Abstract
The hypothesis to be explored in this chapter is based on the assumption that the posterior parietal cortex (PPC) is directly involved in representing a subset of the spatial features associated with spatial information processing and plays an important role in perceptual memory as well as long-term memory encoding, consolidation, and retrieval of spatial information. After presentation of the anatomical location of the PPC in rats, the nature of PPC representation based on single spatial features, binding of visual features associated with visual spatial attention, binding of object-place associations associated with acquisition and storage of associations where one of the elements is a spatial component, and binding of ideothetic and allothetic information in long-term memory is discussed. Additional evidence for a PPC role in mediation of spatial information in long-term storage is offered. Finally, the relationship between the PPC and the hippocampus from a systems and dynamic point view is presented.
Keywords: parietal cortex, metric, topological, hippocampus
Anatomy
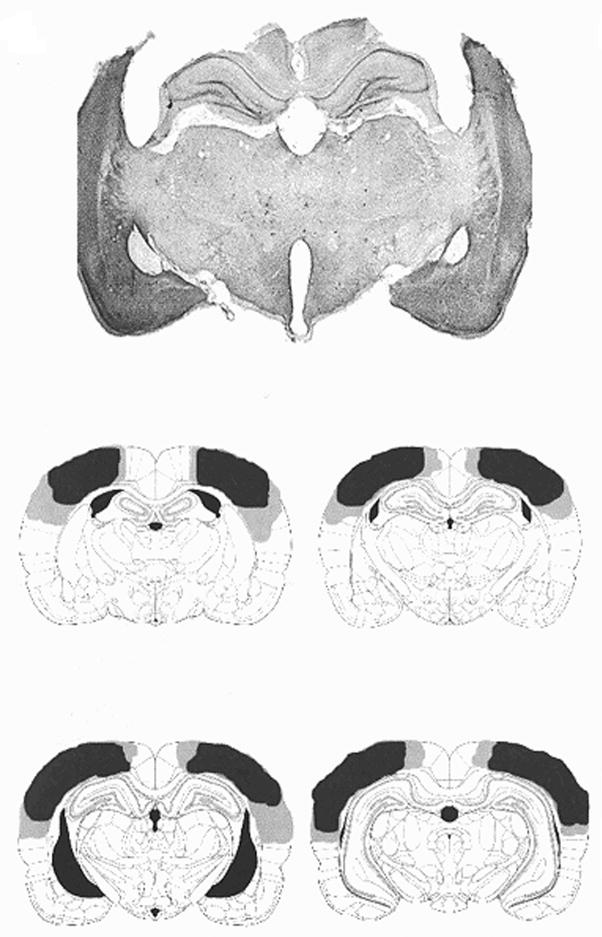
Reep, Chandler, King, and Corwin (1994) have delineated the boundaries of the rodent posterior parietal cortex (PPC). They define the rodent PPC as cortical tissue that has pronounced connections with lateral posterior thalamus lateral dorsal thalamus and posterior nuclei but no input from ventrobasal complex or dorsal lateral geniculate (Reep et al., 1994). It should be noted that rats have no true pulvinar, but it is likely that the homologous structure is the lateral posterior thalamus. With these criteria, the PPC region of the rat is approximately 3.5–4.5 mm caudal to bregma, and 1.5–5 mm lateral to midline (Reep et al., 1994). This region of rodent cortex has connections with ventrolateral orbital and medial orbital cortex, medial agranular cortex and retrosplenial cortex. These patterns of thalamo-cortical and cortico-cortical connections are similar to those in human and non-human primates, and there now seems to be general agreement among investigators of this anatomical definition of rat PPC. For a more detail description of the neuroanatomy of the PPC (see Reep and Corwin’s article in this special issue). In most of our studies the damaged area includes the PPC delineated by Reep et al. (1994), but in addition we usually damage the somatosensory cortex barrel fields with no damage to retrosplenial and dorsal hippocampus. We are also likely to damage some of the white matter, but the degree of damage is highly variable. In summary, we make somewhat larger lesions then have been reported by other investigators who have lesioned the PPC. As a sign that our lesions are probably not too large we do find double dissociations between the PPC and hippocampus (Kesner, 2000; Goodrich-Hunsaker, Hunsaker and Kesner, 2005). A photomicrograph (multiplied by 12.5) of a representative section of a parietal cortex lesioned rat and a series of sections showing minimum and maximum damage within a group of PPC lesioned rats based on Paxinos and Watson’s (1997) atlas of the rat brain is shown in Figure 1.
Figure 1.
A photomicrograph (multiplied by 12.5) of a representative section of a parietal cortex lesioned rat and schematic drawings of the largest (/light/ /gray)/ and the smallest (/dark gray/) damage within a group of PPC lesioned rats based on Paxinos and Watson’s (1997) atlas of the rat brain.
Nature of memory representations of single spatial features
It is important to first determine the nature of memory representation of spatial features and determine whether the PPC mediates all or some subset of these features. Spatial features include the processing of ideothetic information based on vestibular (translational and rotational accelerations), proprioceptive (feedback from muscles tendons and joints), and visual (linear and radial optic flow) cues and processing of allothetic information, such as object (landmark) information based primarily on visual as well as other sensory cues (auditory, somatosensory, olfactory). Short-term copies of locomotor commands (efference copies) may also contribute (Berthoz, 1999). Furthermore, the integration of ideothetic sensory information can aid in determining direction as well path integration, whereas the integration of spatial relationships between landmarks can aid in determining spatial location, topological, metric, and distance aspects of spatial representations. Since almost all of the tasks used require motor movement, ideothetic information is often a part of the processing of spatial representations, but the importance of the ideothetic contribution can be minimized by emphasizing distal space and providing opportunity for developing different viewpoints of the environment to create allothetic representations.
Ideothetic spatial representations
Does the PPC play a role in mnemonic processing of ideothetic spatial representations based primarily in rats on head direction information? In rats, neurons have been found within the parietal cortex that encode spatial location and head direction information and many of these cells are sensitive to multiple cues, including visual, proprioceptive, sensorimotor, and vestibular cue information (Chen, Lin, Barnes, & McNaughton, 1994a; Chen, Lin, Green, Barnes, & McNaughton, 1994b; McNaughton, Chen, & Marcus, 1991). Fore example, as nicely summarized by Chen and Nakamura (1998), single-unit recording data suggest that rat parietal cortex may be involved in head direction orientation representations and spatial memories. A small percentage of cells in PPC respond selectively to the rat’s head orientation (Chen et al., 1994a,b). These head direction cells persist after the removal of visual cues (either by physically removing the cues or turning off the lights), and a subset associate angular motion with head orientation (Chen et al., 1994a,b). Therefore, it appears that parietal cortex cells are responsive to an interaction between visual and sensory-motor inputs. Some of these PPC cells maintain short-term mnemonic information for head direction (Chen et al., 1994a,b) and the spatial location of a tone (Nakamura & Takarajima, 1996). However, in recent studies PPC lesions do not markedly alter the firing characteristics of head direction cells in the anterior thalamus (Calton, Turner, Cyrenne, Lee, & Taube, 2008). PPC lesions studies using a delayed matching-to-sample task for head direction in the dark have not yet been carried out, but hippocampal lesions or vestibular rotations between the study and test phase produce profound deficits (DeCoteau, Hoang, Huff, Stone, & Kesner, 2004). Thus, it appears that the PPC may play a role in mediating head direction information, but more research will be needed.
Does the PPC play a role in mnemonic processing of path integration or dead reckoning information? Whishaw, McKenna, and Maaswinkel (1997) have defined the process of path integration as the means by which an animal can determine its current environmental location as a function of keeping track of its own movements through space in relation to a known starting point or reference point and by integrating signals derived from its own locomotor movements over time. Path integration is assumed to be based on processing of ideothetic information and appears to depend on vestibular signals generated by angular and linear acceleration during movements. This ideothetic information can also be integrated with allothetic cues or landmarks visible in the environment. Support for the idea that ideothetic information is sufficient comes from the finding that when visual cues are removed or otherwise unavailable (e.g., in darkness), there continues to be stable firing of both place cells (Jeffery, Donnett, Burgess, & O’Keefe, 1997; Quirk, Muller, & Kubie, 1990) and head direction cells (Golob & Taube, 1999), and furthermore, animals can still navigate effectively (Etienne & Jeffrey, 2004). One can measure the operation of path integration by allowing the animal to leave its home base, explore the platform to find a hidden food and carry it back to the home base and then one can determine the accuracy in finding the home base. Save, Guazelli, and Poucet (2001), and Parron and Save (2004) have shown that PPC lesions resulted in inaccurate returns to home base. It should be noted that similar disruptive effects of path integration have been reported for hippocampus and entorhinal cortex (Parron & Save, 2004; Save et al., 2001; Whishaw & Jarrard, 1996). Thus, the data indicate that the PPC plays an important role in path integration probably in cooperation with the hippocampus.
Allothetic spatial representations-topological vs. metric
It is proposed that there exist two fundamental and basic features of allothetic space—metric and topological relationships between stimuli. Metric relationships are defined as the relationship of angles and distances between objects as well as linear and angular distances, whereas topological relationships are represented by a connectedness relationship between objects that cannot be affected by metric modifications (Gallistel, 1990; Herrmann & Poucet, 2001; Kuipers & Levitt, 1988; Poucet, 1993). Topological spatial information is based on associations between objects that involve relationships such as connectivity and containment (Gallistel, 1990; Herrmann & Poucet, 2001; Kuipers & Levitt, 1998; Poucet, 1993). According to Poucet (1993), “…topology is a geometry originally based on the notions of continuity and limit, from which are derived the relations of compactedness, neighborhood, enclosure, and connectivity.” Therefore, metric transformations are created by altering distances and angles between objects, whereas topological transformations involve either stretching or contracting the entire environment as a whole or disrupting particular relationships of enclosure or connectivity (Gallistel, 1990). Topological information is a crude description of space, because distances and angles have no effect (Gallistel, 1990; Poucet, 1993). Based upon behavioral experiments, Poucet (1993) has demonstrated that topological information, though crude in its representations of space, is essential to animals’ spatial representations. Also, since animals encode geometric relationships, they might extrapolate overall geometric structures as well, implying the use of topological information processing. In one experiment, Gallistel (1990) trained rats to choose arms in a rectangular orientation in the order of their baited magnitudes up to asymptote. Intermittent test trials were administered between the control trials that altered the rectangular formation. During a counterclockwise rotation (i.e. topological shift) of the baited arms, the rats’ ability to choose arms on the basis of the bait magnitudes were destroyed (Gallistel, 1990). As a result, topological features of space are a significant feature to spatial representations. Therefore, metric transformations are created by altering distances and angles between objects, whereas topological transformations involve either stretching or contracting the entire environment as a whole or disrupting particular relationships of enclosure or connectivity (Gallistel, 1990).
To test the hypothesis that the PPC processes topological spatial information (i.e. spatial configuration of objects), but not metric spatial information (i.e. spatial distances between objects), PPC and controls lesioned rats were tested for novelty detection on both a metric and topological task. The topological task consisted of four different objects placed in a square orientation. On the first day after habituation to the four objects, the first two objects were switched and once the animals habituated to that change, the back two objects were switched. Re-exploration was used as a measure of novelty detection. On the next day, the topological task was repeated with new objects. The metric task consisted of two different objects placed 68 cm apart on a cheese board. After habituation, the objects were moved to a separation of 38 cm on the first day and to a separation of 98 cm on the second day. Again re-exploration was used as a measure of novelty detection. The PPC lesioned group displayed a marked disruption of object re-exploration relative to controls and dorsal hippocampal lesioned rats during the topological reorganizations, but had re-exploration similar to controls for the metric changes, suggesting that the PPC is essential to processing of topological, but not metric information (Goodrich-Hunsaker, Hunsaker, & Kesner, 2005). In contrast, different rats with dorsal hippocampus lesions tested in the same task displayed a marked disruption of object re-exploration relative to PPC and control rats during the distance changes, but had re-exploration similar to controls for the topological changes suggesting that the dorsal hippocampus is essential for processing of metric, but not topological information (Goodrich-Hunsaker et al., 2005).
Further support for a PPC role in supporting topological spatial processing comes from a variety of sources. Since topological information is most likely based on connectedness or proximity between or among visual cues, one would expect a PPC involvement when proximal cues are important and less involved when distal cues are essential. Save and Poucet (2000) presented data that support this role of the PPC in that in the Morris water maze PPC lesioned rats were impaired in finding a hidden platform when three salient cues were located in the pool close to the correct location (proximal cues), but they were not impaired when only room cues (distal cues) were available to find the platform. Kolb and Walkey (1987) showed that PPC lesioned rats were impaired in finding a platform location in a landmark task in which the rats had to associate a visual cue with a site that was spatially discontiguous and where the relevant cue moved relative to the rest of the extra maze cues. This impairment manifested itself in the adoption of a looping strategy to locate a hidden platform. In monkeys posterior parietal cortex lesions disrupted spatial pattern discrimination only when the essential cue and site of reinforcement were separated (Mendoza & Thomas, 1975). Similar results were obtained by Pohl (1973) when the landmark cue was discontiguous with the manipulandum.
Additional evidence comes from the findings that PPC lesioned rats and inferior parietal cortex lesioned monkeys are impaired in learning of a variety of mazes especially when the mazes employed do not provide for many extra maze cues and thus promoting the use of ideothetic as well local topological spatial cues (Barrow & Latto, 1996; Boyd & Thomas, 1977; Rogers & Kesner, 2007). Furthermore Nitz (2006) recorded from PPC cells in rats and found that many cells displayed sustained increases in neural activity as a function of distance between proximal points on the maze providing cellular evidence of topological transformations that involve stretching or contracting entire routes through the maze.
In humans, patient RM who had a bilateral parietal cortex lesion displayed an impairment for learning topological relationships. RM was asked to determine if a large dot was outside or inside a circle. RM was unable to learn this task, averaging 49% correct (18 out of 37 trials) (Robertson, Treisman, Friedman-Hill, & Grabowecky 1997). Similar observations show identical behavioral results with PPC lesioned rats (Goodrich-Hunsaker, Hunsaker, & Kesner., 2008, in press). Initially, rats were trained to discriminate between either a ball inside a ring or outside a ring. After reaching criterion, the rats received PPC lesions and when retested they were unable to make the discrimination. It should be noted that control rats and rats with dorsal hippocampal lesions had no serious difficulty in performing the topological task. Besides task performance, latencies to displace the first object and place preference were measured in the present study. The latencies among the rats were not different; rats also did not spend time exploring around the board. The task was not a navigational task. Rats took only enough time to exit the black box, displace an object, and then return back to the black box. Also, since the animals made rapid decisions and stayed on task, it was concluded that there were no attentional deficits after PPC lesions, at least not as far as task performance was concerned. Place preference was also recorded. Hemineglect is a common neuropathology of PPC lesions and it was expected that hemineglect could lead to place preferences. However, that was not the case in the present study. The above mentioned evidence suggests that the deficits seen in PPC lesioned animals were due to deficits in topological information processing, not by attentional or motivational confounds resulting from the lesion.
It is possible that PPC lesioned rats were impaired in object discrimination. However, previous research suggests that PPC lesioned rats are not impaired in object discrimination (Long, Mellem, & Kesner, 1998). For each trial, Long et al. (1998) presented PPC lesioned rats with one of two possible objects located in various spatial locations. One object was always rewarded, the other object never rewarded. Location of the object never mattered. PPC lesioned rats were able to learn the go/no-go paradigm by only displacing the rewarded object (Long et al., 1998). Other studies with PPC lesions also do not show a deficit for object discrimination (Davis & McDaniel, 1993; Kolb, Buhrmann, McDonald, & Sutherland, 1994), but see (Boyd & Thomas, 1977; McDaniel & Wall, 1988). However, this paradigm only involved simple object discrimination. In another study, PPC lesioned rats were also able to detect object changes in a multiple object scene discrimination task (DeCoteau & Kesner, 1998). For this object discrimination task, rats were presented four objects in a square configuration. There were a total of 8 objects. Rats were rewarded when the correct four objects were presented. Rats were not rewarded when one of the correct objects was replaced with a non-rewarded object. This was a go/no-go paradigm. PPC lesioned rats were able to learn when to displace the four rewarded objects (i.e. go) and when not to displace the objects if there was a non-rewarded object present (i.e. no go; DeCoteau & Kesner, 1998). Thus, in general the results of previous research support the idea that the PPC does not subserve object discrimination (DeCoteau & Kesner, 1998; Long et al., 1998).
Another factor that may contribute to topological deficits observed in PPC lesioned rats is that they may be unable to discriminate the distances between objects. Long & Kesner (1996) found that rats with PPC lesions similar to those in the present study were able to perform a go/no-go successive match-to-sample task. For the study phase, rats were presented with two different objects at either a separation of 2 cm or 7 cm. For the test phase, the same two objects were presented again. If the distance between the two objects (2 cm or 7 cm) matched the study phase distance, the objects were rewarded. If the distance between the two objects (2 cm or 7 cm) did not match the study phase distance, the objects were not rewarded. In contrast, hippocampal lesions produce a profound deficit. Since the latter task is a metric task, the distance between the two objects did not appear to play an important role for PPC processing of metric spatial information, but it was important for the hippocampus. All the above mentioned data appear to be consistent with the idea that the PPC plays an important role in processing topological, but not metric (distance), spatial information. Further evidence that the PPC may not play an important role in tasks that emphasize an important metric component was reported by (Long & Kesner, 1998). Rats were given training trials that consisted of two phases, a study and a test phase. For any given study phase the stimulus was placed either 40 cm or 80 cm from the door that separated the rat from the stimulus. The back wall of the apparatus was movable, thus allowing the distance between the object and the back wall to always be 5 cm in either study phase condition. The design of the apparatus also allowed the back walls and start door to be shifted across spatial locations, thus avoiding the potential use of absolute spatial location to solve the task. The absolute spatial location was pseudorandomly shifted between two locations each day, such that each day every trial was run in one or the other absolute spatial location. The rat was located at one end of the apparatus, with vision and access blocked by a door. The purpose of the study phase was to present the rat with a distance to be traveled and to be remembered; thus, there was always a food reward (one half of a piece of Froot Loop cereal) located under the object in the study phase of the experiment. Upon opening of the door, the rat had to displace the object at the other end of the box to uncover the food reward, and thus end the study phase of the trial. The test phase began after the rat had consumed the food reward and was again isolated in one end of the apparatus. In the test phase, the rat was presented with a distance that either represented a match of the distance presented in the study phase (reward condition) or a distance that did not match the study phase (nonrewarded condition). The latency was recorded from the opening of the door until the rat displaced the stimulus. Running speed was calculated for each trial by dividing the latency of the rat to displace the stimulus by the distance to be traveled for that particular trial (40 or 80 cm). Whenever the stimulus in the test phase represented a match of the traveled distance to be remembered, the rat received a whole piece of Froot Loop cereal for displacing the stimulus. Whenever the stimulus in the test phase represented a mismatch of the stimulus in the study phase, the rat did not receive reinforcement. Therefore, the rats needed to learn not to displace a stimulus in a mismatch test phase. At the end of the test phase the rat was again confined to the start location, where a 30-s ITI was introduced. This ITI was given to help the rat distinguish between the study and test phase of the trial. The rats were then randomly assigned to either a PPC, hippocampus or cortical control lesion group. The results indicated that the PPC and cortical control groups had no difficulty in performing the task, but the hippocampal lesioned rats were impaired. It is probably the case that this task is a metric task and thus involved only the hippocampus, but not the PPC. In a different matching-to-sample task for a single spatial location out of 4 possible locations provided the same pattern of results with no PPC group impairment, but an impairment for the hippocampus group (Long & Kesner, 1998).
Thus, in general it appears that the PPC represents topological, but not metric information. In contrast, it appears that the hippocampus represents metric, but not topological information.
Binding within the visual modality
It has been suggest by Treisman (1998) that the binding of different features of objects may be realized by the utilization of spatial attention to locations that can aid in the selection of features that are currently active in corresponding locations and suppression of those features that are in other locations to prevent erroneous binding. Furthermore, the parietal cortex may play a very important role in ensuring that errors labeled as illusory conjunction errors do not appear in a variety of tasks including search tasks. Thus, the PPC may be directly involved in perceptual binding between, for example, a shape and a color or a shape and a size requiring spatial attention. Support for this idea comes from the finding of the performance of patient RM with bilateral parietal cortex damage in that he had difficulty in binding problems between shape and color and shape and size. When shown two different colored letters, RM made many errors in the form of illusory conjunctions combining the shape of one letter with the color of the other (Friedman-Hill, Robertson, & Treisman, 1995). Similarly, in a visual search task requiring the detection of a target based on the conjunction of two features RM made many errors, but RM had no difficulty in detecting a target based on one feature (Robertson et al., 1997). Using an fMRI study, Donner and colleagues (2002) reported greater activity in the right parietal cortex during conjunction search as compared to feature search even when controlled for overall search difficulty. Similar results with parietal cortex activation in the conjunction search vs. the feature search for simultaneous presented information was reported by Shafritz, Gore, and Marois (2002).
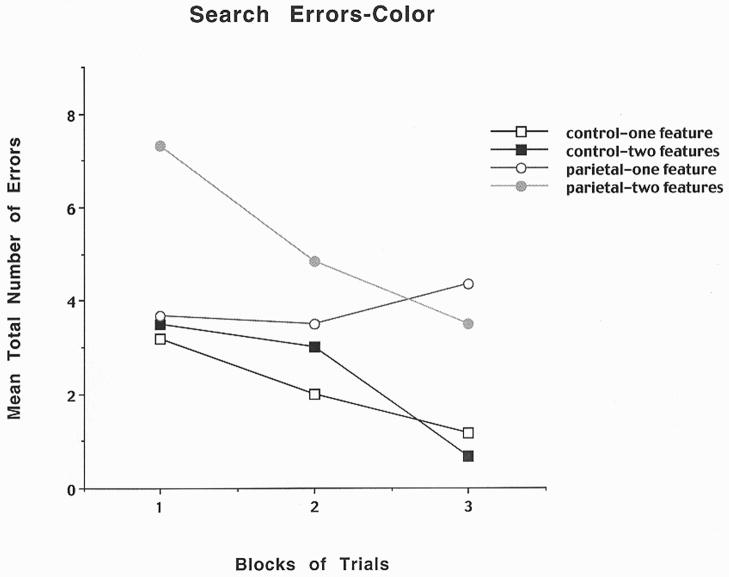
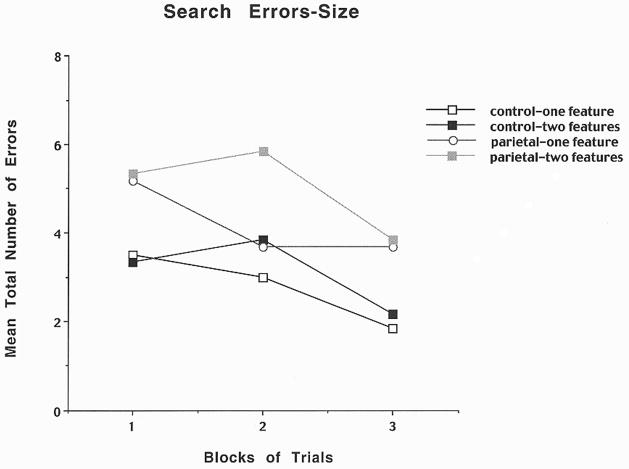
In order to determine whether PPC lesions relative to sham lesions in rats result in the production of illusory conjunction errors, a visual search paradigm was used to examine acquisition of a visual object that varied either only on features of color or height (one feature) or the combination of color and height (two features). The rats were initially pre-trained to displace an object in order to obtain food located in the well underneath the object. The apparatus was a cheese board maze, and the stimuli were three-dimensional wooden block objects 2″ in diameter that differed from each other in both color (black or white), and size (4″ or 6″ in height). For each trial, a randomly targeted object covered a baited food-well in one of the five randomly assigned spatial locations. The inter-trial interval was 30 seconds. The number of errors per trial was recorded and the food reward was Froot Loops breakfast cereal. The testing session consisted of a one feature trial followed by a two feature trial for a total of 60 trials at 10 trials per day for six consecutive days. In the one feature condition the subject was required to locate the targeted object among four other objects that differed in either color or height, i.e. if the target object was a small black block, then four small white blocks for the color condition and four tall black blocks for the size condition would surround that object. In the two feature condition, the subject was required to locate the targeted object among four other objects that differed in both color and height, i.e. if target object was a small black block, then two small white blocks and two tall black blocks would randomly surround that object. The target object for each animal was randomly predetermined and remained consistent throughout the experiment, and the placing of the objects varied on each trial. The rule to be learned in order to obtain a food reward was to always discriminate between the size and colored objects in order to displace the targeted object. The results for color errors are shown in Figure 2 and indicate that PPC lesioned rats relative to controls made only a few errors in detecting the one feature component of the task, but they made many errors throughout all three blocks of trials for the two feature condition suggesting the appearance of illusory conjunction errors. The results for size errors are shown in Figure 3 and indicate that for the PPC lesioned rats relative to controls there was an increase in errors for the two feature condition, suggesting illusory conjunction errors, but there were also some errors in the one feature condition. Thus, it appears that the PPC supports the binding of visual features within a single object or landmark, a process which has been assumed to be mediated by spatial attention.
Figure 2.
Mean number of search errors for one or two features for object color for control and parietal cortex lesioned rats as a function of blocks of trials. Each block consisted of 20 trials.
Figure 3.
Mean number of search errors for one or two features for object size for control and parietal cortex lesioned rats as a function of blocks of trials. Each block consisted of 20 trials.
Binding across modality (object-place)
One possible role for the rodent parietal cortex could be to bind across modalities to maintain the association between landmark and spatial location information. In other words, the parietal cortex may not be involved in memory for a single landmark or a single spatial location, but rather in the processing that assigns a specific landmark to a specific spatial location. To test this hypothesis, rats with small lesions of the parietal cortex were tested in an object/spatial location paired-associate task that required concurrent memory for both object and spatial location information. In addition, memory for landmark only or spatial location only information was also assessed. A deficit in the paired associate task (which requires memory for both landmark and spatial location information), in the absence of deficits in either the landmark or the spatial location only memory, would support the idea that the PPC is involved in the memory for the combination of landmark and spatial location information. The results indicated that small lesions of the PPC as defined by Reep et al. (1994) and larger PPC lesions disrupted learning of the object-place paired-associate task, but did not disrupt the learning of a spatial or object discrimination (Long et al., 1998). It should be noted that lesions of the hippocampus and especially the CA3 subregion of the hippocampus also disrupted object-place paired associate learning (Gilbert & Kesner, 2002, 2003).
Binding across modality (ideothetic-allothetic)
It has been suggested that learning to find a specific location in a water maze or a dry land version of the water maze may be a function of an interaction between ideothetic and allothetic cues and thus the PPC could play a role by binding of ideothetic and allothetic cues (Save, Poucet, Forman, & Thinus-Blanc, 1998). Support for this suggestion comes from the findings that PPC lesions disrupt both acquisition and retention of the Morris water maze and the dry land version of the water maze (DiMattia & Kesner, 1988; Hoh, Kolb, Eppel, Vanderwolf, & Cain, 2003; Kesner, Farnsworth, & Kametani, 1992; Kolb & Walkey, 1987). The magnitude of this effect is smaller in some studies (see Kolb, Sutherland, & Whishaw, 1983; Save & Moghaddam, 1996).
Further support for the binding of ideothetic and allothetic information in long term memory comes from the following study (Rogers & Kesner, 2007). They trained rats in two versions of a modified Hebb-Williams maze to test the role of the PPC in processing ideothetic and allothetic information during acquisition and retention. In the first version, unlike traditional Hebb-Williams mazes, the maze was made of 1.3 cm Plexiglas, measuring 25 cm in height with a 7.5-cm strip, also painted black, placed on the bottom of the barriers. This spatial arrangement allowed the rat to use extra maze cues. Extra maze cues included two posters, a map, and a hanging doll. Given that this maze allowed for the use of extra maze cues, learning the maze may be primarily based on allothetic, but also some ideothetic cues. We will label this task as an allocentric task. The second maze used in these experiments was the same modified Hebb-Williams maze mentioned above; however, the walls were 50.8 cm high, made of .6 cm red Plexiglas. The apparatus was kept in a well-lit room with no windows or extramaze cues. This maze is considered to be based primarily on ideothetic and local topological cues, because the walls were raised, made opaque, any there were few, if any, extra maze cues. We will label this task as an egocentric task.
Bilateral lesions were made to PPC before maze testing (acquisition) or after maze testing (retention). The results indicated that during acquisition lesions of the PPC impaired egocentric maze acquisition, but they had no difficulty in learning the allocentric version of the maze task. Similar deficits following PPC lesions were reported by Boyd & Thomas (1977) during acquisition of the standard Hebb-Williams maze which did not give the rats an opportunity to use extra maze cues. During retention, lesions of the PPC produced a significant impairment on both maze versions, suggesting that the PPC may be combining both ideothetic and allothetic information during normal learning of the maze, but after a PPC lesion the combined information may not available to the animal. In contrast, it should be noted that during acquisition lesions of the dorsal hippocampus impaired allocentric, but not egocentric maze acquisition. During retention lesions of the dorsal hippocampus produced short-lived, transient impairments on both maze versions. These results suggest that during acquisition, the hippocampus and PPC process spatial information in parallel; however, long-term retention of spatial information requires the PPC with the dorsal hippocampus necessary for retrieval and/or access but not necessarily storage.
Other examples of a role for PPC in storing spatial information into long-term memory based also on retention tests include a study by Kesner, DiMattia, and Crutcher (1987) who have shown that in an 8-arm maze PPC lesions placed in rats after training on 4 unbaited and 4 baited arms resulted in a deficit in the reference, semantic, or knowledge-based long-term memory, but not working, episodic, or event-based intermediate-term memory components of the task. If one assumes that the presentation of unbaited arms reflects the operation of the knowledge-based long term memory system and that the presentation of baited arms reflect the operation of the event-based or intermediate- term memory system, then lesions of the PPC only disrupt long-term memory, but not intermediate term memory. It should be noted that using the same task, lesions of the hippocampus disrupt only the intermediate, but not the long-term memory components of the task, suggesting that for spatial information the PPC and hippocampus can operate independent of each other. Additional support can be found in human studies where it has been shown that based on PET scans the PPC as well as the hippocampus is activated while performing a memorized sequence of saccadic eye movements (Berthoz, 1999) and while generating a verbal description of routes through London by taxi drivers (Maguire, Frackowiak, & Firth, 1997).
Perceptual memory based on spatial information could represent a process reflective of the operation of the knowledge-based long-term memory system. In PPC damaged patients there is a deficit in spatial perceptual repetition priming without a loss in intermediate or working memory for spatial information (Ellis, Sala, & Logie, 1996). In order to examine whether similar results can be obtained in rats two spatial continuous recognition training procedures designed to query either perceptual or intermediate memory in rats were designed. A continuous recognition procedure was used to train rats on a 12-arm radial maze. Each rat was allowed to visit a sequence of 12 arms per day in an order predetermined for that trial. Of the 12 arms visited, either 3 or 4 of the arms were repeated within the running sequence. The arms selected for repetition varied according to lag (0–6), or the number of arms that occurred between the first visit to an arm and its repetition. In order to gain access to each arm, the animal was required to orient to a cue on the Plexiglas door at the entrance of the arm. Once the animal oriented to the cue, the door was lowered and the latency for the animal to reach the end of the arm was measured. Six groups of rats were trained, three on a perceptual memory training procedure and three on an intermediate or episodic memory training procedure. The perceptual memory group received reinforcement at the end of each arm regardless of whether the arm was a novel arm or a repeated arm. This group showed decreased latencies when visiting repeated arms displaying a repetition priming effect. The intermediate or episodic memory group received reinforcement only when visiting an arm for the first time in a given sequence. This group showed increased latencies for repeated arms. After training, rats received PPC, hippocampus, or sham-operated and cortical control lesions. After retesting, the results indicated that relative to control and pretraining performance, the PPC lesioned rats were impaired in the perceptual memory condition, but showed no deficits in the intermediate or episodic memory condition. In contrast, the hippocampal lesioned rats were impaired in the intermediate or episodic memory condition, but showed no deficits in the perceptual memory condition (Chiba, Kesner, & Jackson, 2002).
Thus, a double dissociation appears to exist between PPC and hippocampus for memory operations associated with the knowledge-based memory system vs. memory operations (episodic and intermediate memory) associated with the event-based memory system for spatial information, suggesting that the two neural circuits mediated by the hippocampus and PPC can operate independent of each other. This functional independence would require that spatial information could reach the hippocampus and PPC via separate neural pathways. Indeed spatial information that reaches the dorsal lateral thalamus in the rat can be directed to the hippocampus via connections with the pre and parasubiculum and medial entorhinal cortex and the PPC via direct connections. In the rat there are no direct connections between the PPC and hippocampus. The parietal cortex and the hippocampus can interact via the entorhinal cortex or retrosplenial cortex and pre and parasubiculum (Kohler, 1985; Van Groen & Wyss, 1990; Witter, Holtrop, & Loosdrecht, 1988).
In order to have an even better measure of perceptual memory as an indicator of the knowledge-based long-term memory system, a new paradigm was generated to measure positive as well as negative repetition priming in rats similar to paradigms used with humans. In this paradigm rats were trained on a black object and a white object discrimination in the dry land version of the water maze. On every trial rats were started from a black start box and trained to find food beneath the black or white object. The two objects could appear in one of 8 spatial locations on the maze with three locations to the right, three locations to the left and two locations straight head relative to the start box. The locations of the black and white objects were randomly varied with respect to the right and the left. During training, successive locations were not presented. Latency from opening the door until the object was displaced was measured. The animals were trained (16 trials per day) until they did not make any errors and latency to respond was consistent and rapid. After training the rats received in the positive priming condition on 4 of the 16 trials an immediate repetition of the prior location. Based on 48 repetition trials, all rats in the positive priming condition ran more quickly to the repeated location. This facilitation manifested itself only for an immediate repetition, because if any other spatial location occurred prior to a specific location repetition, the facilitation did not occur. In the negative priming condition, it is assumed that rats not only actively attend to the positive stimulus, but also actively inhibit responding to the negative stimulus (Neill & Mathis, 1995). Thus, in the negative priming condition on 4 of the 16 trials, there was not only an immediate repetition of the prior location, but also the black and white object locations were reversed. Based on 48 repetition trials, all rats in the negative priming condition ran more slowly to the repeated location, because the correct location had resulted in some inhibition on the previous trial. This inhibition manifested itself only for an immediate repetition, because if any other spatial location occurred prior to a specific location repetition, the inhibition did not occur. After training, rats received PPC lesions and then were retested. The results indicate that PPC lesioned rats are impaired for both positive and negative priming (Kesner, 2000). In the positive priming paradigm different rats received lesions of the hippocampus (Kesner, 2000). The results indicate that rats with hippocampal lesions show normal positive priming. Thus, it appears that the PPC, but not the hippocampus, is directly involved in perceptual memory for spatial location information. In additional research it was shown that PPC lesions did not disrupt positive priming for objects, whereas TE2 lesions produced a marked impairment, suggesting that the PPC affects primarily spatial, but not object, perceptual memory.
Interactions and dissociations of PPC and hippocampus function
Although the hippocampus and PPC are not directly linked anatomically (Burwell, 2000; Lavenex & Amaral, 2000; Witter et al., 2000), it appears that both regions process spatial information. It is thus important to determine whether the two regions can operate independent of each other or need to interact with each other in processing spatial information. The data presented thus far suggest that with respect to the nature of spatial processing the PPC supports topological spatial information which emphasizes the importance of proximity of local landmark cues, whereas the hippocampus supports metric spatial information which emphasizes the importance of distance between local landmark cues (Goodrich-Hunsaker et al, 2005; Save & Poucet, 2000). With respect to the utilization of allothetic cues, the hippocampus appears to be more important than the PPC in emphasizing distal space and providing opportunity for developing different viewpoints of the environment to create allothetic representations. However, both the PPC and the hippocampus are important for the integration of ideothetic and allothetic cues in supporting head direction and path integration information (Whishaw & Jarrard, 1996; Save et al., 2001).
With respect to binding of information, the PPC, but not the hippocampus, appears to be important for unimodal visual binding, but for cross-modal binding involving spatial information both the PPC and hippocampus play an important role, in that lesions of each of the two areas produces a deficit in learning object- place associations and learning to find a specific location in a dry land version of the water maze (Gilbert & Kesner, 2002; Long et al., 1998). In order to examine whether there is an interaction between the PPC and the hippocampus for learning object-place associations, a disconnection procedure was employed to test the potential interactions between the hippocampus and PPC (for similar procedures, see Warburton, Baird, Morgan, Muir, & Aggelton, 2001). In this approach, unilateral lesions were made to either the hippocampus or PPC. In one group, unilateral lesions were made in contralateral hemispheres and in the second group lesions were made in the same hemisphere. An assumption made is that the right and left hemispheres operate in parallel. Crossed lesions (i.e., unilateral lesions in contralateral hemispheres), therefore, would disrupt communication within each of the two hemispheres, thus disconnecting the two brain regions. It was hypothesized that if the hippocampus and PPC interact, then crossed lesioned animals should be markedly impaired compared to animals with lesions on the same side.
Following recovery from surgery, animals were trained on an object-place paired associate task (for details see Gilbert & Kesner, 2002). Two paired-associates were reinforced which consist of one particular object (A) in one particular location (1) and a different object (B) in a different location (2). Rats should learn that if an object was presented in its paired location then the rat should displace the object to receive a reward (Go). However, the rat should withhold displacing the object if the object is not in its paired location (No-Go). Difference scores calculated from the latencies between Go/No-Go stimuli assessed learning; increased latencies indicate better learning (since animals learn not to run during non-rewarded trials, hence “No-Go”). The results indicated that rats with crossed lesions were considerably more impaired than animals with lesions on the same side, that is, animals with crossed lesions had a lower latency difference score, thus indicating that they failed to learn the object-place associations. Subsequent tests indicated that all animals learned simple discriminations (objects and places). From these data it can be concluded that the hippocampus and PPC are likely to interact with each other during new learning of object-place paired associates (Rogers & Kesner, 2007). Using a very similar disconnection procedure it can be shown that for the spatial navigation to find a specific location in the dryland version of the water maze, there was a deficit for the contralateral, but not ipsilateral lesions suggesting that acquisition of the dryland version of the water maze also required an interaction between the PPC and the hippocampus (Rogers & Kesner, 2007). Thus, it appears that during acquisition the PPC and hippocampus interact in binding object and places as well binding of ideothetic and allothetic cues. In summary, the PPC and hippocampus support both independent (topological vs. metric) and (spatial perceptual vs. spatial episodic memory) and interactive functions (path integration and cross model binding of spatial information).
It has been suggested that even though both the PPC and hippocampus process ideothetic and allothetic cues, the PPC places proportionally a greater emphasis on ideothetic cues to realize egocentric based spatial information, whereas the hippocampus places proportionally a greater emphasis on allothetic cues to realize allocentric based spatial information. From a temporal point of view this emphasis on egocentric and allocentric processing of spatial information would suggest the possibility that short-term spatial information is first encoded in the PPC in an egocentric framework to guide action and then processed in long-term memory in the hippocampus in an allocentric framework (Burgess, Jeffery, & O’Keefe, 1999; Save & Poucet, 2000). This model suggests that long-term memory for spatial information resides in the hippocampus, a view also championed by Nadel & Moscovitch (1997). Some support for this view comes from the finding of long-term retrograde amnesia gradients observed following dysfunction of the hippocampus (for a review see Nadel & Moscovitch, 1997).
There are, however, other views of possible temporal relationships between the hippocampus and PPC. Some models assume that the long-term store for spatial information is located in the PPC and not in the hippocampus. Then, it is assumed that new spatial information is first processed in the PPC and if a mismatch between current input and long-term store occurs, then information is transferred for further processing in the hippocampus followed by consolidation and transfer back to PPC (Save & Poucet, 2000). Alternatively, spatial information could first be processed in the hippocampus and then transferred to the PPC. Finally, other models assume parallel processing of information with spatial information processed independently in both PPC and hippocampus followed by subsequent transfer of information to PPC. The data that have been presented thus far do not differentiate well among these views of temporal interactions between the hippocampus and PPC, but there are some data that support the idea that information is first processed in the hippocampus and then transferred to the PPC. Cho, Kesner, and Brodale (1995) and Cho and Kesner (1996) showed that rats with PPC lesions have a nongraded retrograde amnesia for four, but not two, previously learned spatial discriminations prior to surgery, whereas the entorhinal cortex produce a retrograde amnesia gradient for two or four previously learned spatial discrimination problems. These studies suggest the possibility that information is first processed in the hippocampus and entorhinal cortex and finally stored in the PPC. It appears that the entorhinal cortex also plays an important intermediate role in processing spatial information. This hypothesis is consistent with the idea that the entorhinal cortex and parahippocampal region serve as an intermediate term storage system (Eichenbaum, 1994). Also, it should be noted that topographical amnesia and spatial location memory deficits have been reported for patients with parahippocampal lesions (Bohbot et al., 1998; Habib & Sirigu, 1987; Nunn, Polkey, & Morris, 1998). Also based on PET scans and MRI data, it has been shown that the parahippocampal gyrus and entorhinal cortex are activated when subjects need to process spatial location information (Aguirre, Detre, Alsop, & D’Esposito, 1996; Johnsrude, Owen, Crane, Milner, & Evans, 1999; Milner, Johnsrude, & Crane, 1997). Thus, other neural regions (e.g. parahippocampal cortex, entorhinal cortex) may also contribute to the long-term representation of spatial information. It has been shown that patterns of place cell activity recorded in the CA1 region of the hippocampus in a behavioral task were similar in cellular activity in the PPC during slow wave sleep. It is suggested that during sleep the neuronal states experienced in the hippocampus in the waking state are replayed and transferred to the PPC as part of the consolidation process (Qin, McNaughton, Skaggs, & Barnes, 1997). These results support the idea that information is first processed in the hippocampus and then transferred to the PPC perhaps via intermediate neural sites which would include the entorhinal cortex and parahippocampal regions.
In a different study Izquierdo et al. (1997), showed that injections of AP5 (a glutamate antagonist) or muscimol (a GABA agonist) injected into the hippocampus immediately after inhibitory avoidance training produce retrograde amnesia, whereas the same injections into the entorhinal cortex or PPC produce retrograde amnesia only 180 min after inhibitory training. Furthermore, using CNQX (an AMPA antagonist) injected into the hippocampus 1 day after training, entorhinal cortex 1 or 31 days after training, and parietal cortex 1, 31 or 60 days after training temporarily disrupts retrieval of learned information. These data suggest that during learning the hippocampus is first involved followed by activation of the PPC perhaps via intermediate neural sites which would include the entorhinal cortex and parahippocampal regions and during retrieval the information is stored in long-term memory primarily in the PPC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cerebral Cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- Barrow CJ, Latto R. The role of inferior parietal cortex and fornix in route following and topographic orientation in Cynomolgus monkeys. Behavioural Brain Research. 1996;75:99–112. doi: 10.1016/0166-4328(96)00177-5. [DOI] [PubMed] [Google Scholar]
- Berthoz A. Hippocampal and parietal contribution to topokinetic and topographic memory. In: Burgess N, Jeffery KJ, O’Keefe J, editors. The hippocampal and parietal foundations of spatial cognition. New York: Oxford University Press; 1999. pp. 381–403. [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Boyd MG, Thomas RK. Posterior association cortex lesions in rats: Mazes, pattern discrimination and reversal learning. Physiological Psychology. 1977;5:455–461. [Google Scholar]
- Burgess N, Jeffery KJ, O’Keefe J. Integrating hippocampal and parietal functions: A spatial point of view. In: Burgess N, Jeffery KJ, O’Keefe J, editors. The hippocampal and parietal foundations of spatial cognition. Oxford: Oxford University Press; 1999. pp. 3–29. [Google Scholar]
- Burwell R. The parahippocampal region: Corticocortical connectivity. In: Scharfman HE, Witter MP, Scharcz R, editors. The parahippocampal region. New York: The New York Academy of Sciences; 2000. pp. 25–42. [DOI] [PubMed] [Google Scholar]
- Calton JL, Turner CS, Cyrenne DLM, Lee BR, Taube JS. Landmark control and updating of self-movement cues are largely maintained in head direction cells after lesions of the posterior parietal cortex. Behavioral Neuroscience. 2008;122:827–840. doi: 10.1037/0735-7044.122.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Lin L-H, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex: II. Contributions of visual and ideothetic information to the directional firing. Experimental Brain Research. 1994a;101:24–34. doi: 10.1007/BF00243213. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin L-H, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex: I. Anatomical distribution and behavioral modulation. Experimental Brain Research. 1994b;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Chen LL, Nakamura K. Head-centered representation and spatial memory in rat posterior parietal cortex. Psychobiology. 1998;26:119–127. [Google Scholar]
- Chiba AA, Kesner RP, Jackson P. Two forms of spatial memory: A double dissociation between the parietal cortex and the hippocampus in the rat. Behavioral Neuroscience. 2002;116:874–883. doi: 10.1037//0735-7044.116.5.874. [DOI] [PubMed] [Google Scholar]
- Cho YH, Kesner RP. Involvement of entorhinal cortex or parietal cortex in long-term spatial discrimination memory in rats: Retrograde amnesia. Behavioral Neuroscience. 1996;110:436–442. doi: 10.1037//0735-7044.110.3.436. [DOI] [PubMed] [Google Scholar]
- Cho YH, Kesner RP, Brodale S. Retrograde and anterograde amnesia for spatial discrimination in rats: Role of hippocampus, entorhinal cortex and parietal cortex. Psychobiology. 1995;23:185–194. [Google Scholar]
- Davis BK, McDaniel WF. Visual memory and visual spatial functions in the rat following parietal and temporal cortex injuries. Physiology & Behavior. 1993;53:145–151. doi: 10.1016/0031-9384(93)90023-9. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Hoang L, Huff L, Stone A, Kesner RP. Effects of hippocampus and medial caudate nucleus lesions on memory for direction information in rats. Behavioral Neuroscience. 2004;118:540–545. doi: 10.1037/0735-7044.118.3.540. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP. Effects of hippocampal and parietal cortex lesions on the processing of multiple-object scenes. Behavioral Neuroscience. 1998;112:68–82. doi: 10.1037//0735-7044.112.1.68. [DOI] [PubMed] [Google Scholar]
- DiMattia BV, Kesner RP. Spatial cognitive maps: Differential role of parietal cortex and hippocampal formation. Behavioral Neuroscience. 1988;102:471–480. doi: 10.1037//0735-7044.102.4.471. [DOI] [PubMed] [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage. 2002;15:16–25. doi: 10.1006/nimg.2001.0951. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampal system and declarative memory in humans and animals: experimental analysis and historical origins. In: Schacter DL, Tulving E, editors. Memory systems 1994. Cambridge, MA: MIT Press; 1994. pp. 147–201. [Google Scholar]
- Ellis AX, Della Sala S, Logie RH. The bailiwick of visuo-spatial working memory: evidence from unilateral spatial neglect. Cognitive Brain Research. 1996;3:71–78. doi: 10.1016/0926-6410(95)00031-3. [DOI] [PubMed] [Google Scholar]
- Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill S, Robertson L, Treisman A. Parietal contributions to visual feature binding: Evidence from a patient with bilateral lesions. Science. 1995;269:853–855. doi: 10.1126/science.7638604. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Gilbert PE, Kesner RP. Role of the rodent hippocampus in paired-associate learning involving associations between a stimulus and a spatial location. Behavioral Neuroscience. 2002;116:63–71. doi: 10.1037//0735-7044.116.1.63. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Localization of function within the dorsal hippocampus: The role of the CA3 subregion in paired-associate learning. Behavioral Neuroscience. 2003;117:1385–1394. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Taube JS. Head direction cells in rats with hippocampal or overlying neocortical lesions: Evidence for impaired angular path integration. Journal of Neuroscience. 1999;19:7198–7211. doi: 10.1523/JNEUROSCI.19-16-07198.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Howard BP, Hunsaker MR, Kesner RP. Human topological task adapted for rats: Spatial information processes of the parietal cortex. Neurobiology of Learning and Memory. 2008 doi: 10.1016/j.nlm.2008.05.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behavioral Neuroscience. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Habib M, Sirigu A. Pure topographical disorientation: A definition and anatomical basis. Cortex. 1987;23:73–85. doi: 10.1016/s0010-9452(87)80020-5. [DOI] [PubMed] [Google Scholar]
- Hermann T, Poucet B. Exploratory patterns of rats on a complex maze provide evidence for topological coding. Behavioural Process. 2001;54:155–162. doi: 10.1016/s0376-6357(00)00151-0. [DOI] [PubMed] [Google Scholar]
- Hoh TE, Kolb B, Eppel A, Vanderwolf CH, Cain DP. Role of the neocortex in the water maze task in the rat: A detailed behavioral and Golgi-Cox analysis. Behavioural Brain Research. 2003;138:81–94. doi: 10.1016/s0166-4328(02)00237-1. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Quillfeldt JA, Zanatta MS, Quevedo J, Schaeffer E, Schmitz PK, Medina JH. Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. European Journal of Neuroscience. 1997;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Jeffery KJ, Donnett JG, Burgess N, O’Keefe JM. Directional control of hippocampal place fields. Experimental Brain Research. 1997;117:131–142. doi: 10.1007/s002210050206. [DOI] [PubMed] [Google Scholar]
- Johnsrude IS, Owen AM, Crane J, Milner B, Evans AC. A cognitive activation study of memory for spatial relationships. Neuropsychologia. 1999;37:829–841. doi: 10.1016/s0028-3932(98)00136-5. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral analysis of the contribution of the hippocampus and parietal cortex to the processing of information: Interactions and dissociations. Hippocampus. 2000;10:483–490. doi: 10.1002/1098-1063(2000)10:4<483::AID-HIPO15>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Kesner RP, DiMattia BV, Crutcher KA. Evidence for neocortical involvement in reference memory. Behavioral and Neural Biology. 1987;47:40–53. doi: 10.1016/s0163-1047(87)90145-2. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Farnsworth G, Kametani H. Role of parietal cortex and hippocampus in representing spatial information. Cerebral Cortex. 1992;1:367–373. doi: 10.1093/cercor/1.5.367. [DOI] [PubMed] [Google Scholar]
- Kohler C. Intrinsic projections of the retrohippocampal region in the rat brain. I. The subicular complex. Journal of Comparative Neurology. 1985;236:504–522. doi: 10.1002/cne.902360407. [DOI] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, Sutherland RJ. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cerebral Cortex. 1994;6:664–680. doi: 10.1093/cercor/4.6.664. [DOI] [PubMed] [Google Scholar]
- Kolb B, Sutherland RJ, Whishaw IQ. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behavioral Neuroscience. 1983;97:13–27. doi: 10.1037//0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- Kolb B, Walkey J. Behavioural and anatomical studies of the posterior parietal cortex in the rat. Behavioural Brain Research. 1987;23:127–145. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- Kuipers BJ, Levitt TS. Navigation and mapping in large-scale space. AI Magazine. 1988 summer;9:25–42. [Google Scholar]
- Lavenex P, Amaral D. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Long JM, Kesner RP. The effects of dorsal vs. ventral hippocampal, total hippocampal, and parietal cortex lesions on memory for allocentric distance in rats. Behavioral Neuroscience. 1996;110:922–932. doi: 10.1037//0735-7044.110.5.922. [DOI] [PubMed] [Google Scholar]
- Long JM, Kesner RP. Effects of hippocampal and parietal cortex lesions on memory for egocentric distance and spatial location information in rats. Behavioral Neuroscience. 1998;112:480–495. doi: 10.1037//0735-7044.112.3.480. [DOI] [PubMed] [Google Scholar]
- Long JM, Mellem JE, Kesner RP. The effects of parietal cortex lesions on an object/spatial location paired-associate task in rats. Psychobiology. 1998;26:128–133. [Google Scholar]
- Maguire EA, Frackowiak RSJ, Firth CD. Recalling routes around London: Activation of the right hippocampus in taxi drivers. Journal of Neuroscience. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel WF, Wall TT. Visospatial functions in the rat following injuries to striate, prestriate, and parietal neocortical sites. Psychobiology. 1988;16:251–260. [Google Scholar]
- McNaughton BL, Chen LL, Marcus EJ. “Dead reckoning,” landmark learning, and the sense of direction: A neurophysiological and computational hypothesis. Journal of Cognitive Neuroscience. 1991;3:190–202. doi: 10.1162/jocn.1991.3.2.190. [DOI] [PubMed] [Google Scholar]
- Mendoza JE, Thomas RK., Jr Effects of posterior parietal and frontal neocortical lesions in the squirrel monkey. Journal of Comparative and Physiological Psychology. 1975;89:170–182. doi: 10.1037/h0076657. [DOI] [PubMed] [Google Scholar]
- Milner B, Johnsrude I, Crane J. Right medial temporal-lobe contribution to object-location memory. Philosophical transactions of the Royal Society of London Series B, Biological Sciences. 1997;35:1469–1474. doi: 10.1098/rstb.1997.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Consolidation, retrograde amnesia and the hippocampal formation. Current Opinion in Neurobiology. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Takarajima A. Recognition of pattern position and shape by population vector in the spatial spreading associative neural network. IEEE International Conference on Evolutionary Computation; 1996. pp. 780–785. [Google Scholar]
- Neill WT, Mathis KM. Transfer-inappropriate processing: Negative priming and related phenomena. Psychological Learning & Motivation. 1995;38:1–44. [Google Scholar]
- Nitz DA. Tracking route progression in the posterior parietal cortex. Neuron. 2006;49:747–756. doi: 10.1016/j.neuron.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Nunn JA, Polkey CE, Morris RG. Selective spatial memory impairment after right unilateral temporal lobectomy. Neuropsychologia. 1998;36:837–848. doi: 10.1016/s0028-3932(98)00030-x. [DOI] [PubMed] [Google Scholar]
- Parron C, Save E. Evidence for entorhinal and parietal cortices involvement in path integration in the rat. Experimental Brain Research. 2004;159:349–359. doi: 10.1007/s00221-004-1960-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. San Diego: Academic Press; 1997. [Google Scholar]
- Pohl W. Dissociation of spatial discrimination deficits following frontal and parietal lesions in monkeys. Journal of Comparative and Physiological Psychology. 1973;82:227–239. doi: 10.1037/h0033922. [DOI] [PubMed] [Google Scholar]
- Poucet B. Spatial cognitive maps in animals: New hypotheses on their structure and neural mechanisms. Psychological Review. 1993;100:163–182. doi: 10.1037/0033-295x.100.2.163. [DOI] [PubMed] [Google Scholar]
- Qin YL, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1997;352:1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL. The firing of hippocampal place cells in the dark depends on the rats’ recent experience. Journal of Neuroscience. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JV. Rat posterior parietal cortex: Topography of cortico-cortical and thalamic connections. Experimental Brain Research. 1994;100:67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Robertson L, Treisman A, Friedman-Hill S, Grabowecky M. The interaction of spatial and object pathways: Evidence from Balint’s syndrome. Journal of Cognitive Neuroscience. 1997;9:295–317. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Kesner RP. Hippocampal-parietal cortex interactions: Evidence from a disconnection study in the rat. Behavioural Brain Research. 2007;179:19–27. doi: 10.1016/j.bbr.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Guazzelli A, Poucet B. Dissociation of the effects of bilateral lesions of the dorsal hippocampus and parietal cortex on path integration in the rat. Behavioral Neuroscience. 2001;115:1212–1223. doi: 10.1037//0735-7044.115.6.1212. [DOI] [PubMed] [Google Scholar]
- Save E, Moghaddam M. Effects of lesions of the associative parietal cortex on the acquisition and use of spatial memory in egocentric and allocentric navigation tasks in the rat. Behavioral Neuroscience. 1996;110:74–85. doi: 10.1037//0735-7044.110.1.74. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B. Involvement of the hippocampus and associative parietal cortex in the use of proximal and distal landmarks for navigation. Behavioural Brain Research. 2000;109:195–206. doi: 10.1016/s0166-4328(99)00173-4. [DOI] [PubMed] [Google Scholar]
- Save E, Poucet B, Forman N, Thinus-Blanc C. The contribution of the associative parietal cortex and hippocampus to spatial processing in rodents. Psychobiology. 1998;26:153–161. [Google Scholar]
- Shafritz KM, Gore JC, Marois R. The role of the parietal cortex in visual feature binding. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10917–10922. doi: 10.1073/pnas.152694799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. Feature binding, attention and object perception. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 1998;353:1295–1306. doi: 10.1098/rstb.1998.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. The connections of presubiculum and parasubiculum in the rat. Brain Research. 1990;518:227–243. doi: 10.1016/0006-8993(90)90976-i. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird A, Morgan A, Muir JL, Aggelton JP. The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: Evidence from a disconnection study in the rat. Journal of Neuroscience. 2001;21:7323–7330. doi: 10.1523/JNEUROSCI.21-18-07323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Jarrard LE. Evidence for extrahippocampal involvement in place learning and hippocampal involvement in path integration. Hippocampus. 1996;6:513–524. doi: 10.1002/(SICI)1098-1063(1996)6:5<513::AID-HIPO4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, McKenna JE, Maaswinkel H. Hippocampal lesions and path integration. Current Opinion in Neurobiology. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- Witter MP, Holtrop R, van de Loosdrecht AA. Direct projections from the periallocortical subicular complex to the fascia dentata in the rat. An anatomical tracing study using Phaseolus vulgaris leucoagglutinin. Neuroscience Research Communications. 1988;2:61–68. [Google Scholar]
- Witter MP, Naber P, van Haeften T, Machielsen W, Rombouts S, Barkhof F, Scheltens P, Lopes da Silva F. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;110:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]