Abstract
Theta oscillations (4-8 Hz) are often modulated in human electroencephalogram (EEG) studies of memory, whereas overlapping frequencies dominate rodent hippocampal EEG. An emerging parallelism between theta reactivity and hippocampal functional magnetic resonance imaging activation has suggested a homology between theta activity in humans and rodents, representing a process of cortico-hippocampal interaction involved in memory. In the present study, we investigated EEG reactivity during performance of a relational memory task that induces a negative hippocampal blood oxygenation level dependent (BOLD) signal change, compared to a nonrelational control condition. Relational trials induced theta increases and alpha decreases. Low Resolution Electromagnetic Brain Tomography estimates localized theta and alpha modulation to frontal midline and parietal midline cortices, respectively, both of which exhibit negative BOLD responses in this task. Thus, theta and alpha dynamics are dissociable from positive BOLD activation, and may, in fact, colocalize with negative BOLD responses.
Keywords: fMRI, Alpha, Default mode, Negative BOLD, LORETA
Theta oscillations are an aspect of electroencephalogram (EEG) and magnetoencephalogram (MEG) signals that have attracted increasing attention from cognitive neuroscientists in recent years. Oscillatory activity in the frequency range of approximately 4-8 Hz is defined as the theta band in humans, whereas the same term in the animal literature may refer to activity up to about 12 Hz, especially in the rodent. Theta oscillations dominate the EEG signal recorded from intracranial electrodes in the rodent hippocampus and have been extensively investigated in that system with regard to their generation, pharmacological manipulation, and relationship to neuronal spiking and cognitive information processing. In the human literature, electrophysio-logical oscillations in the theta range have been observed in an ever-increasing list of cognitive tasks, leading to the question of whether theta oscillations recorded from the human scalp and the rodent hippocampus are related phenomena.
Increased oscillatory power in the theta band is commonly observed in human EEG and MEG studies employing comparisons related to performance of memory tasks, including contrasts between successful and unsuccessful memory encoding (Klimesch, Doppelmayr, Russegger, & Pachinger, 1996; Sederberg, Kahana, Howard, Donner, & Madsen, 2003), successful and unsuccessful memory retrieval (Burgess & Gruzelier, 2000; Guderian & Duzel, 2005), working memory load (Jensen & Tesche, 2002), sentence processing (Bastiaansen, van Berkum, & Hagoort, 2002), and spatial navigation (de Araujo, Baffa, & Wakai, 2002; Ekstrom et al., 2005). (For reviews, see Kahana, Seelig, & Madsen, 2001; Klimesch, Doppelmayr, Schwaiger, Auinger, & Winkler, 1999). This pattern of findings has intriguing parallels in the human functional magnetic resonance imaging (fMRI) literature, related to activation of the hippocampal formation and parahippocampal gyrus in similar memory comparisons. Notably, numerous studies have detected positive blood oxygenation level dependent (BOLD) activations in these structures for successful versus unsuccessful encoding (Otten, Henson, & Rugg, 2001; Reber et al., 2002) and retrieval (Gabrieli, Brewer, Desmond, & Glover, 1997; Meltzer & Constable, 2005; Stark & Squire, 2000), although frontal and parietal cortices are also sometimes activated by analogous contrasts. As animal studies have shown that hippocampal theta oscillations play a key role in the encoding of spatial memories in rodents (Hasselmo, Hay, Ilyn, & Gorchetchnikov, 2002; O'Keefe & Recce, 1993), several authors have theorized that theta oscillations in human EEG/MEG may reflect dynamic interactions between hippocampal and cortical structures related to the formation and recall of episodic memories (Bastiaansen & Hagoort, 2003; Buzsaki, 1996; Miller, 1991).
In their thorough review of EEG theta findings related to memory, Bastiaansen and Hagoort (2003) note this pattern of parallelism between findings of theta power increases in human EEG studies and findings of hippocampal BOLD activation in memory tasks, suggesting that theta may serve as a window into hippocampal function available in human EEG studies, with the caveat that, at present, the empirical evidence for a relationship between human EEG theta power and hippocampal function is only indirect and equivocal. Although the involvement of theta responses in memory tasks does suggest homology with hippocampal function, it is generally acknowledged that human EEG activity detected in scalp recordings is unlikely to be generated in the hippocampus, as that is a deep structure with a closed geometry and hence is unlikely to contribute appreciably to the electromagnetic fields detectable at the surface, which are dominated by synchronous activity occurring in radially aligned neurons of the cortex (Nunez & Srinivasan, 2006). Source localization algorithms applied to theta power increases in cognitive tasks have generally implicated frontal midline sources such as medial prefrontal cortex and/or anterior cingulate (Asada, Fukuda, Tsunoda, Yamaguchi, & Tonoike, 1999; Ishii et al., 1999; Luu, Tucker, & Makeig, 2004; Onton, Delorme, & Makeig, 2005), and these findings have been corroborated by direct intracranial recordings in human (Uchida, Maehara, Hirai, Kawai, & Shimizu, 2003; Wang, Ulbert, Schomer, Marinkovic, & Halgren, 2005) and monkey (Tsujimoto, Shimazu, & Isomura, 2006). Some studies of theta rhythm recorded in human intracranial recordings have detected task-modulated theta power at a wide variety of neocortical locations (Caplan, Madsen, Raghavachari, & Kahana, 2001; Meltzer, Zaveri et al., 2008; Raghavachari et al., 2001; Sederberg et al., 2003), while an analysis of theta power and phase correlations between electrodes located at varying distances from each other has suggested that the generators of theta rhythm are of local neocortical origin (Raghavachari et al., 2006), arguing against the idea that theta power throughout the brain reflects volume conduction from a single hippocampal generator or that cortical theta reflects phase-locked neural interactions with hippocampal circuits. Nonetheless, intracranial studies have also detected task-modulated theta oscillations in the hippocampus (Ekstrom et al., 2005; Fell et al., 2003), which may be generated independently.
Given the above evidence, few authors presently believe that human theta responses in scalp EEG are generated in the hippocampus, but the idea that they reflect cortico-hippocampal interactions is alive and well (e.g., Moore, Gale, Morris, & Forrester, 2006), driven largely by the parallelism between findings of increased theta and hippocampal BOLD responses in memory tasks. However, that parallelism may not be an invariable finding. For one thing, this parallelism has arisen from meta-analyses of various studies that have employed scalp EEG, intracranial EEG, or fMRI, but seldom have these methods been combined for study of the same task. The memory tasks that have been reported to activate the hippocampus or drive theta responses are related, but seldom identical. Recently, our laboratory has conducted a series of fMRI studies on a task known as transverse patterning, which may present an opportunity for a definitive dissociation between hippocampal BOLD responses and EEG theta.
In a transverse patterning problem (Spence, 1952), pairs of stimuli are presented, and in each pair, one stimulus is designated correct and one is designated incorrect. To solve the problem, one must learn the following rule: A is correct when paired with B, B is correct when paired with C, and C is correct when paired with A. This is identical to the childhood game “Rock, Paper, Scissors,” in that each stimulus is ambiguous, but by attending to the relations between the stimuli, the problem can be solved. Hippocampal damage has been shown to severely disrupt performance of this task in rats (Alvarado & Rudy, 1995; Dusek & Eichenbaum, 1998; but see Bussey, Clea Warburton, Aggleton, & Muir, 1998), monkeys (Alvarado, Wright, & Bachevalier, 2002), and humans (Rickard & Grafman, 1998; Rickard, Verfaellie, & Grafman, 2006; cf. Reed & Squire, 1999), relative to a control condition in which the same stimuli are consistently designated as correct or incorrect regardless of their pairing. Hereafter, we refer to such a control condition as “elemental,” and the task condition involving the transverse patterning problem as “configural” (Figure 1).
Figure 1.

The transverse patterning task. A: The stimuli and valences for the configural condition, which involved solving the transverse patterning problem. B: The elemental condition, which served as a control condition. Only two pictures were seen at a time, as described in the text.
Despite the evidence that the hippocampus is involved in forming and recalling configural associations, our initial fMRI study (Astur & Constable, 2004) revealed that continuous blocks of configural task performance elicit a robust negative signal change in the hippocampus, relative to both the elemental condition and to fixation. One possible explanation of this apparently paradoxical finding was that the hippocampus may be tonically activated during periods of the less demanding elemental and fixation conditions due to the occurrence of more spontaneous cognitive activity unrelated to the task, an explanation that has been proposed to account for the commonly observed deactivations in medial prefrontal cortex and other regions known as the “default mode network” (Gusnard & Raichle, 2001; Shulman et al., 1997). As a followup study, we reimplemented the task in a mixed block/event-related design to distinguish between sustained deactivation on the block level (attributable to task-independent processes) and transient negative signal changes directly induced by stimulus presentation. The results of this study (Meltzer, Negishi, & Constable, 2008) were striking and unexpected. Sustained signal changes on the block level were not observed. Rather, negative BOLD signal changes were almost entirely attributable to a negative-going event-related transient signal. However, this transient signal was systematically delayed relative to the positive transient signals observed in other regions, peaking at 10-12 s poststimulus, whereas positive transients occurred in the typical range of 4-6 s. Regions exhibiting an enhanced negative transient signal included the hippocampus as well as the typical “default mode” regions such as medial prefrontal cortex, posterior cingulate, and lateral parietal regions.
Given the results of the two fMRI studies described above, the contrast between the configural and elemental versions of this task provides an elegant means to probe for a potential dissociation between hippocampal BOLD activation and EEG theta responses. The configural condition reliably induces a negative event-related signal change in the hippocampus. A theory based on a strict parallelism between hippocampal BOLD activation and EEG theta would therefore predict that configural trials should not elicit a greater theta power response than elemental trials. However, a finding of greater theta power on configural trials, though breaking the hypothesized parallelism, would be in agreement with previous studies of theta responses. Given that the configural condition is more cognitively demanding (as indexed by accuracy and reaction time), we would predict a larger theta response in this condition. Furthermore, configural trials demand retrieval from memory of information involving pairwise relationships between stimuli, a condition that has been suggested to involve theta mechanisms on both empirical (Caplan & Glaholt, 2007) and theoretical grounds (Borisyuk & Hoppensteadt, 1998; Jensen & Lisman, 2005).
To evaluate the reactivity of human EEG theta power in the transverse patterning task, we have conducted EEG recordings on a new cohort of 16 subjects performing the same task that was employed in the original fMRI study of Astur and Constable (2004). Here we report robust event-related responses in both theta and alpha frequency ranges that distinguish between the configural and elemental conditions. Additionally, we have conducted source localization estimates on the observed changes in theta and alpha power and compared them directly to the previously presented fMRI data to provide additional insight on the potential relationship between BOLD and EEG.
Methods
Participants
Sixteen participants were recruited from the Yale University Community, ranging from 18 to 28 years old. All participants gave informed consent, had normal or corrected vision, and were paid for their participation. The study protocol was approved by the Yale University Human Investigation Committee.
Behavioral Task
Two different conditions were contrasted in the behavioral task. In the configural condition, participants were exposed to the transverse patterning problem (A+ vs. B-; B+ vs. C-; C+ vs.A-), and in the elemental condition, participants were exposed to a control problem (U + vs. V -; W + vs. X -; Y + vs. Z -). Note that no relational solution is required for the control problem and that this problem can be solved by animals with hippocampal damage (Alvarado & Rudy, 1995; Reed & Squire, 1999; Rickard & Grafman, 1998). Subjects were told that they would see two pictures, that they were to select which one is the “winner,” and that the computer would give auditory feedback about whether or not their choices were correct. To choose a stimulus, the participant pushed one of two buttons corresponding to the presentation side of the image (left or right side). Correct responses were followed by a pleasant chime, and incorrect responses were followed by a buzzer sound. Stimuli were presented once every 4 s and remained on the screen for 3 s, which was the maximum time allowed for response and feedback. During EEG recording (after the training period described below), stimuli were presented in alternating blocks of 12 from each condition. Each run contained 96 trials, in eight blocks, and each subject performed seven runs. Runs alternately started with the configural or elemental condition.
Training
Subjects were trained on the task prior to EEG recording, using a staged training paradigm that has been shown to be optimal for teaching the transverse patterning task to humans while generally ensuring that subjects adapt a configural strategy for solving it (Astur & Sutherland, 1998). Subjects were trained on one pair from each set (configural and elemental) at a time, until all six pairs had been encountered. Subjects then did a set of all stimuli in random order, but blocked into sets of six at a time from each condition. At this point, most subjects were at nearly 100% performance on the elemental condition but not on the configural condition. We then discussed the logical structure of the configural condition explicitly with subjects, using the rock-paper-scissors analogy. Subjects were then given extra rounds of practice on the configural condition alone, until they reached near-perfect performance. All subjects reached this point within 20 min.
EEG Recording
EEG was recorded from 30 scalp sites with a Quik-cap and Synamps amplifier (Neuroscan, El Paso, TX) and sampled at 250 Hz, using a forehead ground and digitally linked earlobe electrodes as a reference. Bipolar leads were used to monitor blinks and eye movements. Continuous EEG data was epoched into individual trials from - 500 to 3500 ms relative to stimulus presentation and manually screened for severe artifacts. Contaminated trials (<10%) were removed from further analysis. Additionally, trials with incorrect or absent behavioral responses were excluded. Independent Component Analysis (using EEGLAB software; Delorme & Makeig, 2004) was then used to model and remove blinks and eye movements from the data.
For evaluation of oscillatory activity modulated by the task, two methods of spectral analysis were used. First, time-frequency decomposition was applied to all single trials in order to evaluate the event-related modulations in spectral power induced by stimulus presentation in each condition. Each trial was decomposed into 200 overlapping Hanning-window segments. Log power at each point in time-frequency space was averaged across trials (within condition), and from each point was subtracted the average log power during the prestimulus baseline period, producing a measure known as the “event-related spectral perturbation,” or ERSP (Makeig, 1993). ERSP decompositions were averaged across subjects, within condition, at each electrode site.
Although time-frequency analysis provides a measure of the temporal evolution of oscillatory power, the use of smaller time windows within the trial results in a loss of frequency resolution compared to traditional stationary Fourier analysis. To obtain a more specific estimate of the frequency ranges in which the two conditions differed, we also applied multitaper spectral analysis to each trial (Percival & Walden, 1993) using the matlab program “pmtm” applied to the entire time length of the trial. The resulting spectra were averaged to produce an average spectrum for each subject, condition, and electrode.
LORETA Source Localization
To estimate the intracranial sources of the observed changes in EEG theta and alpha power, we used Low Resolution Electromagnetic Brain Tomography (LORETA), implemented in the freely available LORETA-KEY software (http://www.unizh.ch/keyinst/NewLORETA/LORETA01.htm; Pascual-Marqui, Michel, & Lehmann, 1994). This technique produces a distributed solution by minimizing the L2-norm of the difference between the predicted forward solution and the observed data, weighted by a Laplacian operator, which has the effect of producing as smooth an estimate as possible. While numerous strategies for three-dimensional localization of EEG activity are available (Michel et al., 2004), the use of a smoothly distributed inverse solution facilitates combining results across subjects, similar to the smoothing of fMRI data in multisubject studies. The use of LORETA in the frequency domain to localize oscillatory activity is described fully by Frei et al. (2001). Briefly stated, the algorithm incorporates estimation of the cross-spectra between each electrode pair and is not based simply on the measured power spectrum of each electrode.
A LORETA inverse transformation was computed from the standard locations of the electrodes in the elastic cap, using a standardized three-shell spherical head model registered to reference space (the MNI brain). EEG epochs consisting of 512 samples were extracted from the time range of 250-2300 ms poststimulus, which was the time of maximum difference between the two conditions, as found in the time-frequency analysis (see Results section). The cross-spectral matrix was computed for each epoch and averaged within subject and condition, resulting in a LORETA map for each frequency band. LORETA maps were then exported to analyze format (www.ihb.spb.ru/~pet_lab/L2S/L2SMain.htm), and imported into AFNI (afni.nimh.nih.gov/afni) for further analysis and comparison with fMRI data. LORETA maps were averaged across frequencies into theta and alpha bands, and then a difference image for each subject and frequency band was computed by subtracting the elemental condition map from the configural condition. The resulting difference maps were then averaged across subjects and superimposed on a common reference anatomical brain image for visualization.
FMRI
FMRI was performed on a 3T Siemens Trio scanner. As the results of the fMRI experiment have been reported elsewhere (Meltzer, Negishi, & Constable, 2008), we will describe only the essential details here, for comparison to the present results. The behavioral task was the same, except that instead of a constant 4-s interstimulus interval, the time between trials varied pseudo-randomly from 3.1 to 7.75 s, in integer multiples of the scanning repetition time (1.55 s). This was done so that shape of the event-related hemodynamic responses could be empirically calculated through deconvolution while maintaining the block structure of alternation between conditions. The use of a mixed block/rapid-event-related design allowed for the calculation of average net signal change during the alternating blocks, as well as the magnitude of the event-related responses in both conditions that contribute to the net signal change. In this report, we present the average LORETA localization of EEG theta and alpha power changes with the cohort-level fMRI results from the previous study.
Results
Behavioral
As expected, the configural condition was considerably more difficult than the elemental condition, as indicated by measures of reaction time and accuracy, even though all subjects were well above chance on both conditions. Mean reaction time in the configural condition was 1166 ms, and in the elemental condition, 725 ms; paired t test, t(15) = 11.0, p<.001. Mean accuracy was 95.5% for configural and 99.7% for elemental; paired t test, t(15) = -4.54, p<.001.
EEG Time-Frequency Analysis
To assess the extent to which presentation of the stimuli induced changes in EEG spectral power, measures of ERSP (Makeig, 1993) were computed at each electrode for every subject, averaged separately within the configural and elemental conditions. As electrode FZ is the scalp location most commonly associated with frontal midline theta power, we hypothesized that configural stimuli would induce greater increases in theta power than elemental stimuli at that location and at nearby electrodes, although both conditions would induce increases in theta power over the prestimulus baseline. Both of these predictions were confirmed. Figure 2a,b resents time-frequency spectrograms in the configural and elemental conditions at location FZ, averaged over trials and subjects, along with the difference between the two conditions (Figure 2c). After stimulus presentation, power in the delta and theta bands (approximately 0-6.5 Hz) increases, whereas power in the alpha and beta bands (approximately 7-23 Hz) decreases. The amplitude and duration of both the theta increase and the alpha decrease are greater in the configural condition than in the elemental condition. For assessment of the statistical significance of these effects across scalp locations and subjects, we defined the frequency bands of theta as 4-7 Hz and alpha as 8.5-11.5 Hz in order to clearly separate the two bands. The ERSP values for each condition were then averaged across timepoints (0-3000 ms) and trials to generate a theta and an alpha value for each electrode and subject. A paired t test of values at electrode FZ revealed a significant increase in theta for configural versus elemental trials, t(15) = 2.89, p = .011, and a decrease in alpha, t(15) = -3.86, p<.002. To assess the extent to which these effects were present elsewhere on the scalp, we further averaged ERSP values across electrodes into six regions: frontal, central, parietal, occipital, left-temporal, and right-temporal (shown in Figure 2d). Values were submitted to repeated-measures analysis of variance (ANOVA). In the theta band, there were significant main effects of condition, F(1,15) = 10.55, p = .005, and region, F(3.3,49) = 3.38, p = .022, Greenhouse-Geiser corrected, but no Condition × Region interaction, F(2.3,34) = .893, n.s., indicating that the greater theta increase for configural versus elemental trials was fairly evenly distributed across the scalp, as illustrated in the topographical plot in Figure 2e. In the alpha band, there were significant main effects of condition, F(1,15) = 24.1, p<.001, and region, F(2.1,31.8) = 4.66, p = .015, as well as a significant Condition × Region interaction, F(2.98,44.6) = 11.97, p<.001, indicating that the greater alpha desynchronization effects for the configural condition were especially concentrated in the parietal and occipital regions (Figure 2f). Averaged time courses of theta and alpha power at electrodes FZ and PZ are presented in Figure 3, so that the reader may more easily evaluate the differences in amplitude across conditions and scalp locations.
Figure 2.
A: Spectrogram of event-related changes in spectral power, for the configural condition at electrode FZ, across time points and frequencies. Color indicates the amount of event-related spectral perturbation (increase or decrease in power compared to a prestimulus baseline, as defined in the text). B: Spectrogram for the elemental condition. C: The difference between the two conditions. D: Illustration of the 32-electrode layout and the six regions defined for the purposes of computing an ANOVA for effects of condition and location on the ERSP values. E: Topographic map of ERSP difference for all electrodes in the theta band (3-6 Hz), configural minus elemental. F: Topographic map of ERSP differences in the alpha band (8.5-11.5 Hz).
Figure 3.
A: ERSP time courses at the frontal-midline electrode FZ, for the theta band. B: ERSP time courses for the theta band at the parietalmidline electrode PZ. C: Time course of the alpha band, electrode FZ. D: Time course of the alpha band, electrode PZ. E: Average multitaper power spectra in the two conditions at electrode FZ. F: Power spectra at electrode PZ.
Multitaper Spectral Analysis
In addition to time-frequency analysis, we also employed conventional stationary spectral analysis over the entire trial. This was done to maximize the frequency resolution of the estimated power spectra, so that the frequency range of the observed effects could be determined with greater precision. Power spectra at electrodes FZ and PZ are presented in Figure 3e,f. During performance of the configural condition, there is greater power in the theta band and reduced power in the alpha band. The plot of power spectra at the frontal electrode Fz (Figure 3e) demonstrates that the primary differences between the two conditions are indeed maximal in the classical theta band, in the range of 4-7 Hz, despite the fact that the event-related increases in power extend to even lower ranges. As in the ERSP analysis, repeated-measures ANOVA revealed significant effects of condition, theta band: F(1,15) = 12.1, p = .003, alpha band: F(1,15) = 18.07, p = .001, and region, theta band: F(2.2,21.4) = 23.69, p<.001, alpha band: F(2.5,37.2) = 20.27, p<.001, and an interaction effect for alpha only, theta band: F(2.5,37) = 1.06, n.s., alpha band: F(3.0,44.8) = 9.29, p<.001. Topographic maps resembled those in the ERSP analysis (data not shown).
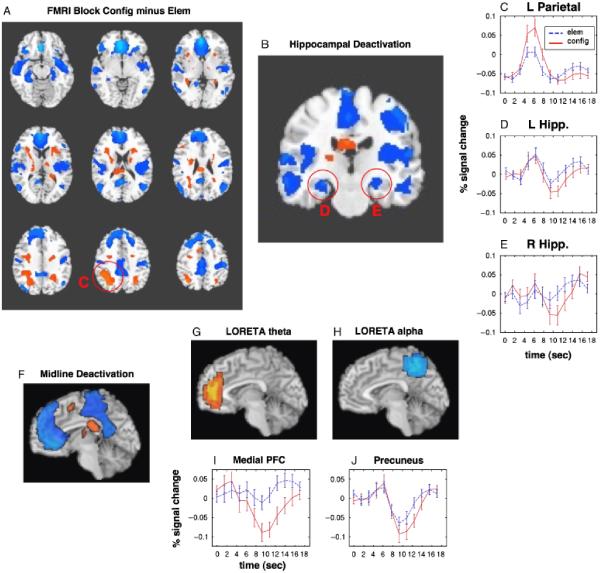
LORETA Compared with fMRI
For comparison with the present data, we present a brief summary of the findings of a previous fMRI study of this task (Meltzer, Negishi, & Constable, 2008), which employed a mixed block/event-related design to evaluate the contributions of evoked hemodynamic transient signals to block-design results. Figure 4a displays the average net signal change for the block design comparison of configural minus elemental task performance. Robust signal decreases are observed in bilateral medial and lateral temporal regions, as well as in “default mode” regions including medial prefrontal cortex, posterior cingulate/precuneus, and lateral parietal regions. Signal decreases in the configural condition mainly resulted from enhanced late negative transients time-locked to stimulus presentation, whereas positive activations were mainly due to increased event-related responses in the more typical early 4-6-s time range. Figure 4b presents a coronal view of the same subtraction, highlighting the robust bilateral deactivation of the hippocampus. Examples of the event-related hemodynamic responses are shown in Figure 4c-e. In Figure 4c, a parietal region positively activated in the configural-minus-elemental subtraction is seen to exhibit a typical positive hemodynamic response, and in Figure 4d,e, the hippocampal deactivations are seen to arise from an event-related negative transient that peaks several seconds later than the positive activations.
Figure 4.
A: Axial overview of fMRI block-design subtraction of configural minus elemental task performance. Thresholded at p<.001 voxel-wise, with cluster-size correction for family-wise error rate of p<.05. Red circles highlight activation clusters for which the averaged time courses are presented in separate panels, indicated by the adjacent letter. B: Coronal view of hippocampal fMRI deactivation for the block design subtraction. C: Averaged event-related BOLD time courses in a positively activated cluster in left parietal cortex. D: BOLD time courses in the left hippocampus. E: BOLD time courses in the right hippocampus. F: Mid-sagital view of the block-design subtraction, showing deactivation in midline default-mode regions. G: Average LORETA difference image of EEG theta power, configural minus elemental conditions, thresholded at 65% of the maximum. H: Average LORETA difference image of EEG alpha power, configural minus elemental conditions, thresholded at 65% of the maximum. I: Average event-related BOLD time courses in the medial prefrontal region identified by the LORETA analysis of panel g. J: Average event-related BOLD timecourses in the parietal midline (precuneus) region identified by the LORETA analysis of panel h.
Despite the fact that the configural condition induced greater theta power increases than the elemental condition over much of the scalp, as indicated by the lack of a significant Condition × Region interaction in the theta band, the difference between the conditions was maximal at frontal electrodes. Accordingly, the average LORETA difference image for theta power during configural versus elemental epochs was maximal in the ventral medial prefrontal cortex. As both theta and alpha LORETA estimates were maximal in midline regions, a midline view of the extensive fMRI deactivation is presented in Figure 4f for comparison. The LORETA result for localization of theta increases is shown in Figure 4g. This figure displays the difference image at an arbitrary threshold of 65% of the maximum difference between conditions. In the fMRI study, the region exhibiting the theta power increase was characterized by a strong net signal decrease in the configural condition. The event-related time courses of BOLD signal for this region in the two conditions (averaged across all the voxels in the thresholded LORETA image) are displayed in Figure 4i, demonstrating that the difference in signal levels between conditions is driven mainly by a large negative transient in the configural condition, although a small response is also seen in the elemental condition. This is in a relatively late time range, more commonly associated with the poststimulus undershoot than with the primary positive BOLD response. Additionally, there is a small initial positive transient in the configural condition.
The LORETA difference image for alpha power (Figure 4h, thresholded at 65% maximum) indicated a maximal decrease in posterior midline cortex, encompassing the occipital-parietal junction, precuneus, and posterior cingulate. Much of this region also exhibited a negative net BOLD signal change in the configural condition, as shown in Figure 4f. BOLD time courses for this region are shown in Figure 4g. In this region, there is a small initial positive transient response in the typical BOLD time range of 4-6 s, which is equal in magnitude in the two conditions. In this region, as in many others, the difference between conditions is driven by the larger late negative transient in the configural condition, resulting in a net negative signal change.
Although the LORETA difference images presented here were derived from a direct comparison of the configural and elemental conditions, taken from a poststimulus time range, it was also possible to estimate the anatomical localization of event-related theta increase and alpha decrease by comparing the prestimulus baseline period with the poststimulus period in each condition separately. This was done and yielded approximately the same results as the comparison between conditions (data not shown). Theta increases in both conditions localized maximally to medial prefrontal cortex, whereas alpha decreases localized maximally to the precuneus midline region.
Discussion
The primary goal of this study was to determine whether or not configural trials of the transverse patterning task would elicit increases in EEG theta power, thus providing a counterexample to the noted parallelism between human hippocampal BOLD activation and EEG theta. To our knowledge, this is the first study to investigate both phenomena with exactly the same cognitive paradigm, and the results offer a clear dissociation: EEG theta and hippocampal BOLD are both sensitive to the contrast between configural and elemental memory retrieval in the transverse patterning task, but with opposite directionality: Theta power is phasically increased in configural processing, whereas the hippocampal BOLD signal is phasically suppressed. In light of this, arguments based on the aforementioned parallelism would seem to be invalid, as that parallelism has been shown to be violated when put to the test.
Nonetheless, a considerable amount of evidence does support a relationship between hippocampal theta and neuronal activity in cortex. While it has long been known that hippocampal neurons exhibit specific patterns of spike timing related to the phase of ongoing theta rhythm, with behavioral significance (Bland & Oddie, 2001; Hasselmo, 2005), several rodent studies have recently demonstrated that neurons in other brain regions also exhibit such a relationship to hippocampal theta, even though theta periodicity is not necessarily evident in the local field potential of those regions (Hyman, Zilli, Paley, & Hasselmo, 2005; Jones & Wilson, 2005; Siapas, Lubenov, & Wilson, 2005). Notably, the medial prefrontal cortex has repeatedly been identified as a site of such interactions. These findings have provided convincing evidence to support theta as a mechanism for long-distance integration of hippocampal and cortical function. Additionally, numerous theoretical studies have proposed mechanisms by which information can be encoded and transmitted via neuronal interactions with large-scale oscillatory field potentials in the theta frequency range (Axmacher, Mormann, Fernandez, Elger, & Fell, 2006; Lisman, 2005; Yamaguchi, Aota, Sato, Wagatsuma, & Wu, 2004).
How then to interpret the present results? We have shown that configural processing in the transverse patterning task elicits phasic increases in theta power. That finding is in good agreement with previous studies that have shown theta EEG responses to be differentially engaged under conditions of greater mnemonic or general cognitive demand. Although hippocampal BOLD often responds in a similar manner as EEG theta, in this case it does not. This dissociation suggests that hippocampal BOLD activation and EEG theta increases may be independent phenomena, which may happen to respond in a similar fashion in some task comparisons but are not necessarily locked together. However, our results do not necessarily imply that the hippo-campus has no role in human cortical theta. Based on our findings, we draw a more limited conclusion: Human cortical theta does not depend on an increase in hippocampal metabolism and/or firing rate, quantities that are generally assumed to be correlated with the BOLD signal.
Several studies have suggested that theta rhythm in the hippocampus is not linked to any increase in overall firing rate or metabolic rate. Two studies using invasive recording in rodents in conjunction with indirect metabolic indicators have shown an inverse correlation between theta rhythm and metabolism in portions of the hippocampus (Sanchez-Arroyos, Gaztelu, Zaplana, Dajas, & Garcia-Austt, 1993; Uecker, Barnes, McNaughton, & Reiman, 1997). A recent study of human hippocampal recordings during a navigation task found significant task modulation of both neuronal firing rates and theta power, but essentially no correlation between these quantities (Ekstrom et al., 2007). Furthermore, a lack of correspondence between firing increases and theta is also evident in cortex. A recent study in awake behaving primates showed that spiking activity in visual cortex during the delay period of a working memory task displayed temporal patterns phase-locked with ongoing theta activity, despite no net increase (or even a slight decrease) in the number of spikes during that period (Lee, Simpson, Logothetis, & Rainer, 2005). Finally, in humans, simultaneous invasive recordings of local field potentials and multi-unit activity (spikes) from the dorsal anterior cingulate during various cognitive tasks have revealed a poststimulus increase in theta power along with a simultaneous evoked potential and a decrease in multi-unit firing (Wang et al., 2005).
Ever since the pioneering study of Logothetis, Pauls, Augath, Trinath, and Oeltermann (2001), which employed simultaneous measures of local field potential, firing rates, and BOLD, numerous authors have explored the idea that the BOLD signal is highly correlated with local field power. However, such correlations have proven to be quite frequency dependent. Correlations between BOLD and power in higher frequencies such as the gamma band have been overwhelmingly positive (Foucher, Otzenberger, & Gounot, 2003; Lachaux et al., 2007; Mukamel et al., 2005; Niessing et al., 2005). In lower frequencies such as the alpha band, numerous studies have reported negative correlations between EEG power and BOLD (Goldman, Stern, Engel, & Cohen, 2002; Laufs et al., 2003; Moosmann et al., 2003). This pattern of frequency-dependent correlations makes sense from a cognitive viewpoint, given that gamma oscillations typically increase under conditions of greater cognitive demand whereas alpha oscillations usually display the opposite pattern of reactivity (Bastiaansen & Hagoort, 2003; Brookes et al., 2005; Kilner, Mattout, Henson, & Friston, 2005). However, the theta band seems to be a notable exception, given that it is of lower frequency than the alpha band but is generally associated positively with cognitive demand. Several EEG-fMRI studies have reported negative correlations between EEG theta power and BOLD in medial prefrontal cortex (Martinez-Montes, Valdes-Sosa, Miwakeichi, Goldman, & Cohen, 2004; Mizuhara, Wang, Kobayashi, & Yamaguchi, 2004; Scheeringa et al., 2007; but see Sammer et al., 2007 for a contradictory finding).
Although the EEG-fMRI studies mentioned above have explored correlations between spontaneous fluctuations of theta power and BOLD, they suggest that event-related BOLD responses may also be negatively correlated with theta responses. We observed such a correlation across subjects in a recent study of individual variability in the Sternberg working memory task, in which subjects exhibiting frontal midline theta increases with greater memory load also tended to deactivate medial prefrontal cortex (Meltzer, Negishi, Mayes, & Constable, 2007). The results of the present study also support such a relationship, although the fMRI and EEG studies were conducted in separate subject cohorts. Source localization was a secondary goal of this study, as we did not take advantage of the most advanced (and costly) methods for EEG source estimation, which include the use of individualized head models, high density electrode arrays, and individually measured electrode coordinates. Accordingly, we employed a low-resolution estimation technique that is relatively insensitive to modest amounts of spatial error, the LORETA algorithm. Nonetheless, the source estimates obtained in this study for both theta and alpha reactivity closely match those of other invasive and noninvasive studies that have used more sophisticated estimation techniques, indicating that the results would not be likely to differ significantly had we used other methods. The theta power increases observed in configural versus elemental trials localized maximally to medial prefrontal cortex, which also exhibited a profound event-related decrease in BOLD signal in the fMRI experiment. Therefore, these results along with others suggest that theta synchronization and negative BOLD could represent a common underlying neural process, perhaps involving a phasic inhibition of ongoing neuronal activity. The role of negative BOLD responses in cognition is not well understood, but is a topic of much contemporary interest (Gusnard & Raichle, 2001; McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003).
The observed decreases in alpha rhythm in this study are similar to the theta increases, in that they are time-locked to stimulus presentation and are larger in the configural condition. However, they are quite spatially distinct, being maximal at a posterior midline location, suggesting that the alpha decrease and theta increase do not reflect a simple shift in peak frequency of a single oscillator, but rather represent distinct neuronal populations. The observed parietal-occipital maximum of task-induced alpha modulation matches that seen in several other studies (Ciulla, Takeda, & Endo, 1999; Tuladhar et al., 2007; Vanni, Revonsuo, & Hari, 1997; Yamagishi et al., 2003). Resting state EEG-fMRI studies have mainly observed a negative correlation between cortical BOLD and alpha power (Goldman et al., 2002; Laufs et al., 2003; Moosmann et al., 2003), although high interindividual variability (Laufs et al., 2006) and instances of positive correlation (Goncalves et al., 2006) have also been reported. A negative correlation between alpha and BOLD makes intuitive sense, as alpha power is typically suppressed by performance of cognitive tasks. Therefore, one might suppose that suppression of alpha power occurs in the same structures that exhibit a positive BOLD response in a cognitive paradigm. However, that hypothesis is not supported by the present data. In the posterior parietal midline region of maximum alpha decrease, we observed an overall BOLD deactivation in the configural condition, driven by an event-related negative transient, exactly as seen in the medial prefrontal region of maximal theta increase. There was no evidence of any spatial correspondence of theta or alpha dynamics with positive BOLD responses such as those seen in bilateral parietal regions.
In summary, the results of this study indicate that neither EEG theta increase nor alpha decrease in the transverse patterning task correspond to positive BOLD response in the regions where they are generated, despite the fact that both EEG phenomena are typically associated with the engagement of cognitive processes. These findings underscore the need to interpret negative results in fMRI cautiously, whether they be in the form of negative BOLD signals or the absence of a significant change altogether. Theta increases and alpha decreases are widely considered to represent neuronal activity related to information processing, and so a lack of correspondence to positive BOLD implies that not all information processing yields a detectable hemodynamic signal. Although dissociations between EEG/MEG and fMRI/PET are widely acknowledged, numerous attempts are underway to perform data fusion between these modalities under the assumption that they index the same underlying neuronal activity with varying degrees of spatial and temporal resolution. Findings of dissociation such as the present report suggest that, given our present state of knowledge, noninvasive electrophysiological and hemodynamic imaging methods yield nonoverlapping and complementary information about the neural basis of cognition and should be interpreted accordingly.
Acknowledgments
This research was supported by NIH R01-NS38467 and by a predoctoral fellowship from the American Epilepsy Society (J.A.M.). We thank Ulrich Schridde for helpful comments on the manuscript and Robert Astur for development of the transverse patterning task.
REFERENCES
- Alvarado MC, Rudy JW. Rats with damage to the hippocampal-formation are impaired on the transverse-patterning problem but not on elemental discriminations. Behavioral Neuroscience. 1995;109:204–211. doi: 10.1037//0735-7044.109.2.204. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Wright AA, Bachevalier J. Object and spatial relational memory in adult rhesus monkeys is impaired by neonatal lesions of the hippocampal formation but not the amygdaloid complex. Hippocampus. 2002;12:421–433. doi: 10.1002/hipo.1115. [DOI] [PubMed] [Google Scholar]
- Asada H, Fukuda Y, Tsunoda S, Yamaguchi M, Tonoike M. Frontal midline theta rhythms reflect alternative activation of prefrontal cortex and anterior cingulate cortex in humans. Neuro-science Letters. 1999;274:29–32. doi: 10.1016/s0304-3940(99)00679-5. [DOI] [PubMed] [Google Scholar]
- Astur RS, Constable RT. Hippocampal dampening during a relational memory task. Behavioral Neuroscience. 2004;118:667–675. doi: 10.1037/0735-7044.118.4.667. [DOI] [PubMed] [Google Scholar]
- Astur RS, Sutherland RJ. Relational learning in humans: The transverse patterning problem. Psychobiology. 1998;26:176–182. [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Research. Brain Research Reviews. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Event-induced theta responses as a window on the dynamics of memory. Cortex. 2003;39:967–992. doi: 10.1016/s0010-9452(08)70873-6. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, van Berkum JJ, Hagoort P. Event-related theta power increases in the human EEG during online sentence processing. Neuroscience Letters. 2002;323:13–16. doi: 10.1016/s0304-3940(01)02535-6. [DOI] [PubMed] [Google Scholar]
- Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: The case for its role in sensorimotor integration. Behavioural Brain Research. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- Borisyuk RM, Hoppensteadt FC. Memorizing and recalling spatial-temporal patterns in an oscillator model of the hippocampus. Biosystems. 1998;48:3–10. doi: 10.1016/s0303-2647(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Brookes MJ, Gibson AM, Hall SD, Furlong PL, Barnes GR, Hillebrand A, et al. GLM-beamformer method demonstrates stationary field, alpha ERD and gamma ERS co-localisation with fMRI BOLD response in visual cortex. NeuroImage. 2005;26:302–308. doi: 10.1016/j.neuroimage.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Burgess AP, Gruzelier JH. Short duration power changes in the EEG during recognition memory for words and faces. Psychophysiology. 2000;37:596–606. [PubMed] [Google Scholar]
- Bussey TJ, Clea Warburton E, Aggleton JP, Muir JL. Fornix lesions can facilitate acquisition of the transverse patterning task: A challenge for “configural” theories of hippocampal function. Journal of Neuroscience. 1998;18:1622–1631. doi: 10.1523/JNEUROSCI.18-04-01622.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Glaholt MG. The roles of EEG oscillations in learning relational information. NeuroImage. 2007;15:15. doi: 10.1016/j.neuroimage.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Madsen JR, Raghavachari S, Kahana MJ. Distinct patterns of brain oscillations underlie two basic parameters of human maze learning. Journal of Neurophysiology. 2001;86:368–380. doi: 10.1152/jn.2001.86.1.368. [DOI] [PubMed] [Google Scholar]
- Ciulla C, Takeda T, Endo H. MEG characterization of spontaneous alpha rhythm in the human brain. Brain Topography. 1999;11:211–222. doi: 10.1023/a:1022233828999. [DOI] [PubMed] [Google Scholar]
- de Araujo DB, Baffa O, Wakai RT. Theta oscillations and human navigation: A magnetoencephalography study. Journal of Cognitive Neuroscience. 2002;14:70–78. doi: 10.1162/089892902317205339. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and trans-verse patterning guided by olfactory cues. Behavioral Neuroscience. 1998;112:762–771. doi: 10.1037//0735-7044.112.4.762. [DOI] [PubMed] [Google Scholar]
- Ekstrom A, Viskontas I, Kahana M, Jacobs J, Upchurch K, Bookheimer S, et al. Contrasting roles of neural firing rate and local field potentials in human memory. Hippocampus. 2007;17:606–617. doi: 10.1002/hipo.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Fell J, Klaver P, Elfadil H, Schaller C, Elger CE, Fernandez G. Rhinal-hippocampal theta coherence during declarative memory formation: Interaction with gamma synchronization? European Journal of Neuroscience. 2003;17:1082–1088. doi: 10.1046/j.1460-9568.2003.02522.x. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Otzenberger H, Gounot D. The BOLD response and the gamma oscillations respond differently than evoked potentials: An interleaved EEG-fMRI study. BMC Neuroscience. 2003;4:22. doi: 10.1186/1471-2202-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei E, Gamma A, Pascual-Marqui R, Lehmann D, Hell D, Vollenweider FX. Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA) Human Brain Mapping. 2001;14:152–165. doi: 10.1002/hbm.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Jr., Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. NeuroReport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves SI, de Munck JC, Pouwels PJ, Schoonhoven R, Kuijer JP, Maurits NM, et al. Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: Inter-subject variability. NeuroImage. 2006;30:203–213. doi: 10.1016/j.neuroimage.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Guderian S, Duzel E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus. 2005;15:901–912. doi: 10.1002/hipo.20125. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews. Neuroscience. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm? Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15:936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Hay J, Ilyn M, Gorchetchnikov A. Neuromodulation, theta rhythm and rat spatial navigation. Neural Networks. 2002;15:689–707. doi: 10.1016/s0893-6080(02)00057-6. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, et al. Medial prefrontal cortex generates frontal midline theta rhythm. NeuroReport. 1999;10:675–679. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends in Neurosciences. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biology. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Current Opinion in Neurobiology. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Mattout J, Henson R, Friston KJ. Hemo-dynamic correlates of EEG: A heuristic. Neuroimage. 2005;28:280–286. doi: 10.1016/j.neuroimage.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T. Theta band power in the human scalp EEG and the encoding of new information. NeuroReport. 1996;7:1235–1240. doi: 10.1097/00001756-199605170-00002. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T. `Paradoxical' alpha synchronization in a memory task. Brain Research. Cognitive Brain Research. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, et al. Relationship between task-related gamma oscillations and BOLD signal: New insights from combined fMRI and intracranial EEG. Human Brain Mapping. 2007;1:1. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, et al. Where the BOLD signal goes when alpha EEG leaves. NeuroImage. 2006;31:1408–1418. doi: 10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, et al. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45:147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Lisman J. The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15:913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: Neurophysiological mechanisms of action regulation. Clinical Neurophysiology. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalography and Clinical Neurophysiology. 1993;86:283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- Martinez-Montes E, Valdes-Sosa PA, Miwakeichi F, Goldman RI, Cohen MS. Concurrent EEG/fMRI analysis by multiway Partial Least Squares. NeuroImage. 2004;22:1023–1034. doi: 10.1016/j.neuroimage.2004.03.038. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Constable RT. Activation of human hippocampal formation reflects success in both encoding and cued recall of paired associates. NeuroImage. 2005;24:384–397. doi: 10.1016/j.neuroimage.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Constable RT. Biphasic hemo-dynamic responses influence deactivation and may mask activation in block-design fMRI paradigms. Human Brain Mapping. 2008;29:385–399. doi: 10.1002/hbm.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Mayes LC, Constable RT. Individual differences in EEG theta and alpha dynamics during working memory correlate with fMRI responses across subjects. Clinical Neurophysiology. 2007;118:2419–2436. doi: 10.1016/j.clinph.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, et al. Effects of working memory load on oscillatory power in human intracranial EEG. Cerebral Cortex. 2008;18:1843–1855. doi: 10.1093/cercor/bhm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clinical Neuro-physiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Miller R. Cortico-hippocampal interplay and the representation of contexts in the brain. Springer-Verlag; Heidelberg: 1991. [Google Scholar]
- Mizuhara H, Wang LQ, Kobayashi K. A long-range cortical network emerging with theta oscillation in a mental task. NeuroReport. 2004;15:1233–1238. doi: 10.1097/01.wnr.0000126755.09715.b3. [DOI] [PubMed] [Google Scholar]
- Moore RA, Gale A, Morris PH, Forrester D. Theta phase locking across the neocortex reflects cortico-hippocampal recursive communication during goal conflict resolution. International Journal of Psychophysiology. 2006;60:260–273. doi: 10.1016/j.ijpsycho.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, et al. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. NeuroImage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: The neurophysics of EEG. Oxford University Press; Oxford: 2006. [Google Scholar]
- O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. NeuroImage. 2005;27:341–356. doi: 10.1016/j.neuroimage.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: Relationship between findings from across- and within-task comparisons. Brain. 2001;124:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. International Journal of Psychophysiology. 1994;18:49–65. doi: 10.1016/0167-8760(84)90014-x. [DOI] [PubMed] [Google Scholar]
- Percival DB, Walden AT. Spectral analysis for physical applications. Cambridge University Press; Cambridge: 1993. [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, et al. Gating of human theta oscillations by a working memory task. Journal of Neuroscience. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working-memory task: Evidence for local generators. Journal of Neurophysiology. 2006;95:1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Siwiec RM, Gitelman DR, Parrish TB, Mesulam MM, Paller KA, et al. Neural correlates of successful encoding identified using functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:9541–9548. doi: 10.1523/JNEUROSCI.22-21-09541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired transverse patterning in human amnesia is a special case of impaired memory for two-choice discrimination tasks. Behavioral Neuroscience. 1999;113:3–9. doi: 10.1037//0735-7044.113.1.3. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Grafman J. Losing their configural mind. Amnesic patients fail on transverse patterning. Journal of Cognitive Neuroscience. 1998;10:509–524. doi: 10.1162/089892998562915. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Verfaellie M, Grafman J. Transverse patterning and human amnesia. Journal of Cognitive Neuroscience. 2006;18:1723–1733. doi: 10.1162/jocn.2006.18.10.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammer G, Blecker C, Gebhardt H, Bischoff M, Stark R, Morgen K, et al. Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Human Brain Mapping. 2007;28:793–803. doi: 10.1002/hbm.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Arroyos R, Gaztelu JM, Zaplana J, Dajas F, Garcia-Austt E. Hippocampal and entorhinal glucose metabolism in relation to cholinergic theta rhythm. Brain Research Bulletin. 1993;32:171–178. doi: 10.1016/0361-9230(93)90071-i. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. International Journal of Psychophysiology. 2007;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. Journal of Neuroscience. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Spence KW. The nature of response in discrimination learning. Psychology Review. 1952;59:89–93. doi: 10.1037/h0063067. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. Journal of Neuroscience. 2000;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. Journal of Neurophysiology. 2006;95:2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- Tuladhar AM, Huurne NT, Schoffelen JM, Maris E, Oostenveld R, Jensen O. Parieto-occipital sources account for the increase in alpha activity with working memory load. Human Brain Mapping. 2007;31:31. doi: 10.1002/hbm.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Maehara T, Hirai N, Kawai K, Shimizu H. Theta oscillation in the anterior cingulate and beta-1 oscillation in the medial temporal cortices: A human case report. Journal of Clinical Neuroscience. 2003;10:371–374. doi: 10.1016/s0967-5868(03)00025-0. [DOI] [PubMed] [Google Scholar]
- Uecker A, Barnes CA, McNaughton BL, Reiman EM. Hippocampal glycogen metabolism, EEG, and behavior. Behavioral Neuroscience. 1997;111:283–291. doi: 10.1037//0735-7044.111.2.283. [DOI] [PubMed] [Google Scholar]
- Vanni S, Revonsuo A, Hari R. Modulation of the parieto-occipital alpha rhythm during object detection. Journal of Neuroscience. 1997;17:7141–7147. doi: 10.1523/JNEUROSCI.17-18-07141.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. Journal of Neuroscience. 2005;25:604–613. doi: 10.1523/JNEUROSCI.4151-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, Goda N, Anderson SJ, Yoshida Y, Kawato M. Attentional modulation of oscillatory activity in human visual cortex. NeuroImage. 2003;20:98–113. doi: 10.1016/s1053-8119(03)00341-0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Aota Y, Sato N, Wagatsuma H, Wu Z. Synchronization of neural oscillations as a possible mechanism underlying episodic memory: A study of theta rhythm in the hippocampus. Journal of Integrative Neuroscience. 2004;3:143–157. doi: 10.1142/s0219635204000488. [DOI] [PubMed] [Google Scholar]