Abstract
Since only 30% of alcoholics develop alcoholic liver disease (ALD), a factor other than heavy alcohol consumption must be involved in development of alcohol-induced liver injury. Animal and human studies suggest that bacterial products such as endotoxin are the second key co-factor and oxidant-mediated gut leakiness is one of the sources of endotoxemia. Probiotics have been used to prevent and treat diseases associated with gut-derived bacterial products and disorders associated with gut leakiness. Indeed, “probiotic” Lactobacillus has been successfully used to treat alcohol-induced liver injury in rats. But, the mechanism of action of the potential beneficial effects of Lactobacillus in alcohol liver injury is not known. We hypothesized that probiotics could preserve normal barrier function in an animal model of ALD by preventing alcohol-induced oxidative stress and thus prevent development of hyperpermeability and subsequent alcoholic steatohepatitis. Male Sprague-Dawley rats were gavaged with alcohol twice daily (8gm/kg) for 10 weeks. In addition, alcoholic rats were also treated with once daily gavage of either 2.5 107 live Lactobacillus GG (LGG) or vehicle. Intestinal permeability (baseline and 10wk) was determined using a sugar bolus and GC analysis of urinary sugars. Intestinal and liver tissues were analyzed for markers of oxidative stress and inflammation. In addition livers were assessed histologically for severity of alcoholic steatohepatitis (ASH) and total fat (steatosis). Alcohol-LGG fed rats had significantly (p≤ .05) less severe ASH than alcohol-vehicle fed rats. LGG also improved alcohol-induced gut leakiness and significantly blunted alcohol-induced oxidative stress and inflammation in both intestine and the liver. LGG probiotic gavage significantly ameliorated alcoholic steatohepatitis in rats. This improvement was associated with reduced markers of intestinal and liver oxidative stress and inflammation and preserved gut barrier function. Our study provides a scientific rationale to test probiotics for treatment and/or prevention of alcoholic liver disease in man.
Keywords: alcohol, alcoholic liver disease, Lactobacillus GG, oxidative stress, intestinal permeability
Introduction
Alcoholic liver disease (ALD) remains as an important cause of morbidity and mortality with more than 75,000 deaths annually worldwide and incidence increasing in the last decade (Gramenzi et al., 2006; Tsukamoto, 2007). However, only about 30% of heavy drinkers develop alcoholic steatohepatitis (ASH) and clinically significant ALD (Gramenzi et al., 2006). Thus, heavy alcohol drinking is required but not sufficient for development of ASH and other factor(s) are required. Several animal and human studies have suggested that the gut-derived bacterial product, endotoxin is the required co-factor. For example, ALD patients and animal models of chronic alcoholism have abnormally high plasma endotoxin levels (Bode et al., 1987; Fukui et al., 1991; Lumsden et al., 1988; Thurman, 1998; Tilg and Diehl, 2000).
The cause of alcohol-induced endotoxemia is not well established but several studies suggest that alcohol-induced increased intestinal permeability to gut derived endotoxin is the possible mechanism (Fukui et al., 1991; Keshavarzian et al., 1999; Parlesak et al., 2000; Rao et al., 2004; Tilg and Diehl, 2000; Worthington et al., 1978). If endotoxemia and intestinal hyperpermeability are the key factors in development of alcoholic steatohepatitis, then intervention aiming at ameliorating endotoxemia and gut leakiness should prevent alcoholic steatohepatitis. Indeed, studies in animal models of ALD have revealed that treatment with antibiotics to sterilize the gut and thus eliminate this source of endotoxin can prevent alcohol-induced liver injury (Adachi et al., 1995). Moreover, Lactobacillus GG supplementation significantly decreased severity of liver injury in a rat model of alcoholic steatohepatitis (Nanji et al., 1994). We also showed that oats supplementation prevented both increased intestinal permeability and steatohepatitis in chronically alcohol fed rats (Keshavarzian et al., 2001). These studies strongly support the notion that gut bacteria play a key role in the pathogenesis of alcohol induced liver injury and that gut leakiness may be one mechanism that allows proinflammatory bacterial products to reach the liver and initiate the proinflammatory cascade required to cause ASH.
One mechanism whereby alcohol induces intestinal permeability in vivo may be oxidative stress. Antioxidants have been shown to normalize intestinal permeability in alcoholic patients (Varella Morandi Junqueira-Franco et al., 2006). We have also shown that alcohol increases intestinal epithelial cell permeability in vitro through an oxidant stress mediated mechanism (Banan et al., 1999; Banan et al., 1998). In those studies we demonstrated that alcohol stimulates activation of the key transcription factor NF-kB which in turn stimulates upregulation of inducible nitric oxide synthase (iNOS) (Banan et al., 2000; Banan et al., 2007). Our data support iNOS production of reactive nitrogen species (e.g. peroxynitrite) as the principal source of ethanol induced oxidative stress. The resulting oxidative stress causes increased carbonylation and nitrotyrosination of cellular proteins including the actin and microtubule cytoskeletons which when disrupted results in loss of tight junction integrity and increased paracellular permeability (Farhadi et al., 2006). This model has recently been supported independently by studies examining alcohol-induced epithelial permeability in the lung (Brown et al., 2004; Polikandriotis et al., 2007). Thus we propose that oxidative stress is the key element driving alcohol-induced intestinal hyperpermeability and anti-oxidants may be effective therapeutic agents to prevent ASH.
Probiotics are microorganisms that when taken by the host have beneficial effects on the host beyond their simple nutritive value (Ewaschuk and Dieleman, 2006). They can change the gut microbiota profile and thereby change the gut lumen favoring an anti-inflammatory milieu resulting in decreased production of pro-inflammatory bacterial products and also improved barrier integrity. One widely studied probiotic bacterium is Lactobacillus rhamnosus GG (LGG) (Di Caro et al., 2005; Mattar et al., 2001; Nanji et al., 1994; Zhang et al., 2006). Probiotics including LGG have been shown to have several beneficial effects on intestinal function including stimulating intestinal development and mucosal immunity, ameliorating diarrhea, prolonging remission in ulcerative colitis and pouchitis, and maintaining and improving intestinal barrier function (Bruzzese et al., 2004; Ewaschuk and Dieleman, 2006; O'Hara and Shanahan, 2006; Resta-Lenert and Barrett, 2003; Sartor, 2004; Versalovic, 2007). LGG has also been shown to reduce intestinal oxidative stress (Tao et al., 2006).
To determine whether we can exploit these favorable characteristics of LGG in order to prevent alcohol-induced leaky gut and liver injury, we studied the effects of daily LGG on intestinal permeability, intestinal and liver oxidative stress, and severity of steatohepatitis in our rat model of alcohol-induced leaky gut and steatohepatitis. We hypothesized that LGG would reduce alcohol-induced oxidative stress and preserve barrier function and thus prevent liver disease in alcohol fed rats. Using our model of chronic alcohol gavage, we show in this study that treatment with LGG significantly reduces markers of intestinal oxidative stress, normalizes barrier function, and ameliorates alcoholic steatohepatitis.
Materials and Methods
Culture of Lactobacillus rhamnosus GG
Lactobacillus rhamnosus Gorbach-Goldin (LGG) (ATCC 531030) were cultured in Lactobacillus MRS broth (Difco, BD, Sparks, MD) at 37°C in accordance with ATCC guidelines. Bacteria were harvested from MRS broth by centrifugation and CFU counted by dilution and streaking on MRS agar plates (Difco) at 37°C overnight. LGG were then centrifuged and resuspended at a dilution of 2.5×107/ml in PBS and 1ml gavage was used for once-a-day daily treatment.
Animal Subjects
Male Sprague-Dawley rats (Zivic-Miller Laboratories, Zelienople, PA; n = 17; 250-300 g, initial body weight) were acclimated for 6 to 7 days, at 22 ± 1°C with a 12:12-h dark-light cycle. During acclimatization, rats were given water and standard laboratory food (rat chow) ad libitum. During the experiment, alcohol or dextrose were administered intragastrically by gavage twice daily (6h between doses). A 12-gauge gavage needle was used (Popper & Sons, New Hyde Park, NY). Alcohol-fed rats received alcohol gavage (∼2-3 ml) twice daily starting with an initial dose of 2 g/kg/day. This dose was progressively increased during weeks 1 and 2 to a maintenance dose of 8 g/kg/day (solutions maximally contained 50-60% alcohol with each gavage containing 4g/kg that is 1/2 the daily dose) that was continued for 8 more weeks. Control rats received an isocaloric amount of dextrose, also by gavage. Rats also received intragastric feedings of a slurry of either powdered rat chow (vehicle) or Lactobacillus rhamnosus Gorbach-Goldin (LGG) (ATCC, #53103)(2.5×107 live/once daily). All rats also had regular rat chow available (ad libitum) throughout the 10-week period. Rats were weighed daily. Four treatment groups were studied: 1) alcohol + vehicle alone (ALC-V) (n = 11); 2) alcohol + Lactobacillus GG (ALC-LGG) (n = 9); 3) dextrose control (CON) (n = 5); and 4) dextrose + Lactobacillus GG (CON-LGG) (n = 3). Gut permeability was measured (as described below) at baseline and at the end of 10 weeks. Blood samples were taken for blood alcohol levels (BAL)1 h after gavage because we previously showed peak BAL one hour after gavage (Keshavarzian et al., 2001). Another recent study supports our own data showing peak BAL I hour after gavage (Walker and Ehlers, 2008). Samples were taken 3 weeks after initiation of alcohol (i.e., after the final ethanol dose of 8 g/kg/day had been administered for at least 5 days). BAL values were in agreement with values we have previously published for this model of 398 ± 70 mg/100 ml (Keshavarzian et al., 2001).
At 10 weeks, the animals were humanely killed by CO2 inhalation, followed immediately by cardiac puncture (for blood collection), and laparotomy for the collection of intestinal and liver tissues. All animal protocols and practices were reviewed and approved in advance by the Rush University Institutional Animal Care and Use Committee (IACUC) in accordance with guidelines set forth by the Office of Laboratory Animal Welfare (OLAW), NIH, and the publications: U.S. Government Principles for the Utilization and Care of Vertebrate Animals used in Testing, Research, and Training, and the Guide for the Care and Use of Laboratory Animals (Guide). Every effort was made to minimize animal pain or discomfort.
Intestinal Permeability
Intestinal permeability in rats was assessed after an 8 h fast as previously described by us (Keshavarzian et al., 1994). We gavaged 2.0 ml of a solution containing: sucralose 15 mg/kg, lactulose 107 mg/kg, mannitol 30 mg/kg, and sucrose 570 mg/kg. Two tests of Intestinal permeability were performed in each rat- one at baseline and one 10 weeks after daily alcohol feeding. Rats were housed individually in metabolic cages, and urine was collected for the first 5 h. To promote urine output, each rat received 10 ml of a Ringer-lactate solution subcutaneously, just prior to sugar administration. Only rats with adequate urinary output (2.4 ml or more) were included in the analysis. Then we measured the concentration of the four sugars (as well as the lactulose/mannitol ratio) in 5 hour urine using gas chromatography as described (Farhadi et al., 2003). We used percent excretion of the urinary sucralose (% of oral dose recovered, typically 1-4%) as our primary index of increased whole gut permeability (Meddings and Swain, 2000).
Quantitative Slot-Immunoblotting for oxidation (carbonylation) and nitration (nitrotyrosination)
Oxidation and nitration of mucosal proteins were assessed by measuring protein carbonyl and protein nitrotyrosine formation using a slot-blotting method and densitometry (Image J, NIH) we previously described (Banan et al., 2004; Keshavarzian et al., 2003).
Urinary and colonic total NO measurements
We measured the urinary or tissue nitric oxide (NO) as the sum of urinary nitrite (NO2) and nitrate (NO3) levels using the Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI). Assays were performed using the method and standard curve provided by the manufacturer.
Colonic and liver myeloperoxidase (MPO)
Colonic and liver MPO were measured using the Myeloperoxidase Assay Kit (Cytostore, Calgary, Alberta, Canada). Tissue samples were flash frozen and stored at -80°C until thawing on ice. Thawed samples were weighed and homogenized on ice. Samples were processed according to manufacturers instructions and final values expressed as MPO units/mg tissue.
Liver Histology and Liver Fat
Liver Histology. Formalin fixed liver tissues were processed for staining with H&E and then studied by light microscopy. The assessment was done by a GI pathologist (SJ) who was blinded to group assignments. Histological slides were assessed for scoring of liver disease by grading steatosis, necrosis, inflammation & fibrosis (Keshavarzian et al., 2001). Severity of steatosis (% of liver cells containing fat) was scored 1+ to 4+, where the # of liver cells with fat were, respectively, <25%, 26-50%, 51-75%, or > 75%. Necrosis was quantified as the # of necrotic foci/mm2 and severity graded on a scale of 0-4. Severity of inflammation was scored as the # of inflammatory cells/mm2 and severity graded on a scale of 0-4. Severity of fibrosis was scored on a scale of 0 to 4 using trichrom staining. Two scoring systems were calculated: A total histological score (max=16) that included all 4 components of histological assessment; A necroinflammatory score (max=8) that included only inflammation and necrosis scales. When assessing the pathological slides, at least 3 different sections were studied in each rat. Steatohepatitis was defined as the presence of spotty necrosis and liver cell necrosis with inflammatory cells.
Liver Fat Content. Total liver fat was measured gravimetrically as described (Folch et al., 1957) and used by us (Farhadi et al., 2003).
Results
Lactobacillus GG gavage ameliorates alcohol-induced liver disease
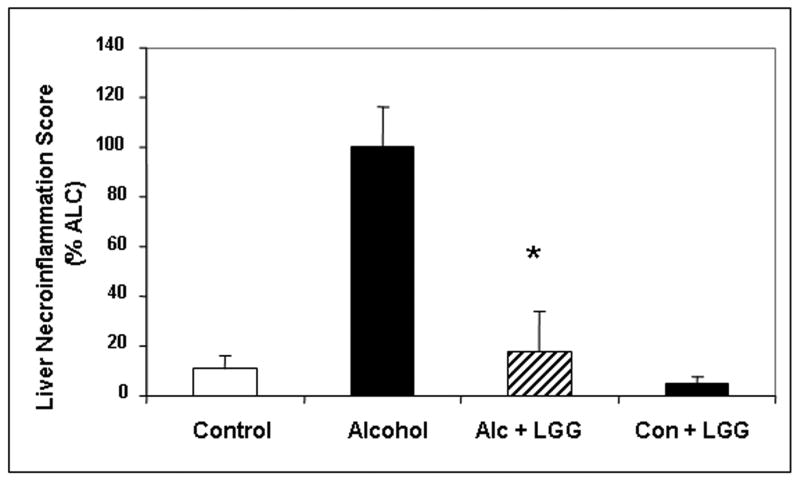
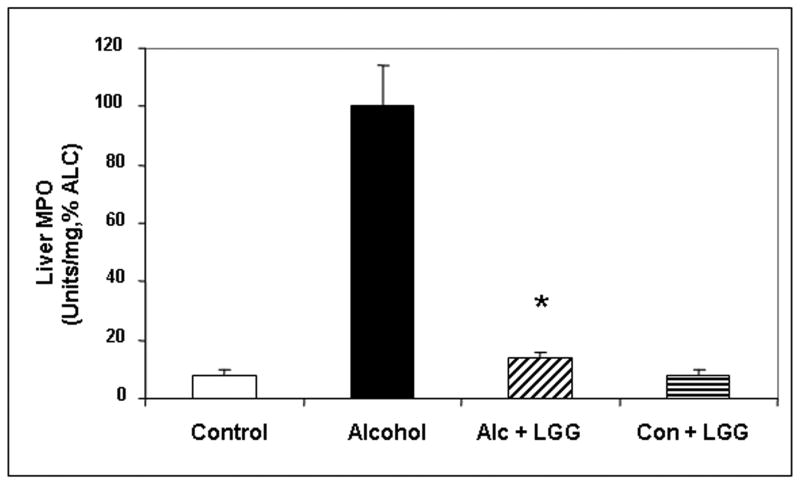
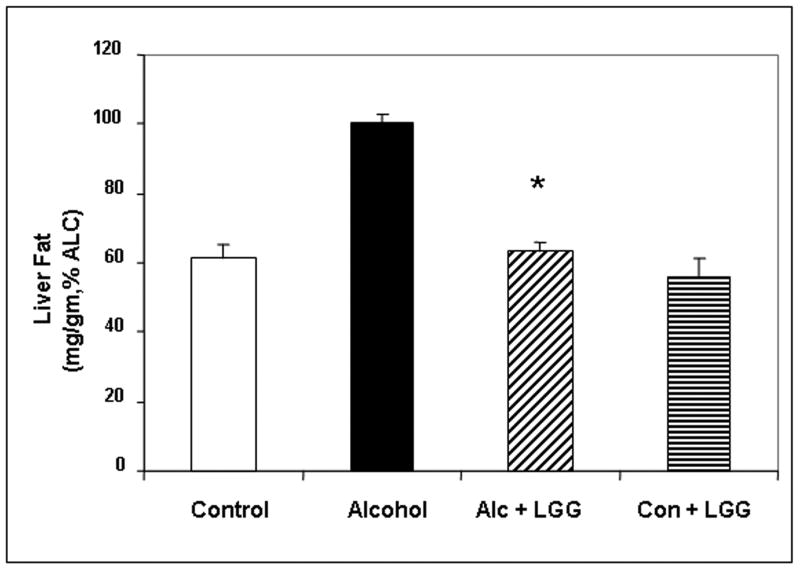
Daily LGG treatment significantly improved severity of alcohol-induced steatohepatitis. Total histology score was more than 3-fold the value for livers of ALC-V fed rats (7.6± .95) compared to ALC-LGG fed rat livers (2.16± 1.45). Indeed, the ALC-LGG fed rat liver histology mean score was not significantly different from the dextrose-V fed (CON) rats (1.4± .92). The improvement in total histology score by LGG was primarily due to improvement (6-fold) in necroinflammatory components of the histopathological scoring system (Fig.1). Indeed, when severity of liver inflammation was assessed by biochemical measurement of hepatic myeloperoxidase (MPO), an index of the magnitude of neutrophil infiltration into the liver, LGG significantly improved the severity of hepatic inflammation (Fig. 2a). The LGG effectively prevented a 7-fold increase in liver MPO levels in alcohol fed rats. It should be noted that LGG also significantly decreased the degree of fatty liver and ALC-LGG fed rats had near normal amounts of fat in their livers (Fig. 2b).
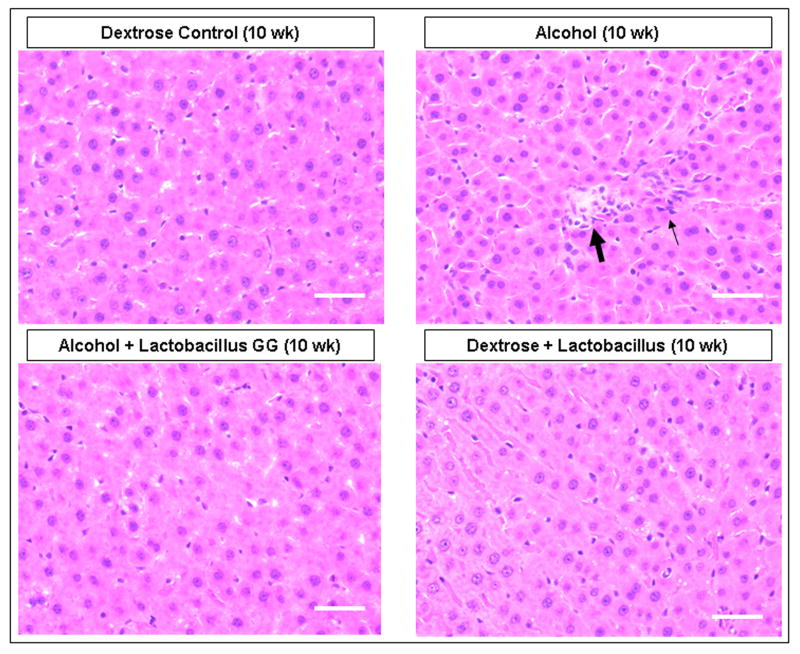
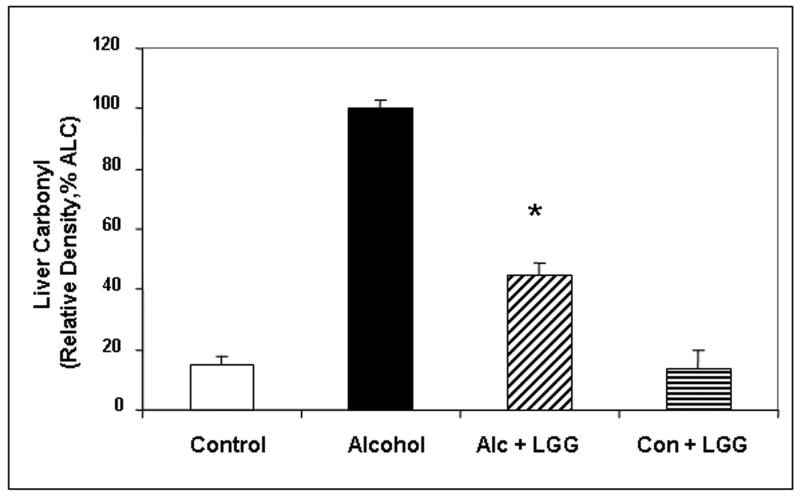
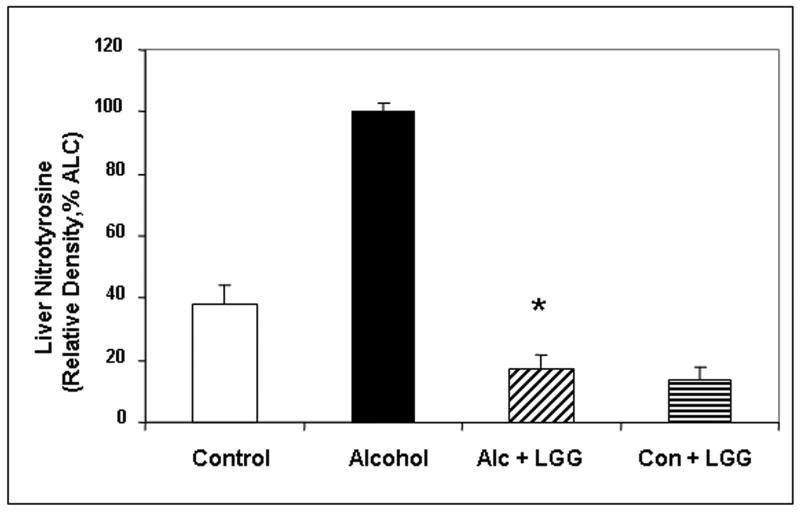
Figure 1. The effect of Lactobacillus GG on the severity of alcoholic steatohepatitis assessed by the total liver histology and liver necroinflammation scores.
After 10 weeks liver tissues from rats in the four gavage treatment groups were processed, stained (H&E) and assessed histologicalIy and total histology scores and necroinflammation scores were calculated as described in Methods. Fig.1a shows representative liver histology slides from each of the 4 treatment groups (400×, bar = 20μm). Large arrow denotes area of focal necrosis and inflammatory cell infiltration, small arrow is additional inflammatory cell infiltration. Fig. 1b histogram summarizes the necroinflammatory scores for the four groups. Treatment groups were: Control: isocaloric dextrose; Alcohol: alcohol + vehicle: 8g/kg/day ethanol; Alcohol + LGG (Alc+LGG): ethanol 8g/kg/day + Lactobacillus GG 2.5 × 107/day; Control + LGG (Con+LGG): isocaloric dextrose + Lactobacillus GG 2.5 × 107/day. Data are histology and necroinflammation score means ± standard error of the mean (S.E.) expressed as a percent of the alcohol alone treated group (% ALC). * denotes significance (ANOVA) of p ≤ .05 for alcohol group versus alcohol + LGG group in all figures.
Figure 2. The effect of Lactobacillus GG on the severity of alcoholic steatohepatitis assessed by liver fat content and liver MPO levels.
After 10 weeks liver tissue from the treatment groups described in Fig. 1 was assessed for myeloperoxidase (MPO) (Fig. 2a.) and total fat (mg fat/gram liver) (Fig. 2b) as described in Methods. Data are for means (as % alcohol group) ± S.E.
Lactobacillus GG also ameliorated alcohol-induced oxidative stress in the liver. Alcohol fed rats who also received LGG had significantly less liver tissue protein carbonyl (marker of protein oxidation) and nitrotyrosine (marker of protein nitration) levels (Fig. 3). Liver carbonyl values were reduced by 55% in ALC-LGG treated rats (Fig. 3a). In addition, nitrotyrosine levels in ALC-V fed rat livers were 5.7-fold of ALC-LGG fed rats or dextrose-V fed CON rats (Fig. 3b). Taken together, the histological and necroinflammatory scores, MPO, and oxidative stress measurements confirm that chronic alcohol gavage resulted in significant alcoholic steatohepatitis (ASH) and that LGG gavage significantly reduced the severity of ASH and oxidative stress in livers of alcohol fed rats.
Figure 3. The effect of Lactobacillus GG on the severity of hepatic oxidative stress assessed by liver carbonyl and nitrotyrosine levels.
Liver tissue from the four treatment groups was assessed after 10 weeks for markers of oxidative stress namely liver carbonyl (Fig. 3a) and liver nitrotyrosine (Fig. 3b) by slot blot and densitometry as described in Methods. Data are means of relative density (as % alcohol group) ± S.E.
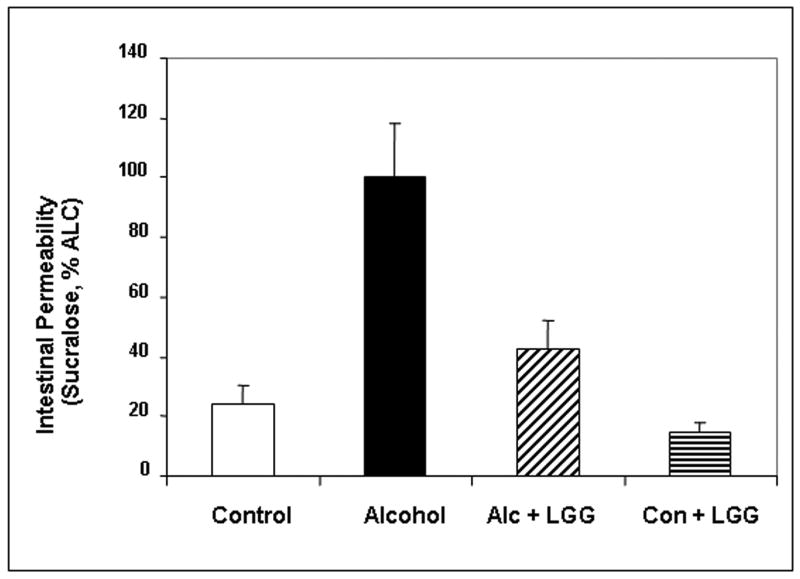
Probiotics ameliorate alcohol-induced intestinal hyperpermeability (leaky gut)
Chronic daily alcohol gavage did not significantly affect urinary sucrose levels (data not shown) demonstrating that our chronic alcohol gavage model does not significantly disrupt gastro-duodenal mucosal integrity. Chronic daily alcohol gavage did not significantly affect lactulose/mannitol ratio (not shown). However, as shown in Fig. 4, after 10 weeks of daily alcohol feeding, the ALC-V fed rats had significantly higher levels of urinary sucralose, an index of whole gut intestinal permeability, than dextrose-V fed control rats. Urinary sucralose levels in alcohol fed rats were about 3-fold higher than dextrose fed rats (4.1 ± .2 % recovered dose in 5 hour urine vs. 1.0 ± 0.2%) indicating alcohol-induced whole gut leakiness. Furthermore, the ALC-LGG fed rats exhibited a robust and significant reduction in urinary sucralose values to less than half that of ALC fed rats (1.8 ± 0.2% recovered dose). Finally, urinary sucralose values in dextrose-LGG fed rats were similar to the values in the dextrose-V fed CON rats showing LGG alone has no effect on “normal” gut permeability.
Figure 4. Effect of Lactobacillus GG on alcohol-induced intestinal permeability.
Whole intestinal permeability to Sucralose was assessed at 10 weeks in the four treatment groups by analysis of 5h urine samples with gas chromatography (Methods). Data are means of percent oral Sucralose dose recovered in urine (as % alcohol group) ± S.E.
Lactobacillus GG gavage prevents alcohol-induced oxidative stress in the small intestine
We next sought to determine possible mechanisms for the observed protection by LGG of alcohol-induced intestinal hyperpermeability by evaluating the effects of LGG on markers of oxidative stress and found that alcohol-induced increased intestinal protein carbonylation and nitrotyrosination epitope expression in alcohol fed rats were significantly (p≤ 0.05) reduced in proximal (duodenum/ jejunum) and distal (ileum) portions of the small bowel by daily administration of intragastric LGG (Table 1). For proximal small bowel (duodenum and jejunum), although LGG treatment significantly decreased carbonylation in alcohol fed rats, the levels were still significantly higher than in dextrose fed rats. LGG decreased ileal carbonyl levels in alcohol fed rats to levels similar to the dextrose fed rats. LGG supplementation significantly reduced nitrotyrosine levels in both small bowel and colon in a similar pattern as with tissue carbonyl. For proximal small bowel (duodenum and jejunum), LGG significantly decreased nitrotyrosine levels by 31% and 28% respectively in the alcohol fed rats, but the levels remained significantly higher than in the dextrose fed rats. For ileum, LGG dropped the nitrotyrosine levels in the alcohol fed rats more robustly by 38% and the levels were no longer significantly different from the levels in the dextrose fed control rats.
Table 1.
LGG Treatment Ameliorates Alcohol-Induced Oxidative Stress and Inflammation. (*p ≤ .05 for ALC-V compared to ALC-LGG)
| Measure | Site | CON | ALC-V | ALC-LGG | CON-LGG |
|---|---|---|---|---|---|
| Carbonyl | Duodenum | 6930 ±862 | 16754 ±483 | 11680 ±849* | 4464 ±592 |
| Jejunum | 1081 ±101 | 8596 ±562 | 2904 ±355* | 1860 ±360 | |
| Ileum | 4862 ±623 | 13983 ±370 | 4795 ±701* | 3949 ±415 | |
| Colon | 5759 ±1297 | 26257±960 | 7184 ±1773* | 6186 ±925 | |
| Nitrotyrosine | Duodenum | 5357 ±774 | 19033 ±686 | 13066 ±985* | 5220 ±811 |
| Jejunum | 5532 ±1026 | 14118 ±810 | 10189 ±715* | 4628 ±730 | |
| Ileum | 5842 ±1304 | 16920 ±931 | 10427±1892* | 1385 ±791 | |
| Colon | 6767 ±1099 | 25599 ±953 | 4573 ±1323* | 1308 ±307 | |
| MPO (U/mg) | Colon | 4.8 ±0.2 | 6.8 ±0.4 | 5.3 ±0.3* | 5.4 ±0.3 |
| Total NO (μM) | Colon | 819 ±99 | 2717 ±175 | 673 ±62* | 386 ±68 |
| Urine | 88 ±20 | 387 ±37 | 119 ±11* | 57 ±3 | |
| Nitrite(NO2)(μM) | Urine | 1.4 ±0.2 | 15.6 ±3 | 2 ±0.4* | 2 ±1.3 |
| Nitrate (NO3)(μM) | Urine | 86 ±20 | 387 ±37 | 117 ±10* | 56 ±3 |
Lactobacillus GG gavage prevents alcohol-induced oxidative stress in the colon
We also assessed oxidative stress markers of carbonylation and nitrotyrosination in the rats' colons (Table 1). Alcohol feeding significantly increased carbonyl expression in the colon and LGG treatment reduced the levels by 73% and dropped carbonyl expression levels to values similar to those in dextrose fed control rats. Alcohol feeding also increased nitrotyrosine levels in the colon and LGG gavage decreased the levels by 82% and normalized the colonic nitrotyrosine values as there was no longer any significant difference between ALC-LGG and dextrose fed control rats. As a further measure of NO mediated oxidative stress we also measured colon tissue total NO (nitrite+nitrate) and found the colonic mucosa NO in ALC-V fed rats was 4-fold higher than dextrose fed rats (Table 1). LGG markedly and significantly decreased NO levels in the colon of alcohol fed rats and NO levels in alcohol- LGG fed rats were not significantly different from dextrose fed rats (Table 1).
Lactobacillus GG gavage prevents alcohol-induced inflammation in the colon
There was no gross structural or histological abnormality in the small bowel or colonic mucosa of alcohol fed rats when they were assessed macroscopically (careful inspection of mucosa) or microscopically (H&E staining; data not shown), However, there was biochemical evidence of mild inflammation in the colon of alcohol fed rats. Myeloperoxidase (MPO) is present in the cytoplasm of myeloid derived cells such as neutrophils and has been successfully used as a reliable marker of tissue inflammation (Malle et al., 2007). We found that alcohol feeding results in a modest (29%) but significant increase in MPO levels in the colon (Table 1). LGG treatment significantly dropped the MPO levels in the colon of alcohol fed rats. Indeed, LGG prevented colonic inflammation induced by alcohol feeding since there was no significant difference in colonic MPO levels between the ALC-LGG and dextrose fed control rats (Table 1).
Lactobacillus GG treatment decreases alcohol-induced systemic oxidative stress markers
As a further measure of systemic alcohol-induced oxidative stress we determined values for urinary NO2 (nitrite), NO3 (nitrate), and total NO (Table 1). Alcohol feeding significantly increased urinary nitrite and nitrate and LGG supplementation prevented the increase. Urinary nitrite (NO2) was up 7.8-fold in the ALC-V fed rats compared to ALC-LGG fed rats, while no significant difference was found between ALC-LGG fed and dextrose fed or dextrose-LGG fed control rats. Also, urinary nitrate (NO3) values in ALC-V fed rats were 3-fold higher than the nitrate values in ALC-LGG fed rats, while no significant differences were found between ALC-LGG fed and dextrose fed or dextrose-LGG fed control rats. Finally, the total NO value in ALC-V fed rats was 3-fold higher than values in ALC-LGG rats and the ALC-LGG total NO was not significantly different than dextrose fed or dextrose-LGG fed control rats (Table 1). Thus treatment with LGG reduced markers of systemic and tissue oxidative stress in the alcohol fed rats.
Discussion
The aims of the current study were to: 1) Determine if LGG treatment could prevent alcohol-induced gut leakiness and development of alcoholic steatohepatitis in our animal model of alcoholic steatohepatitis (ASH); and 2) Identify one possible mechanism for LGG beneficial effects, namely reduction of alcohol-induced intestinal and systemic oxidative stress.
We have previously established that chronic gavage of alcohol in rats represents a valid experimental model of ASH (Keshavarzian et al., 2001). In those studies we showed that prevention of alcohol-induced intestinal hyperpermeability ameliorated alcohol-induced endotoxemia and alcoholic steatohepatitis. In the present study, using the same rat model, we now show that daily gavage with Lactobacillus GG also prevented alcohol-induced gut leakiness and development of alcoholic steatohepatitis in alcohol fed rats. With regard to possible mechanisms for preservation of gut barrier function, our data show that LGG gavage significantly reduced intestinal markers of oxidative stress and inflammation in alcohol-fed rats.
Our finding that LGG prevented ASH in our rat model of alcoholic liver disease confirmed the previous study by Nanji et al who also showed that Lactobacillus supplementation improved fatty liver in rats fed an alcohol Lieber-Decarli diet (36% of calories from alcohol)(Nanji et al., 1994). Although the study did not directly assess the mechanism of action of Lactobacillus, the fact that serum endotoxin was significantly lowered by LGG treatment suggested that the beneficial effects of LGG is through its ability to prevent alcohol-induced endotoxemia (Nanji et al., 1994). Indeed, a probiotic Lactobacillus sp. also prevented endotoxemia and liver damage in a model of acute alcohol exposure and pancreatitis (Marotta et al., 2005).
One possible mechanism of alcohol-induced endotoxemia is gut leakiness. Several studies have demonstrated a role for alcohol-induced increased intestinal permeability to luminal contents (gut leakiness) and the development of liver injury in animal models and in alcoholic subjects (Bode and Bode, 2005; Keshavarzian et al., 1999; Lumeng and Crabb, 2001; Rao et al., 2004). Thus, the observed beneficial effect of LGG in preventing endotoxemia could be due to its ability to improve barrier function. Our data show that gavage with LGG robustly reduced gut leakiness (to sucralose by 56%). These data support our hypothesis that by promoting normal intestinal barrier function LGG treatment might prevent ASH in this model. Indeed, several studies support the notion that Lactobacillus spp. including LGG, can improve gut barrier function in a wide range of pathological conditions. For example, LGG treatment was recently shown to protect against gastric hyperpermeability and injury caused by acute alcohol exposure in a rat model (Lambert et al., 2007). In addition, Lactobacillus sp. treatment prevented liver injury mediated directly by endotoxin without alcohol in another recent study (Osman et al., 2006). LGG reversed increased intestinal permeability caused by cows milk in suckling rats (Isolauri et al., 1993). Treatment with Lactobacillus spp. restored normal intestinal permeability and greatly ameliorated intestinal inflammation in IL-10 knockout mice (Madsen et al., 1999; Schultz et al., 2002). Also, in two different rat models of psychological stress-induced intestinal dysfunction Lactobacillus treatment prevented bacterial translocation and restored normal gut barrier function (Eutamene and Bueno, 2007; Eutamene et al., 2007; Zareie et al., 2006). In vitro and ex-vivo studies also support the protective effect of LGG on intestinal barrier integrity. Lactobacillus spp. promoted normal intestinal epithelial cell (IEC) barrier function (increased TER) and inhibited increased permeability caused by inflammatory cytokines and pathogenic E. coli. (Resta-Lenert and Barrett, 2003; Resta-Lenert and Barrett, 2006; Roselli et al., 2006; Sherman et al., 2005; Zareie et al., 2006). Lactobacilli protected IEC tight junctions from aspirin-induced damage in HT-29 cells as well (Montalto et al., 2004). In a rat intestinal loop model Lactobacilli and other probiotics were shown to directly modulate and improve intestinal permeability (Garcia-Lafuente et al., 2001). Thus, in vitro and in vivo animal models, including our study reported here, have shown that probiotics and especially LGG can preserve barrier function in response to alcohol and diverse other injurious stimuli.
The mechanisms for probiotics beneficial effects on barrier function are not known. Probiotics including LGG have been shown to inhibit inflammatory signaling by producing bacteriocins that inhibit pathogenic bacteria and other proteins that modulate IEC signaling (Menard et al., 2004; Tao et al., 2006; Yan et al., 2007). Probiotics ameliorate inflammation in experimental colitis models using DSS and TNBS (Osman et al., 2006; Peran et al., 2007). In vivo, probiotics may also directly modulate immune cell functions including dendritic cells, macrophages, and T cells (Feleszko et al., 2007). A recent study showed lactobacillus probiotics restored normal neutrophil function in alcoholics with cirrhosis (Stadlbauer et al., 2008). LGG also decreases systemic inflammation markers caused by LPS in rats (Zhang et al., 2006). One significant anti-inflammatory mechanism identified for probiotics including LGG is inhibition of NF-kB activation, a pathway we have identified as being critical to alcohol-induced oxidative stress and intestinal hyperpermeability (Banan et al., 2007; O'Hara and Shanahan, 2006; Petrof et al., 2004; Zhang et al., 2005). For example, gene array analysis of LGG treatment in humans reveals downregulation of NOS and NF-kB family genes in IEC (Di Caro et al., 2005). Our data now support our proposed hypothesis that LGG inhibition of NF-kB activation by alcohol results in decreased iNOS expression and reduced oxidative stress resulting in the reduced markers of oxidative stress. The result is the improved barrier function that led to our observed improvement of steatohepatitis.
Lactobacillus spp. also can modulate epidermal growth factor receptor (EGFR) mediated intracellular signaling (Resta-Lenert and Barrett, 2003), a pathway we and others have shown can also reverse alcohol and E. Coli-induced disruption of intestinal monolayer barrier integrity (Banan et al., 1999; Banan et al., 2000; Resta-Lenert and Barrett, 2003). In addition, LGG has been shown to secrete proteins that protect IEC from oxidative stress by upregulation of heat shock proteins (Tao et al., 2006). That the antioxidant effects observed are specifically related to LGG treatment is supported by the pattern of the carbonyl and nitrotyrosine data in our study. Studies in mice and rats inoculated with Lactobacillus species have noted that although lactobacilli are detectable throughout the digestive tract, due to the ‘washout effect’ of gut motility, ingested lactobacilli were especially prevalent in the more static ileum and colon (Tannock, 2004). In our study, ileum and colon- the areas of intestine previously documented to harbor the greatest levels of ingested Lactobacilli- exhibited the most dramatic evidence of antioxidant benefit and protection by LGG gavage from alcohols effects. Further studies are needed to determine the exact mechanisms of LGG antioxidant effects and preservation of barrier function after chronic alcohol consumption in animals and man.
Another possible mechanism for LGG beneficial effects in our model is the preservation of a normal gut microbiota community. For example, prevention of bacterial overgrowth by pathogenic species could contribute to protection of intestinal barrier integrity as well as reduce the endotoxin load in the gut that could travel to the liver (Versalovic, 2007). An abnormal profile of intestinal microbiota (dysbiosis) has been demonstrated in pathological conditions such as aging, inflammatory bowel disease, and liver disease where endotoxin appears to play a pathogenic role (Fabia et al., 1993; Hopkins et al., 2001; Martinez-Medina et al., 2006; Riordan and Williams, 2006; Seksik et al., 2003). Several studies suggest an abnormal gut microbiota profile may contribute to both alcoholic and nonalcoholic liver disease and that probiotic therapy can have beneficial effects by normalizing the dysbiotic microbiota (Riordan and Williams, 2006). For example, several studies reported beneficial therapeutic effects of Lactobacillus spp. and other probiotics in patients with hepatic encephalopathy and non-alcoholic liver disease (Adawi et al., 2001; Chiva et al., 2002; Liu et al., 2004). More specifically, intestinal bacteria were shown to contribute to alcohol-induced liver injury in a rat model of alcoholic liver disease (Ferrier et al., 2006). Furthermore, a recently published study, using culture technique reported dysbiosis in alcoholics with liver disease (Kirpich et al., 2008) and supports our findings. The authors showed that chronic alcoholics have an abnormal profile of gut microbiota with significantly lower numbers of bifidobacteria and lactobacilli. Patients who received probiotic therapy with bifidobacteria and lactobacillus spp. restored numbers of these species to levels seen in controls and demonstrated significant improvement in liver function. Further studies are needed to directly assess the effect of LGG on gut microbiota in the alcohol fed rats and alcoholic subjects with and without liver disease.
In summary, we showed for the first time that gavage with the probiotic Lactobacillus GG robustly reduces the severity of alcoholic steatohepatitis, alcohol-induced gut hyperpermeability, and alcohol-induced tissue (gut and liver) and systemic oxidative stress in our rat gavage model of ALD. Further, our data suggest that possible underlying mechanisms for beneficial effects of LGG on ASH are the reduction of alcohol-induced oxidative stress and restoration of barrier function.
Acknowledgments
We are grateful to Ms. Jayanthi Rangan for her technical support and Dr. A. Banan for his advice. The study was supported by NIH grant # AA013745 (AK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Adawi D, Ahrne S, Molin G. Effects of different probiotic strains of Lactobacillus and Bifidobacterium on bacterial translocation and liver injury in an acute liver injury model. Int J Food Microbiol. 2001;70:213–220. doi: 10.1016/s0168-1605(01)00550-5. [DOI] [PubMed] [Google Scholar]
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 2000;294:997–1008. [PubMed] [Google Scholar]
- Banan A, Keshavarzian A, Zhang L, Shaikh M, Forsyth CB, Tang Y, Fields JZ. NF-kappaB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol. 2007;41:447–460. doi: 10.1016/j.alcohol.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Banan A, Smith GS, Rieckenberg CL, Kokoska ER, Miller TA. Protection against ethanol injury by prostaglandin in a human intestinal cell line: role of microtubules. Am J Physiol. 1998;274:G111–121. doi: 10.1152/ajpgi.1998.274.1.G111. [DOI] [PubMed] [Google Scholar]
- Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Novel effect of NF-kappaB activation: carbonylation and nitration injury to cytoskeleton and disruption of monolayer barrier in intestinal epithelium. Am J Physiol Cell Physiol. 2004;287:C1139–1151. doi: 10.1152/ajpcell.00146.2004. [DOI] [PubMed] [Google Scholar]
- Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and nonalcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Bruzzese E, Raia V, Gaudiello G, Polito G, Buccigrossi V, Formicola V, Guarino A. Intestinal inflammation is a frequent feature of cystic fibrosis and is reduced by probiotic administration. Aliment Pharmacol Ther. 2004;20:813–819. doi: 10.1111/j.1365-2036.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- Chiva M, Soriano G, Rochat I, Peralta C, Rochat F, Llovet T, Mirelis B, Schiffrin EJ, Guarner C, Balanzo J. Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatol. 2002;37:456–462. doi: 10.1016/s0168-8278(02)00142-3. [DOI] [PubMed] [Google Scholar]
- Di Caro S, Tao H, Grillo A, Elia C, Gasbarrini G, Sepulveda AR, Gasbarrini A. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig Liver Dis. 2005;37:320–329. doi: 10.1016/j.dld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Eutamene H, Bueno L. Role of probiotics in correcting abnormalities of colonic flora induced by stress. Gut. 2007;56:1495–1497. doi: 10.1136/gut.2007.124040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, Corthesy-Theulaz I, Fioramonti J, Bueno L. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137:1901–1907. doi: 10.1093/jn/137.8.1901. [DOI] [PubMed] [Google Scholar]
- Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol. 2006;12:5941–5950. doi: 10.3748/wjg.v12.i37.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabia R, Ar'Rajab A, Johansson ML, Andersson R, Willen R, Jeppsson B, Molin G, Bengmark S. Impairment of bacterial flora in human ulcerative colitis and experimental colitis in the rat. Digestion. 1993;54:248–255. doi: 10.1159/000201045. [DOI] [PubMed] [Google Scholar]
- Farhadi A, Keshavarzian A, Holmes EW, Fields J, Zhang L, Banan A. Gas chromatographic method for detection of urinary sucralose: application to the assessment of intestinal permeability. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:145–154. doi: 10.1016/s1570-0232(02)00787-0. [DOI] [PubMed] [Google Scholar]
- Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006;316:1–7. doi: 10.1124/jpet.105.085449. [DOI] [PubMed] [Google Scholar]
- Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148–1154. doi: 10.2353/ajpath.2006.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Lafuente A, Antolin M, Guarner F, Crespo E, Malagelada JR. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut. 2001;48:503–507. doi: 10.1136/gut.48.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease--pathophysiological aspects and risk factors. Aliment Pharmacol Ther. 2006;24:1151–1161. doi: 10.1111/j.1365-2036.2006.03110.x. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut. 2001;48:198–205. doi: 10.1136/gut.48.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993;105:1643–1650. doi: 10.1016/0016-5085(93)91059-q. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Banan A, Farhadi A, Komanduri S, Mutlu E, Zhang Y, Fields JZ. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. 2003;52:720–728. doi: 10.1136/gut.52.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- Keshavarzian A, Fields JZ, Vaeth J, Holmes EW. The differing effects of acute and chronic alcohol on gastric and intestinal permeability. Am J Gastroenterol. 1994;89:2205–2211. [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ, Cave M. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D, Padfield PJ, McLaughlin J, Cannell S, O'Neill CA. Ochratoxin A displaces claudins from detergent resistant membrane microdomains. Biochem Biophys Res Commun. 2007;358:632–636. doi: 10.1016/j.bbrc.2007.04.180. [DOI] [PubMed] [Google Scholar]
- Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Crabb DW. Alcoholic liver disease. Curr Opin Gastroenterol. 2001;17:211–220. doi: 10.1097/00001574-200105000-00004. [DOI] [PubMed] [Google Scholar]
- Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- Malle E, Furtmuller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marotta F, Barreto R, Wu CC, Naito Y, Gelosa F, Lorenzetti A, Yoshioka M, Fesce E. Experimental acute alcohol pancreatitis-related liver damage and endotoxemia: synbiotics but not metronidazole have a protective effect. Chin J Dig Dis. 2005;6:193–197. doi: 10.1111/j.1443-9573.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil LJ. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis. 2006;12:1136–1145. doi: 10.1097/01.mib.0000235828.09305.0c. [DOI] [PubMed] [Google Scholar]
- Mattar AF, Drongowski RA, Coran AG, Harmon CM. Effect of probiotics on enterocyte bacterial translocation in vitro. Pediatr Surg Int. 2001;17:265–268. doi: 10.1007/s003830100591. [DOI] [PubMed] [Google Scholar]
- Meddings JB, Swain MG. Environmental stress-induced gastrointestinal permeability is mediated by endogenous glucocorticoids in the rat. Gastroenterology. 2000;119:1019–1028. doi: 10.1053/gast.2000.18152. [DOI] [PubMed] [Google Scholar]
- Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53:821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalto M, Maggiano N, Ricci R, Curigliano V, Santoro L, Di Nicuolo F, Vecchio FM, Gasbarrini A, Gasbarrini G. Lactobacillus acidophilus protects tight junctions from aspirin damage in HT-29 cells. Digestion. 2004;69:225–228. doi: 10.1159/000079152. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman N, Adawi D, Molin G, Ahrne S, Berggren A, Jeppsson B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006;6:31. doi: 10.1186/1471-230X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Peran L, Camuesco D, Comalada M, Bailon E, Henriksson A, Xaus J, Zarzuelo A, Galvez J. A comparative study of the preventative effects exerted by three probiotics, Bifidobacterium lactis, Lactobacillus casei and Lactobacillus acidophilus, in the TNBS model of rat colitis. J Appl Microbiol. 2007;103:836–844. doi: 10.1111/j.1365-2672.2007.03302.x. [DOI] [PubMed] [Google Scholar]
- Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology. 2004;127:1474–1487. doi: 10.1053/j.gastro.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Polikandriotis JA, Rupnow HL, Brown LA, Hart CM. Chronic ethanol ingestion increases nitric oxide production in the lung. Alcohol. 2007;41:309–316. doi: 10.1016/j.alcohol.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC) Gut. 2003;52:988–997. doi: 10.1136/gut.52.7.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731–746. doi: 10.1053/j.gastro.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Riordan SM, Williams R. The intestinal flora and bacterial infection in cirrhosis. J Hepatol. 2006;45:744–757. doi: 10.1016/j.jhep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Roselli M, Finamore A, Britti MS, Mengheri E. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br J Nutr. 2006;95:1177–1184. doi: 10.1079/bjn20051681. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Schultz M, Veltkamp C, Dieleman LA, Grenther WB, Wyrick PB, Tonkonogy SL, Sartor RB. Lactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient mice. Inflamm Bowel Dis. 2002;8:71–80. doi: 10.1097/00054725-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Seksik P, Rigottier-Gois L, Gramet G, Sutren M, Pochart P, Marteau P, Jian R, Dore J. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut. 2003;52:237–242. doi: 10.1136/gut.52.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun. 2005;73:5183–5188. doi: 10.1128/IAI.73.8.5183-5188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer V, Mookerjee RP, Hodges S, Wright GA, Davies NA, Jalan R. Effect of probiotic treatment on deranged neutrophil function and cytokine responses in patients with compensated alcoholic cirrhosis. J Hepatol. 2008;48:945–951. doi: 10.1016/j.jhep.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol. 2004;70:3189–3194. doi: 10.1128/AEM.70.6.3189-3194.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H. Conceptual importance of identifying alcoholic liver disease as a lifestyle disease. J Gastroenterol. 2007;42:603–609. doi: 10.1007/s00535-007-2075-3. [DOI] [PubMed] [Google Scholar]
- Varella Morandi Junqueira-Franco M, Ernesto Troncon L, Garcia Chiarello P, do Rosario Del Lama Unamuno M, Afonso Jordao A, Vannucchi H. Intestinal permeability and oxidative stress in patients with alcoholic pellagra. Clin Nutr. 2006;25:977–983. doi: 10.1016/j.clnu.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Versalovic J. Probiotics: intestinal gatekeeping, immunomodulation, and hepatic injury. Hepatology. 2007;46:618–621. doi: 10.1002/hep.21916. [DOI] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.09.017. online advance publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington BS, Meserole L, Syrotuck JA. Effect of daily ethanol ingestion on intestinal permeability to macromolecules. Am J Dig Dis. 1978;23:23–32. doi: 10.1007/BF01072571. [DOI] [PubMed] [Google Scholar]
- Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareie M, Johnson-Henry K, Jury J, Yang PC, Ngan BY, McKay DM, Soderholm JD, Perdue MH, Sherman PM. Probiotics prevent bacterial translocation and improve intestinal barrier function in rats following chronic psychological stress. Gut. 2006;55:1553–1560. doi: 10.1136/gut.2005.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li N, Caicedo R, Neu J. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J Nutr. 2005;135:1752–1756. doi: 10.1093/jn/135.7.1752. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li N, des Robert C, Fang M, Liboni K, McMahon R, Caicedo RA, Neu J. Lactobacillus rhamnosus GG decreases lipopolysaccharide-induced systemic inflammation in a gastrostomy-fed infant rat model. J Pediatr Gastroenterol Nutr. 2006;42:545–552. doi: 10.1097/01.mpg.0000221905.68781.4a. [DOI] [PubMed] [Google Scholar]