) (Fig. 6). The enzyme activity was again inhibited clearly by GM 6001 (P < 0·01), confirming the presence of specific MMP proteolytic activity in the samples. Thus, the presence of functional enzyme activity seems to parallel the MMP expression studies described above (Figs 2 and 3).
Fig. 5.

Spontaneous and tumour necrosis factor (TNF)-α-induced matrix metalloproteinase (MMP) enzyme activity in HT-29 cells. After stimulation with TNF-α (10–9 M) for 24 h, MMP activity in cell supernatant was assessed by measurement of the ability to degrade a fluorescent peptide containing the cleavage sites for MMPs. Incubation with a specific MMP inhibitor GM 6001 was included in all samples, as a control for unspecific degradation of the peptide. The figure shows mean (standard error of the mean) values for six independent experiments. #P < 0·01 compared with activity in unstimulated cultures.
Fig. 6.

Matrix metalloproteinase (MMP) enzyme activity in human colonic epithelial cells from normal and inflamed colonic mucosa. Epithelial cells were isolated from mucosal biopsies from control patients and from uninflamed or inflamed mucosa from patients with ulcerative colitis or Crohn's disease. MMP activity in cell supernatant was assessed by measurement of the ability to degrade a fluorescent peptide containing the cleavage sites for MMPs. Incubation with a specific MMP inhibitor GM 6001 was included in all samples as a control for unspecific degradation of the peptide. The figure shows mean (standard error of the mean) values for five to seven patients. #P < 0·02 compared with specific MMP activity in cells isolated from uninflamed IBD mucosa.
Discussion
In this study, we show that primary epithelial cells isolated from human colonic mucosa express transcripts for a wide range of MMPs, and that MMP-1, -3, -7, -9 and -10 mRNA levels are increased in cells from patients with active IBD. Moreover, we demonstrate that epithelial cell supernatants have functional MMP activity. These data were substantiated by the demonstration of spontaneous and cytokine-induced MMP expression and functional activity in two colonic epithelial cell line models. The expression pattern in these widely used cell models is, however, somewhat different from that seen in isolated human colonic epithelial cells, probably reflecting that they are less representative for the differentiated colonic epithelium because of their malignant origin, as reported previously [30].
Among the MMPs detected, MMP-3, -7 and 10 are stromelysins, known to degrade proteoglycans, collagens, gelatine and laminine. MMP-3 and -10 have been linked strongly to T cell and TNF-α-mediated tissue degradation in the inflamed intestine [7,9,12,22,23], and increased levels are found in mucosal samples from patients with active IBD [3,4,6,7,22,35]. Consistent with this notion, we observed that the transcript levels of MMP-3, -7 and -10 were increased significantly in epithelial cells obtained from inflamed IBD mucosa.
The MMP-3 and -10 mRNA were also detected in epithelial cell lines, and TNF-α treatment, which mimics an inflammatory stimulus, induced a clear increase in both MMPs in HT-29 cells. The detection of MMP-3 transcripts in isolated colonic epithelial cells is also concordant with a single previous study showing MMP-3 protein expression in epithelial cells by immunohistochemistry in severely ulcerated colonic mucosa from patients with UC [23]. MMP-10 has been identified in migrating epithelial cells in an ex vivo model of intestinal ulceration [36], and one study has identified MMP-10 mRNA in ulcerated epithelium of IBD mucosa using in situ hybridization [19].
The MMP-1 transcripts were detected clearly in colonic epithelial cells and, like the stromelysins, the level of this collagenase was also increased significantly in cells from inflamed IBD mucosa. Increased levels of MMP-1 has been reported previously in inflamed IBD mucosa [7,8,20,23,35], but identified mainly in myofibroblasts or mononuclear cells. Our results are, however, in good agreement with two previous studies in humans, reporting MMP-1 expression in enterocytes during intestinal wound healing and in epithelial cells of regenerating areas of necrotizing colitis in children [5,37]. MMP-8, which also belongs to the family of collagenases, was detected mainly, albeit inconsistently, in epithelial cells isolated from inflamed IBD mucosa. MMP-8 has been identified previously in epithelial cells from human oral mucosa and skin, where it has been implicated in chronic inflammation. [24,38,39]. Little is known currently about MMP-8 in intestinal inflammatory conditions, but our findings are in line with a single study in a murine model of IBD, showing an increased MMP-8 expression in the intestinal epithelium in animals with active inflammation [40].
The MMP-9 is a gelatinase that cleaves gelatine, types V, VII and X collagens as well as basement membrane type IV collagen. MMP-9 has been found repeatedly to be up-regulated in immune cells during active flares of IBD in humans [5,6,8,12,22,27,41], and it has also been implicated in regulation of the epithelial barrier function [10,16]. In the present study we found that MMP-9 mRNA was expressed in isolated epithelial cells from normal colon and, like the stromelysins and MMP-1, at significantly higher levels in cells from inflamed IBD mucosa. Unlike the other MMPs, MMP-9 was not detected in the HT-29 or DLD-1 cells. However, the detection of MMP-9 mRNA in primary epithelial cell cultures are in good agreement with two recent studies showing increased epithelial MMP-9 expression in murine models of IBD [16,42]. Although it has also been suggested that regulated MMP-9 expression in goblet cells of the epithelium may play a crucial role in maintaining normal mucosal defence [10,14,16,42,43], further studies are warranted to describe the role of this MMP in human epithelial cell differentiation and immunological function.
The MMPs are secreted as proenzymes, which must undergo proteolytic processing to become fully active. Substrate gel electrophoresis has been used previously to identify MMP activity in colonic mucosa or fetal intestinal explants [5,12,20,22]. This method is based on qualitative identification of the active forms of MMPs based on gelatinase or casein degradation, but is somewhat insensitive and detects mainly MMP-2, -3 and -9 activity [4,44]. To overcome the limitation of this method to assess MMP activities, we measured instead total proteolytic activity directly in epithelial cell supernatants using a synthetic peptide mimicking the cleavage site for MMPs (Mca-Pro-Leu-Gly-Leu-Dap(Dnp)-Ala-Arg-NH2). Unspecific degradation in the samples was blocked by addition of a range of protease inhibitors [2], and HPLC analysis confirmed that the peptide was cleaved at the MMP-specific bond. Using this functional assay, we were able to demonstrate the presence of MMP enzyme activity in cell supernatant from cultures of primary colonic epithelial cells and that the proteolytic activity is increased in cell cultures from patients with active IBD. Addition of a specific MMP inhibitor (GM 6001) [34] confirmed that the proteolytic activity was related to specific MMP activity. Moreover, we found that unspecific degradation of the peptide because of the presence of other protease activities was minimal. We also detected a significant TNF-α-mediated increase in MMP enzyme activity in HT-29 cells in vitro, which is consistent with the increased MMP transcript levels after TNF-α exposure. Interestingly, the peptide cleavage assay technique has been used previously to demonstrate that isolated epithelial cells express the closely related TNF-α-converting enzyme, which belong to the ADAM (a disintegrin and metalloproteinase) family of proteinases [2,29].
In conclusion, we have shown that the expression of several MMPs as well as the levels of functional activity are increased in epithelial cells from patients with active IBD. These results suggest that MMPs released by the intestinal epithelium may be involved in the pathogenesis of IBD by promoting local mucosal damage.
Acknowledgments
The technical assistance of Anne Hallander, Anni Petersen, Birgit Kristensen, Hanne Fuglsang and Vibeke Voxen at the Laboratory of Gastroenterology 5403, Herlev University Hospital, Denmark are greatly appreciated.
References
- 1.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–49. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 2.Brynskov J, Foegh P, Pedersen G, et al. Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut. 2002;51:37–43. doi: 10.1136/gut.51.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis E, Ribbens C, Godon A, et al. Increased production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 by inflamed mucosa in inflammatory bowel disease. Clin Exp Immunol. 2000;120:241–6. doi: 10.1046/j.1365-2249.2000.01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heuschkel RB, MacDonald TT, Monteleone G, Bajaj-Elliott M, Smith JA, Pender SL. Imbalance of stromelysin-1 and TIMP-1 in the mucosal lesions of children with inflammatory bowel disease. Gut. 2000;47:57–62. doi: 10.1136/gut.47.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baugh MD, Perry MJ, Hollander AP, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–22. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 6.von Lampe B, Barthel B, Coupland SE, Riecken EO, Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut. 2000;47:63–73. doi: 10.1136/gut.47.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warnaar N, Hofker HS, Maathuis MH, et al. Matrix metalloproteinases as profibrotic factors in terminal ileum in Crohn's disease. Inflamm Bowel Dis. 2006;12:863–9. doi: 10.1097/01.mib.0000231568.43065.ed. [DOI] [PubMed] [Google Scholar]
- 8.Arihiro S, Ohtani H, Hiwatashi N, Torii A, Sorsa T, Nagura H. Vascular smooth muscle cells and pericytes express MMP-1, MMP-9, TIMP-1 and type I procollagen in inflammatory bowel disease. Histopathology. 2001;39:50–9. doi: 10.1046/j.1365-2559.2001.01142.x. [DOI] [PubMed] [Google Scholar]
- 9.Salmela MT, MacDonald TT, Black D, et al. Upregulation of matrix metalloproteinases in a model of T cell mediated tissue injury in the gut: analysis by gene array and in situ hybridisation. Gut. 2002;51:540–7. doi: 10.1136/gut.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg P, Rojas M, Ravi A, et al. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J Immunol. 2006;177:4103–12. doi: 10.4049/jimmunol.177.6.4103. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald TT, Pender SL. Proteolytic enzymes in inflammatory bowel disease. Inflamm Bowel Dis. 1998;4:157–64. doi: 10.1002/ibd.3780040211. [DOI] [PubMed] [Google Scholar]
- 12.Pender SL, Tickle SP, Docherty AJ, Howie D, Wathen NC, MacDonald TT. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582–90. [PubMed] [Google Scholar]
- 13.Vaalamo M, Mattila L, Johansson N, et al. Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds. J Invest Dermatol. 1997;109:96–101. doi: 10.1111/1523-1747.ep12276722. [DOI] [PubMed] [Google Scholar]
- 14.Medina C, Videla S, Radomski A, et al. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G116–G122. doi: 10.1152/ajpheart.00036.2002. [DOI] [PubMed] [Google Scholar]
- 15.Naito Y, Takagi T, Kuroda M, et al. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium-induced colitis in mice. Inflamm Res. 2004;53:462–8. doi: 10.1007/s00011-004-1281-1. [DOI] [PubMed] [Google Scholar]
- 16.Castaneda FE, Walia B, Vijay-Kumar M, et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Arimura Y, Goto A, et al. Therapeutic implications of the specific inhibition of causative matrix metalloproteinases in experimental colitis induced by dextran sulphate sodium. J Pathol. 2006;209:376–83. doi: 10.1002/path.1978. [DOI] [PubMed] [Google Scholar]
- 18.Kruidenier L, MacDonald TT, Collins JE, Pender SL, Sanderson IR. Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant CXCL7 from intestinal epithelial cells. Gastroenterology. 2006;130:127–36. doi: 10.1053/j.gastro.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol. 1998;152:1005–14. [PMC free article] [PubMed] [Google Scholar]
- 20.McKaig BC, McWilliams D, Watson SA, Mahida YR. Expression and regulation of tissue inhibitor of metalloproteinase-1 and matrix metalloproteinases by intestinal myofibroblasts in inflammatory bowel disease. Am J Pathol. 2003;162:1355–60. doi: 10.1016/S0002-9440(10)63931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saarialho-Kere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg ML. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996;148:519–26. [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkegaard T, Hansen A, Bruun E, Brynskov J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut. 2004;53:701–9. doi: 10.1136/gut.2003.017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuno K, Adachi Y, Yamamoto H, et al. The expression of matrix metalloproteinase matrilysin indicates the degree of inflammation in ulcerative colitis. J Gastroenterol. 2003;38:348–54. doi: 10.1007/s005350300062. [DOI] [PubMed] [Google Scholar]
- 24.Pirila E, Korpi JT, Korkiamaki T, et al. Collagenase-2 (MMP-8) and matrilysin-2 (MMP-26) expression in human wounds of different etiologies. Wound Repair Regen. 2007;15:47–57. doi: 10.1111/j.1524-475X.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 25.Rechardt O, Elomaa O, Vaalamo M, et al. Stromelysin-2 is upregulated during normal wound repair and is induced by cytokines. J Invest Dermatol. 2000;115:778–87. doi: 10.1046/j.1523-1747.2000.00135.x. [DOI] [PubMed] [Google Scholar]
- 26.Ejeil AL, Igondjo-Tchen S, Ghomrasseni S, Pellat B, Godeau G, Gogly B. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J Periodontol. 2003;74:188–95. doi: 10.1902/jop.2003.74.2.188. [DOI] [PubMed] [Google Scholar]
- 27.Tarlton JF, Whiting CV, Tunmore D, Bregenholt S, Claesson MH, Bland PW. The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis. Am J Pathol. 2000;157:1927–35. doi: 10.1016/S0002-9440(10)64831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perner A, Andresen L, Pedersen G, Rask-Madsen J. Superoxide production and expression of NAD(P)H oxidases by transformed and primary human colonic epithelial cells. Gut. 2003;52:231–6. doi: 10.1136/gut.52.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkegaard T, Pedersen G, Saermark T, Brynskov J. Tumour necrosis factor-alpha converting enzyme (TACE) activity in human colonic epithelial cells. Clin Exp Immunol. 2004;135:146–53. doi: 10.1111/j.1365-2249.2004.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen G, Andresen L, Matthiessen MW, Rask-Madsen J, Brynskov J. Expression of Toll-like receptor 9 and response to bacterial CpG oligodeoxynucleotides in human intestinal epithelium. Clin Exp Immunol. 2005;141:298–306. doi: 10.1111/j.1365-2249.2005.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vainer B, Nielsen OH, Horn T. Comparative studies of the colonic in situ expression of intercellular adhesion molecules (ICAM-1, -2, and -3), beta2 integrins (LFA-1, Mac-1, and p150,95), and PECAM-1 in ulcerative colitis and Crohn's disease. Am J Surg Pathol. 2000;24:1115–24. doi: 10.1097/00000478-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen G, Saermark T, Giese B, Hansen A, Drag B, Brynskov J. A simple method to establish short-term cultures of normal human colonic epithelial cells from endoscopic biopsy specimens. Comparison of isolation methods, assessment of viability and metabolic activity. Scand J Gastroenterol. 2000;35:772–80. doi: 10.1080/003655200750023471. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen G, Saermark T, Bendtzen K, Brynskov J. Cultures of human colonic epithelial cells isolated from endoscopical biopsies from patients with inflammatory bowel disease. Effect of IFNgamma, TNFalpha and IL-1beta on viability, butyrate oxidation and IL-8 secretion. Autoimmunity. 2000;32:255–63. doi: 10.3109/08916930008994099. [DOI] [PubMed] [Google Scholar]
- 34.Koon HW, Zhao D, Na X, Moyer MP, Pothoulakis C. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem. 2004;279:45519–27. doi: 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- 35.Wang YD, Mao JW. Expression of matrix metalloproteinase-1 and tumor necrosis factor-alpha in ulcerative colitis. World J Gastroenterol. 2007;13:5926–32. doi: 10.3748/wjg.v13.i44.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salmela MT, Pender SL, Karjalainen-Lindsberg ML, Puolakkainen P, MacDonald TT, Saarialho-Kere U. Collagenase-1 (MMP-1), matrilysin-1 (MMP-7), and stromelysin-2 (MMP-10) are expressed by migrating enterocytes during intestinal wound healing. Scand J Gastroenterol. 2004;39:1095–104. doi: 10.1080/00365520410003470. [DOI] [PubMed] [Google Scholar]
- 37.Bister V, Salmela MT, Heikkila P, et al. Matrilysins-1 and -2 (MMP-7 and -26) and metalloelastase (MMP-12), unlike MMP-19, are up-regulated in necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 2005;40:60–6. doi: 10.1097/00005176-200501000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Impola U, Jeskanen L, Ravanti L, et al. Expression of matrix metalloproteinase (MMP)-7 and MMP-13 and loss of MMP-19 and p16 are associated with malignant progression in chronic wounds. Br J Dermatol. 2005;152:720–6. doi: 10.1111/j.1365-2133.2005.06447.x. [DOI] [PubMed] [Google Scholar]
- 39.Kiili M, Cox SW, Chen HY, et al. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J Clin Periodontol. 2002;29:224–32. doi: 10.1034/j.1600-051x.2002.290308.x. [DOI] [PubMed] [Google Scholar]
- 40.Pirila E, Ramamurthy NS, Sorsa T, Salo T, Hietanen J, Maisi P. Gelatinase A (MMP-2), collagenase-2 (MMP-8), and laminin-5 gamma2-chain expression in murine inflammatory bowel disease (ulcerative colitis) Dig Dis Sci. 2003;48:93–8. doi: 10.1023/a:1021790532723. [DOI] [PubMed] [Google Scholar]
- 41.Stallmach A, Chan CC, Ecker KW, et al. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut. 2000;47:415–22. doi: 10.1136/gut.47.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg P, Ravi A, Patel NR, et al. Matrix metalloproteinase-9 regulates MUC-2 expression through its effect on goblet cell differentiation. Gastroenterology. 2007;132:1877–89. doi: 10.1053/j.gastro.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 43.Pirila E, Sharabi A, Salo T, et al. Matrix metalloproteinases process the laminin-5 gamma 2-chain and regulate epithelial cell migration. Biochem Biophys Res Commun. 2003;303:1012–7. doi: 10.1016/s0006-291x(03)00452-2. [DOI] [PubMed] [Google Scholar]
- 44.Pender SL, Fell JM, Chamow SM, Ashkenazi A, MacDonald TT. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol. 1998;160:4098–103. [PubMed] [Google Scholar]


