Abstract
It has been suggested the hippocampus mediates episodic memory processing involving snapshot memory and temporal sequence learning. To test this theory, rats learned trial-unique sequences of spatial locations along a runway box and were tested on recall by removing one of the locations in the sequence and making the rat choose the correct location to be rewarded. Once animals were able to reliably perform this episodic memory task, they received lesions to either CA3 or CA1. Animals with lesions to either CA3 or CA1 had difficulty with episodic memory processing, although CA1 lesioned animals had a much greater deficit. However, when animals were trained on a non-episodic version of the same task, hippocampal lesions had no effect. These results suggest that CA3 and CA1 both contribute to episodic memory processing since lesions to CA3 or CA1 result in an inability to process spatial information episodically, whereas they have no effect on non-episodic information processing.
Keywords: Spatial Information Processing, CA1, CA3, Episodic Memory, Temporal Context
Introduction
Episodic memory has been described as a collection of mental snapshots bound together into coherent episodes [cf. 1,10,11,13,14,21] One computational model for this process is the temporal context model [5,6] that suggests the hippocampus binds these mental snapshots supplied by the entorhinal cortex into a retrievable spatiotemporal context (this model is conceptually similar to the adaptive resonance theory; cf. [4]). This model has been used to describe the responses of place fields to changes in contextual inputs, as well as to describe episodic learning in humans [5,6]. An alternate theory is that the hippocampus acts as a sequence predictor; that is, the hippocampus continuously anticipates the subsequent input and acts as a match/mismatch comparator between the anticipated and experienced input patterns. This model has been used to describe sequence learning in a single trial [10,11,21], as well as episodic memory processing via integration of a sequence of mental snapshots [10,11]. More recently, interactions between neurophysiological oscillations and episodic memory have been proposed, focusing on path integration, navigation, and spatial sequence learning [1,13,14,15].
The present experiment was designed to evaluate the ability of rats to rapidly learn a short sequence of spatial locations (i.e. a sequence of 4 spatial locations presented only a single time), and to recall portions of the sequence that are omitted during a later test presentations. It is assumed that the animal has to use the immediately preceding element in the sequence to choose the correct, unmarked location. This could be solved either by using the temporal context (i.e. the unmarked location 3 occurred between locations 2 and 4 so that the presentation of either location 2 or 4 would be sufficient to guide recall of location 3 based on spatial and/or temporal adjacencies; cf. [5]) or via sequence prediction (i.e. the location 2 directly activates the stored representation of location 3 to guide the rat toward the correct unmarked spatial location that matches the representation; cf. [10,11,20,21]). Alternately, it has been suggested that rapid recall of the entire sequence during gamma oscillations could guide correct sequential recall in the dentate gyrus-CA3 system [1,13,14].
The present experiment revealed that rats with excitotoxic lesions to either dorsal CA3 or dorsal CA1 were unable to remember the sequence of spatial locations as well as control animals. Control animals were able to remember the correct, unmarked spatial location within the sequence at roughly 80% accuracy, CA3 lesioned animals were able to perform at 65% accuracy, and CA1 animals were only able to perform at 45% accuracy (25% accuracy corresponds to chance in this experiment). However, when lesioned rats were given a non-episodic version of the same task, there were no apparent deficits. This suggests that CA3 and CA1 contribute differentially to the rapid learning and recalling of spatial sequences; a finding supported by both the sequence prediction and temporal context models. Furthermore, the data suggest that both CA3 and CA1 participate in episodic memory processing by binding the spatiotemporal context needed to rapidly form episodic memories [7].
Materials and Methods
Animals
Sixteen male Long Evans rats, approximately 2 months of age and weighing 300-400 g at the start of the experiment were used as subjects. Each rat was housed independently in standard plastic rodent cages in a colony room. The colony was maintained on a 12 h light/dark cycle. All testing was conducted in the light proportion of the light/dark cycle. All rats were free fed and allowed access to water ad libitum. All animal care and experimental procedures conformed to the National Institutes of Health and Institution for Animal Care and Use Committee guidelines for proper care and use of experimental animals. A veterinarian verified the health of the animals weekly.
Experimental Apparatus
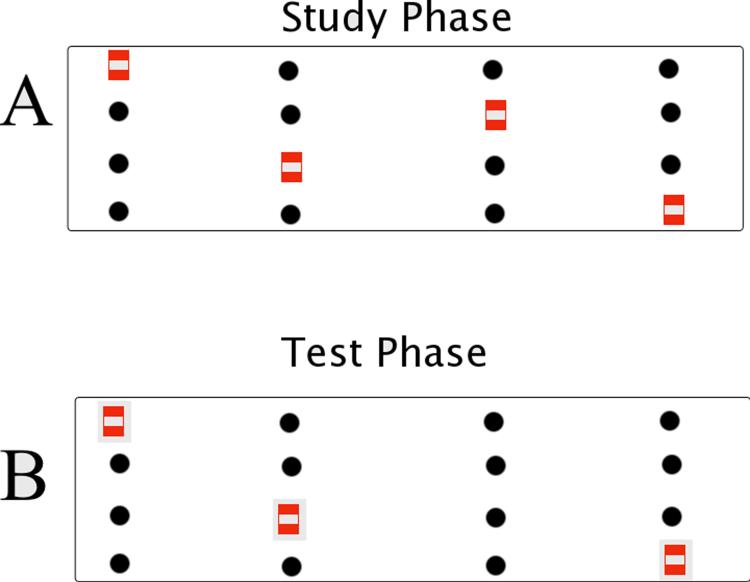
The experimental apparatus was a red Plexiglass box meant to approximate a runway (100 cm length × 30 cm width × 40 cm height) with 1.5 cm diameter holes drilled 1 cm deep into the floor of the box, with 4 holes spanning the width (~ 6.25 cm center to center distance; cf. Fig 1). There were four rows of wells along the length of the box. Four identical neutral blocks (7 cm tall × 2 cm wide) marked the holes containing reward.
Figure 1. Behavioral Apparatus.
A. Study Phase. Animals are presented a sequence of spatial locations along the runway box. B. Test Phase. Animals are given the sequence with a single location unmarked.
Surgical Method
All rats were trained to a criterion of >75% correct on the task prior to receiving surgery. Each rat was randomly assigned to receive a dorsal CA3 lesion (n = 5), a dorsal CA1 lesion (n = 6), or a vehicle control lesion (n = 5; CA3 vehicle n=3, CA1 vehicle n=2). Rats were anesthetized with isoflurane. Each rat was placed in a stereotaxic apparatus (David Kopf Instruments, Tunjana, CA) with an isothermal heating pad to maintain body temperature at 37°C. With its head level, the scalp was incised and retracted to expose bregma and lambda. Bregma and lambda were then positioned in the same horizontal plane to ensure a flat skull surface. Small holes were drilled into the skull and ibotenic acid (Ascent Scientific, Bristol, UK; 6 mg/mL PBS) was infused at a rate of 6 μL/hr via a 26 gauge injection cannula attached to a Hamilton (Reno, NV) syringe and a syringe pump (Cole Parmer, Vernon Hills, IL) at the following coordinates: for the dorsal CA3 lesion (0.05-0.15 μL of ibotenic acid infused into each site)—1) 2.5 mm posterior to bregma, 2.6 mm lateral to midline, and 3.2 mm ventral from dura (0.05 μL), 2) 3.3 mm posterior to bregma, 3.3 mm lateral to midline, and 3.2 mm ventral from dura (0.08 μL), and 3) 4.2 mm posterior to bregma, 4.2 mm lateral to midline, and 3.1 mm ventral from dura (0.15 μL); for the CA1 lesion (0.1-0.15 μL ibotenic acid infused into each site)—3.6 mm posterior to bregma, 1.0, 2.0, and 3.0 mm lateral to midline, and 1.9, 2.1, and 1.8 mm ventral from the dura mater (0.1, 0.1, and 0.15 μL infused). Control lesions were made into the two sub-regions (CA3 and CA1) with phosphate buffer (PBS) vehicle. Following surgery, the incisions were sutured and the rats were allowed to recover for one week before experimentation. They also received acetaminophen (200 mg/100 mL water) in their drinking water as an analgesic.
Behavioral Methods
Animals were initially trained to displace a neutral grey block for a cereal reward (Froot Loops, Kellogg's, Battle Creek, MI) over 7 d until they immediately displaced the block and consumed the reward. Once the animals freely obtained and consumed the reward, pre-surgical training began. Each trial consisted of a study phase made up of the presentation of a linear sequence of four spatial locations marked by neutral blocks (Fig 1A). After a 30 s interval (the approximate time needed to reset the box), the animal was given the test phase. The test phase consisted of the same sequence presented during the study phase, but one of the spatial locations was not marked by a block, but still contained a reward. The unmarked spatial location was pseudo-randomly distributed equally between the first, second, third, and fourth item in the sequence. To receive a reward, the rat had to visit the correct, unmarked spatial location (Fig 1B). If they chose incorrectly, the animal was allowed to self-correct and the trial was recorded as an error. Training continued until all animals reached a criterion of >75% correct. After surgery, the protocol was the same as before. Twenty-eight trials were given post-surgery. To ensure that the task was processed episodically, no sequence was repeated during pre-surgery training or post-surgery testing.
After the post-surgery testing was completed, animals were given a non-episodic version of the same task, that is to say they were given multiple trials using the same sequence so they could learn via trial and error. This task was given to evaluate any possible non-episodic processes that could have contributed to learning the episodic version of the task. The study phases of this fixed sequence were always the same. The unmarked spatial location changed each trial, but it was always within the same repeated sequence of spatial locations. Twenty-eight trials were given.
Data Collection and Statistical Analysis
The dependent variable in this experiment was whether or not the rat explored the correct, unmarked spatial location within the sequence during the test phase. If an animal made an error, the animal was allowed to self-correct and an error was recorded.
The scores were calculated and input into a matrix that was used to calculate a two way repeated measures ANOVA with lesion group (control, CA3, and CA1) as the grouping factor and block (Pre, Post, Fixed) as the repeated within factor. Tukey's HSD post hoc paired comparison tests were run on all significant effects and results were considered significant at p<0.05. The statistical power of all analyses was >0.80. All analyses were run on SPSS 15.0 (SPSS, Inc.; Chicago, IL). To evaluate whether the temporal position of the unmarked location affected the animal's performance, a two-way repeated measures ANOVA was performed on the post condition (the episodic portion of the task) with lesion group as the between grouping factor and temporal position (first, second, third, fourth) as the repeated within factor.
Histological Methods
After all behavioral testing was completed, rats were euthanized with a lethal dose of sodium pentobarbital (70 mg/mL i.p.) and perfused intracardially with 0.9% PBS (pH 6.0) for 2 m followed by 10% (wt/vol) formalin (pH 7.0) for another 5 m. The brains were then extracted and stored in 30% (wt/vol) sucrose formalin at 4° Celsius for 72 h before being frozen and sliced into 40 μm coronal sections with a freezing-stage microtome. Every third section from the tissue block containing the hippocampus was mounted on microscopic slides and nissl stained with cresyl violet for microscopic verification of the lesions. Sections were photographed and imported into ImageJ (v1.35j National Institute of Health; Bethesda, MD) for quantitative lesion analysis. Briefly, the anatomical region in question (CA3 or CA1) was traced using the freehand selection tool on Image J and the area contained within the tracing was calculated. The spared portion of the anatomical region was then traced and the percent damage was calculated based upon those two values. The dentate gyrus was also traced to evaluate nonspecific damage. The percent damage for each animal was calculated from the sections and then averaged across animals.
Results
Histology
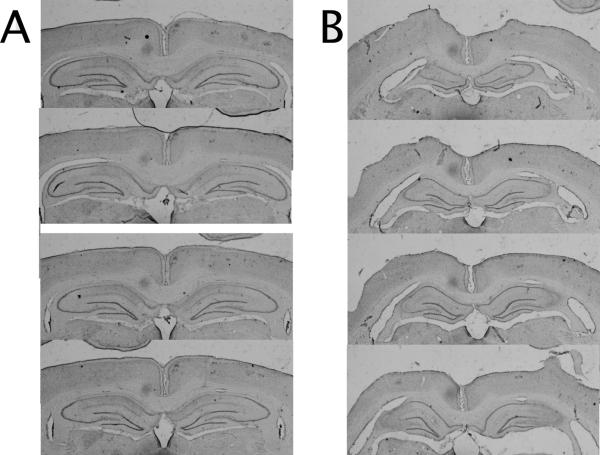
Fig 2 shows the results of the ibotenic-acid induced excitotoxic lesions of the CA1 (Fig 2A) and CA3 (Fig 2B) subregions of the dorsal hippocampus. In no cases did lesions result in extrahippocampal damage to parietal, entorhinal, postrhinal, or perirhinal cortices. CA3 was separated into CA3a,b and CA3c since there is differential damage to these subregions after our lesions, as well as because Lisman and colleagues [13,14] model the back projections from CA3c to the DG that are not as prominent in CA3a,b [cf. 12]. Also, percent damage refers to the dorsal hippocampus and not ventral, so damage was quantified from 2 mm posterior to bregma to 4.5 mm posterior to bregma, after Paxinos and Watson [19]. A quantitative analysis revealed that CA3 lesions resulted in (mean +/- standard error) 79 +/- 6.2% damage to the dorsal CA3 pyramidal cell layer (>90% in CA3a,b and ~50% in CA3c) with 3 +/- 0.9% damage to the upper blade of the dorsal dentate gyrus and 5 +/- 1.2% to dorsal CA2 and dorsal CA1. Lesions of CA1 resulted in 82 +/- 4.3% damage in the dorsal CA1 pyramidal cell layer with 7 +/- 1.7% damage to the underlying upper blade of the dorsal dentate gyrus, 10 +/- 2.2 % damage to dorsal CA2, <1% damage to dorsal CA3a,b, and no damage to CA3c.
Figure 2. Histology.
A. Dorsal CA3 lesion photomicrographs from a single animal. B. Dorsal CA1 lesion photomicrographs from a single animal.
Behavior
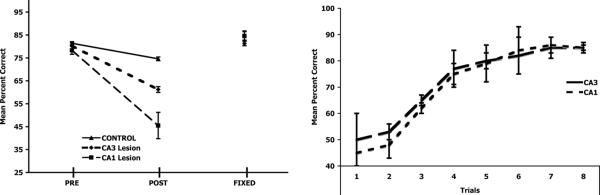
Fig 3A shows the results of the present task. All animals were able to learn the task to criterion in 50 +/- 10 trials prior to surgery (data not shown). Notice that no groups differed prior to surgery (e.g. training) or during the fixed sequence (e.g. the non-episodic version) task. They did differ, however, during the postoperative testing period (e.g. the episodic version). To analyze this data, a two-way repeated measures ANOVA with lesion (control, CA1, CA3) as the between factor and block (Pre, Post, Fixed) as the within factor was performed. There was a significant effect for lesion (F(2,13)=9.98, p=0.002), an effect for blocks (F(2,13)=81.42, p<0.0001), as well as a lesion x blocks interaction (F(4,13)=14.84, p<0.0001). Since the most informative results contained within the analysis were within the lesion x blocks interaction, Tukey's HSD post hoc paired comparisons were performed on lesion group within blocks. Before surgery (Pre), no groups differed (all ps>0.50). This suggests that all groups learned the task to criterion prior to surgery, which was expected, as the animals had not received surgical manipulation prior to training. After surgery, during the episodic task (Post), both CA1 and CA3 lesioned animals made a greater number of errors than the control group (CA1 vs. Control p=0.001; CA3 vs. Control p<0.05). CA1 lesioned animals also made a greater number of errors than CA3 lesioned animals (p<0.05). When animals were given an over-trained, fixed sequence that was non-episodic (Fixed), no groups differed (all ps>0.50). These results suggest that the deficit was one of episodic memory processing due to an inability to rapidly process the temporal sequence of spatial locations, and not a spatial processing deficit per se.
Figure 3. Behavioral Results.
A. There were no preoperative group differences (Pre). Post-operatively (Post), CA3 and CA1 were impaired relative to controls and CA1 was impaired relative to CA3. During the fixed task, no groups differed. B. Acquisition of the fixed condition. Notice that both CA3 and CA1 lesioned animals were able to rapidly learn the fixed task to criterion.
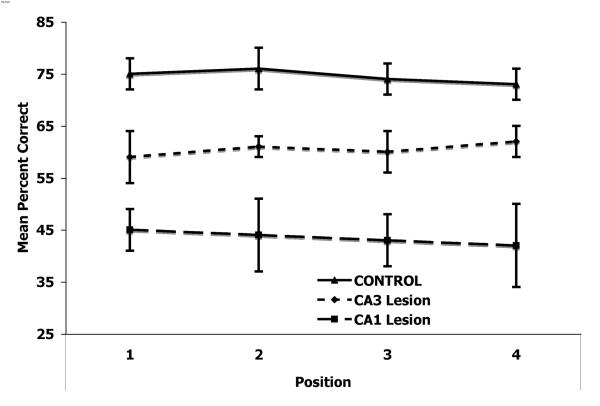
To evaluate whether animals made a greater or lesser number of errors based on temporal sequence, a two way repeated measures ANOVA was run on the Post condition with lesion and temporal position (first, second, third, fourth) as factors revealed no effect for temporal order (F(3,13)=0.55, p=0.59; Fig 4). This is probably due to the short sequence length (4 items) not being sufficiently difficult to dissect out more discrete temporal effects (cf. [5,21]).
Figure 4. Serial Order.
Notice that there were not differences observed as a function of temporal position.
For the fixed condition, all groups showed a relatively steep learning curve, with all animals reaching the pre-surgical criterion by trial 6 +/- 2 (mean +/- SEM) out of the 28 trials given (The first 8 trials are shown in Fig 3B). To assess any potential differences between groups (CA3 and CA1) as a function of block of four trials, a two way repeated measures ANOVA was performed. There were no differences between groups for acquisition of the fixed condition (F(1,13)=1.22, p=0.29), there was a block effect (F(7,13)=7.21, p=0.01), but no lesion group x block interaction, (F(1,13)=2.99, p=0.10). This suggests that CA3 and CA1 lesioned animals learned similarly.
Discussion
Episodic Memory Processing Models
The present data support previous models of episodic memory processing that posit a central role for the hippocampus [1,10,11,17,20,21], since the hippocampus is capable of rapidly encoding (or binding) contextual and temporal information. This has been described as an integration process of either mental snapshots [10,11] or the rapid acquisition of a spatiotemporal context [4,5,20,21]. This role of the hippocampus was verified by testing rats on an episodic version of a temporal sequence for spatial locations task, followed by a non-episodic version of the same task, wherein the only difference was the nature of the information processing (e.g. trial unique or repeated spatial sequences). Whatever the mechanism of episodic memory processing, the present data provide compelling evidence that the hippocampus mediates this form of processing for spatio-temporal information.
The data suggest that CA1 plays a more critical role in the present task than CA3. This could be due to the emphasis placed on temporal processing in the episodic version of the task. Although it is necessary for the animals to rapidly learn the spatial locations and numerous models have suggested that the DG and CA3 primarily mediate the rapid spatial processing important for episodic memory formation [8,10,11,13,14,16,20,21] it does not appear that CA3 is as critical as CA1 for performance of this task. CA3 may be helpful for rapidly processing spatial information and passing it to CA1 for further processing [cf 3,18]. Perhaps the present data can be interpreted as support for the role of CA1 in binding both the spatial and temporal contexts either directly, as postulated by the temporal context model [5,6], or else via parallel processing as postulated by adaptive resonance theory [4].
The present data do not discount, however, that the larger effect of a lesion to CA1 results in not only a disruption of CA1 processing, but also a disruption of CA3 outputs. This would support the theories that suggest DG-CA3 play a critical role in episodic memory processing [1,13,14] since the CA3 outputs to CA1 provide more reliable spatiotemporal information than the direct projections into CA1 form the entorhinal cortex [20]. If this is the case (and the results of the present experiment do not definitively conclude either way), then the DG-CA3 system may rapidly associate spatial sequences in a temporal framework (i.e. immediate adjacencies associated by the recurrent collateral system), which are sent to CA1via the Shaffer collaterals for further temporal processing and comparison with direct entorhinal inputs [cf. 1,10,11,13,14,20,21]. That would mean that a CA1 lesion would act, in effect, as a combined CA1 and CA3 lesion if the Shaffer collaterals carry information required for episodic memory processing and thus explain the greater deficits seen after CA1 lesions than after CA3 lesions.
The only way to know whether CA3 or CA1 show a more critical involvement in this task is to run the task while recording neural firing and comparing the CA3 responses to CA1 responses. The same proviso holds for directly testing the models for temporal context formation and retrieval. Only by directly evaluating neural responses can the precise character of the information processing be elucidated. The present study was limited to a behavioral analysis and a rough determination of the relative roles of CA3 and CA1 for episodic memory processing with an emphasis on temporal context.
Neurophysiological Models
The present data cannot directly provide support for theories of episodic sequence learning that use neurophysiological oscillations as recall mechanisms [cf.1,13,14] since no neural recording accompanied the present experiment. The data clearly indicate that the CA3 system (e.g. CA3 itself or the reciprocal dentate gyrus-CA3 interaction as postulated by Lisman [12,13,14]) is not as critical as CA1 for episodic recall of spatio-temporal sequences. An alternative explanation may be that episodic memory processing is dependent upon the trisynaptic loop, so a lesion anywhere along the loop will result in deficits. This means that a lesion to CA1 would result in deficits in CA1, but also deficits mirroring CA3. This suggests that any sequential replay in the dentate gyrus-CA3 system is not the critical determinant of efficient performance of the episodic task. The data, however, suggest that repetitions of the same sequence allow the sequence to be recalled by extrahippocampal substrates (e.g. neither CA3 nor CA1 mediate recall once consolidated). This data is somewhat difficult to compare to theories that suggest theta phase precession plays a critical role in episodic recall [5,10,11], except to say that the fixed component of the task was initially learned episodically (e.g. during trials 1-5), and then was acquired via a non-episodic, potentially semantic mechanism (e.g. trials 5-thereafter). The data seem to support a proposition made by Buzsaki [1] that repeated paths along a corridor, or else intersections of current and previous pathways, may facilitate the transition from episodic to semantic processing of the spatial information--thus guiding accurate navigation and path integration within the environment and eventual consolidation into the neocortex and out of the hippocampus.
Acknowledgments
This research was supported by NSF Grant IBN-0135273 and NIH Grant RO1 MH065314 awarded to RPK. The authors wish to thank Alan Coltrin, David McPhee, and Jessica Goodman for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic, and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- [2].Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- [3].Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15:808–184. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- [4].Gorchetchnikov A, Grossberg S. Space, time and learning in the hippocampus: how fine spatial and temporal scales are expanded into population codes for behavioral control. Neural Networks. 1997;21:182–203. doi: 10.1016/j.neunet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- [5].Howard MW, Fotedar MS, Datey AV, Hasselmo ME. The temporal context model in spatial navigation and relational learning: toward a common explanation of medial temporal lobe function across domains. Psychological Review. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Howard MW, Natu VS. Place from time: reconstructing position from a distributed representation of temporal context. Neural Networks. 2005;18:1150–1163. doi: 10.1016/j.neunet.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hunsaker MR, Kesner RP. The attributes of episodic memory processing. In: Dere E, Huston JP, Easton A, Nadel L, editors. Handbook of Behavioral Neuroscience Research: Episodic Memory Research. Elsevier; Amsterdam: 2007. In press. [Google Scholar]
- [8].Kesner RP. Neurobiological views of memory. In: RP Kesner RP, Martinez JL Jr, editors. Neurobiology of Learning and Memory. 2nd edition Elsevier; Amsterdam: 2007. [Google Scholar]
- [9].Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci. 2005;120:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- [10].Levy WB. A computational approach to hippocampal function. In: R. D. Hawkins RD, G. H. Bower GH, editors. Computational Models of Learning in Simple Systems. Academic Press; San Diego: 1989. pp. 243–305. [Google Scholar]
- [11].Levy WB. A simplified hippocampal model that learns and uses three kinds of context. In: Bower JM, editor. Computational Neuroscience: Trends in Research. Plenum Press; New York: 1997. pp. 379–383. [Google Scholar]
- [12].Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994:339, 181–218. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- [13].Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- [14].Lisman JE, Talamini LM, Raffone A. Recall of memory sequences by interaction of the dentate and CA3: a revised model of the phase precession. Neural Networks. 2005;18:1201–1211. doi: 10.1016/j.neunet.2005.08.008. [DOI] [PubMed] [Google Scholar]
- [15].Manns JR, ZIlli EA, Ong KC, Hasselmo ME, Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: Relation to theta phase. Neurobiol Learn Mem. 2006;87:9–21. doi: 10.1016/j.nlm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [16].Minai AA, Levy WB. Sequence learning in a single trial. International Journal of Neural Networks. 1993;2:423–426. [Google Scholar]
- [17].Morris RGM. Episodic-like memory in animals: psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. In: Baddeley A, Aggleton JP, Conway MA, editors. Episodic Memory: New Directions in Research. Oxford University Press; Oxford: 2001. pp. 181–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Teckel MF, Sun LD, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002:297, 211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. Academic Press; San Diego: 1997. [DOI] [PubMed] [Google Scholar]
- [20].Rolls ET, Stringer SM, Trappenberg TP. A unified model of spatial and episodic memory. Proc R Soc London, B. 2002:269, 1087–1093. doi: 10.1098/rspb.2002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Treves A. Computational constraints between retrieving the past and predicting the future, and the CA3-CA1 differentiation. Hippocampus. 2004:14, 539–556. doi: 10.1002/hipo.10187. [DOI] [PubMed] [Google Scholar]