Abstract
Apoptotic cell phagocytosis has recently raised considerable interest, particularly due to its intricate molecular mechanisms and negative immunologic impact of incompetent clearance of apoptotic cells. There is a need for simple and reliable methods to clearly determine the internalization of apoptotic cells. Labeling with pHrodo succinimidyl ester (SE), a pH-sensitive fluorescent dye, makes engulfed apoptotic cells detectable due to the increased post-phagocytic light emission. This is a valuable tool for phagocytosis studies via FACS. We designed an ex vivo assay, using apoptotic pHrodo-labeled lymphocytes as prey and anti-CD11b-labeled tissue macrophages. To demonstrate its validity of detecting internalized apoptotic lymphocytes, we used MFGE8-/- macrophages, known to have impaired phagocytic ability. Uptake of apoptotic lymphocytes was accelerated and enhanced in splenic macrophages after stimulation with recombinant MFGE8, while peritoneal macrophages were able to compensate for the delayed uptake. This novel assay is a quick and reliable method to evaluate the internalization of apoptotic cells.
Keywords: pHrodo-SE, Phagocytosis, FACS, MFGE8
Introduction
Since Ilya Mechnikov first described phagocytosis, this phenomenon has been recognized as an important function of immune cells (Klemparskaya, 1983). Although many cell types are capable of phagocytosis, some cells are specialized to do just that very efficiently. These cells, such as neutrophils, macrophages and dendritic cells, help to remove intruding microbes, inert material, cellular debris and apoptotic cells (Stuart and Ezekowitz, 2008). This permits maintenance of tissue and organ function and overall continuity of the organism. The efficiency of phagocytosis is determined by the phagocytosing cell type as well as the phagocytized object. It is well established that phagocytosis of certain microbes elicits a strong proinflammatory response and activation in the phagocytes leading to accelerated phagocytosis and recruitment of other immune cells (Blander and Medzhitov, 2004; Watts, 2004). Such a response is desirable in a local infection because it helps to clear the microbes and to mount an innate and adaptive immune response against the intruder (Schnitzler et al., 1999). On the other hand, during ontogenesis, many cells undergo apoptosis as tissue remodeling takes place. These dead cells need to be removed by phagocytosis without eliciting an inflammation. Generally, this is established by the immunosuppressive effect of apoptotic cell phagocytosis on phagocytes themselves (Huynh et al., 2002; Asano et al., 2004). Hanayama et al. have shown that deficient phagocytosis of apoptotic B-cells in the spleen leads to the generation of anti-DNA autoantibodies and to an lupus erythematosus-like disease in mice (Hanayama et al., 2004). They have shown that macrophages deficient in milk fat globule EGF-factor 8 (MFGE8), an opsonizing protein secreted by certain phagocytes with a high affinity to phosphatidylserine expressed on apoptotic cells, are able to bind apoptotic cells on their surface but are inefficient in the engulfment of these targets. The absence of MFGE8 has been proposed to have detrimental proinflammatory effects in several disease models including autoimmunity, atherosclerosis and intestinal mucosa regeneration after injury, linking engulfment of apoptotic cells and inflammation (Hanayama et al., 2004; Ait-Oufella et al., 2007; Bu et al., 2007). There are several phagocytosis assay protocols, based on microscopic evaluation or FACS analysis with labeled targets. In most cases, these assays are sufficient, however, they may overestimate actual engulfment of targets due to the detection of some surface-bound bacteria or other material. Thus, in some circumstances the determination of true engulfment of apoptotic cells is desirable. The purpose of this report is to demonstrate a novel and simple approach to a phagocytosis assay that determines the engulfment of apoptotic cells by flow cytometry or fluorescent microscope.
Experimental
Materials
Animals
In our study, we used male Sprague-Dawley rats (6-8 weeks old; 250-300g BW; Charles River), C57BL6/J wild-type (WT) mice (6-8 weeks old; 20-25g; Taconic), and C57BL6/J MFG-E8-/- mice (6-8 weeks old; 20-25g; generous gift by S. Nagata, Osaka University, Japan). All experiments were performed according to national guidelines for the use of animals in research and approved by the Animal Care and Use Committee of the Feinstein Institute for Medical Research.
Reagents
We purchased the following reagents: recombinant MFGE8 (mouse or rat, R&D Systems), pHrodo™ succinimidyl ester (SE) (Invitrogen), FITC-anti-rat CD11b/c (OX42; BD Pharmingen), APC-anti-mouse CD11b (Integrin αM chain Mac-1; BD Parmingen), Annexin V-FITC Apoptosis Detection Kit (BD Pharmingen) and the In Situ Cell Death Detection Kit, Fluorescein (Roche). For cell isolation and culture we used collagenase Type 4 (Worthington, 4000 Mandl U/ml stock solution), Phosphate Buffered Saline (PBS) (pH7.2-7.4; GIBCO), ACK lysis buffer for red blood cells (0.15M NH4Cl, 10mM KHCO3, 0.1mM EDTA), 4% paraformaldehyde in PBS (Sigma), DMEM (GIBCO), OPTI-MEM I (reduced serum medium; GIBCO), Hank's Balanced Salt Solution (HBSS) with or without Ca2+ (GIBCO), Fetal Bovine Serum (FBS) (Cellgro), Penicillin/Streptomycin (GIBCO), L-Glutamine (GIBCO), 60 mm and 100 mm tissue culture dishes (FALCON)
Methods
Splenic macrophages
Mice or rats were anesthetized with isoflurane and spleens and thymuses explanted. Harvested spleens were injected with 4-10 ml of 100 U/ml collagenase 4 until it turned pale and swollen. The retained suspension was collected in a tube and set aside on ice. The spleen was then digested with 10 ml of 400 U/ml collagenase 4 and crushed with the backside of a syringe piston. After incubation at 37°C for 1h, spleens were washed with PBS, RBCs lysed with ACK buffer for 5 min, filtered, washed again twice with PBS and resuspended in DMEM. 4 ml of 107/ml splenocytes were added to 60 mm plates for 1h, supernatants removed and replaced with fresh media for another hour. Plates were then thoroughly washed with PBS (three times) and replaced with fresh media. One test plate was used to estimate the number of adhered cells by detachment and manual counting under the microscope.
Peritoneal macrophages
Macrophages were isolated from peritoneal lavage fluid (using ice-cold HBSS without Ca2+, 60 ml per rat and 6 ml per mouse). Peritoneal lavage fluid was washed twice with PBS (1200-1400 rpm for 7 min), followed by resuspension in DMEM. Cells were plated at 106 per 60 mm plate with 4-6 ml DMEM. 2h later, adherent macrophages were thoroughly washed with PBS and replenished with fresh media. The number of adherent cells was estimated by detaching and counting on a hemocytometer.
Thymocytes
The collected thymus was ground between frosted glass slides until completely homogenized without destroying cells. Collected cells were filtered and washed twice with PBS. Cells were then resuspended in complete DMEM at 107 cells/ml and cultured at 37°C in a humidified atmosphere containing 5% CO2.
Induction of apoptosis
0.1μM dexamethasone was added to 107/ml thymocytes in complete DMEM for 16h. This produced almost 100% apoptosis, as assessed by annexin V / propidium iodine (PI) staining and FACS analysis.
Apoptotic cell labeling with pHrodo-SE
After the induction of apoptosis, thymocytes were washed twice with PBS and resuspended in PBS at 106 cells/ml. 1μl of 1 mg/ml pHrodo-SE (stock solution in DMSO) was added per 50 ml of cell suspension (i.e., final concentration 20 ng/ml and 106 cells pHrodo-SE). As the solvent DMSO was diluted by 2×10-8 times, its effect on cellular function is negligible. After incubation for 30 min at RT, cells were washed twice with PBS and resuspended in OPTI-MEM I (e.g., 5×106 cells/ml). Non-apoptotic cells stained in the same manner with pHrodo-SE served as a control.
Phagocytosis assay
For 106 macrophages on a 60 mm plate, 4×106 pHrodo-SE-labeled apoptotic thymocytes were added in 4 ml medium and incubated at 37°C in a humidified atmosphere containing 5% CO2 for 5 to 180 min. pHrodo-SE-labeled non-apoptotic thymocytes coincubated in the same manner served as a control. Phagocytosis was verified under the light microscope prior to labeling and detaching cells. In experiments using MFGE8-/- macrophages, dexamethasone-treated thymocytes were preincubated with 0.5 μg/ml rmMFGE8 for 30 min prior to phagocytosis assay to opsonize the apoptotic cells for phagocytosis. After incubation, cells were thoroughly washed and stained.
Macrophage labeling with FITC-anti-rat-CD11b/c mAb (OX42) or APC-anti-murine-CD11b mAb (M1/70)
Splenic macrophages or peritoneal macrophages were labeled after phagocytosis to minimize the interference with the assay and stress on the macrophages. After phagocytosis, cells were washed three times with PBS and 2.5 μl/ml PBS FITC-anti-CD11b/c (for rat macrophages) or 2.5 μl/ml PBS APC-anti-CD11b (for mouse macrophages) were added for 20 min at RT. Cells were washed again with PBS, detached from the plate by placing them on ice with 1 ml PBS and using a cell lifter. Thus collected cells were transferred to a FACS tube and fixed with 1%PFA and kept at 4°C until analyzed by FACS within the next 3 days. This staining provided a homogenous surface staining of macrophages and was also useful to distinguish cell surfaces of cells during fluorescent microscopy. As peritoneal macrophages tend to adhere firmer to the plate we used Trypsin + EDTA to detach them.
FACS analysis
Following controls were set up for flow cytometry:
| Macrophages: | unstained and stained |
| Thymocytes: | unstained and stained with Annexin V/PI or pHrodo-SE stained with pHrodo-SE + 1M HCl (1-3 μl/ml, positive control) |
| Samples: | unstained and stained macrophages with non-apoptotic or apoptotic pHrodo+ thymocytes |
Using the FACS Calibur, we gated on viable macrophages in FSC/SSC, and used rat CD11b/c (FITC) in FL2 or mouse CD11b (APC) in FL4 and pHrodo in FL3. Stained macrophages and pHrodo-SE-labeled apoptotic cells alone served to determine cutoffs for phagocytosis. In 104 gated collected events, phagocytosis percentage (pHrodo+/CD11b/c) and MFIpHrodo+ were assessed.
Data analysis and statistics
Each experiment was performed in triplicate and experiments were repeated at least three times. Data are presented as means ± SEM. Differences were compared by two-way ANOVA and Student Newman Keuls' test, or Student's t-test.
Results
Approach to a novel phagocytosis assay
A phagocytosis assay based on fluorescent labeling and FACS analysis can be used to assess the phagocytosis of apoptotic cells by macrophages labeled by a fluorescent labeled anti-CD11b antibody. Experiments using TUNEL-labeled apoptotic T cells as prey makes the distinction of attached and engulfed apoptotic cells to the macrophage difficult. Hanayama et al. solved this problem using caspase-activated DNAse-deficient (CAD-/-) T cells as prey. These apoptotic cells become TUNEL-positive only after the post-phagocytic macrophage-mediated degradation of T cell DNA (Hanayama et al., 2002). While this methodology is very elegant, it requires the availability of CAD-/- mice. We hence developed a novel method using another phenomenon of macrophage-mediated modification of engulfed particles. The post-phagocytic fusion of phagosomes and lysosomes leads to a drop in pH within the phagolysosome compartment due to the acidic environment of lysosomes, which can be detected by pH-sensitive dyes. pHrodo-SE (Invitrogen, Carlsbad, CA) is such a fluorescent dye that emits light in the red range at an increased intensity with decreasing environmental pH (Invitrogen, 2008). In a pH neutral environment, the light emission by pHrodo-SE is almost undetectable under the fluorescent microscope. Thus, labeled cells that are non-phagocytized or attached to macrophages are very dim. Once these cells are engulfed, however, the signal becomes brighter because of the acidic environment in the phagosomes of phagocytes. It has been successfully used in labeling latex beads and microbes and is commercially available for phagocytosis assays based on fluorescent microscopy or FACS analysis. This staining procedure has never been used to label eukaryotic cells before and we present, for the first time, a phagocytosis assay using pHrodo-SE-labeled apoptotic cells as prey with fluorescent anti-CD11b-stained macrophages using FACS analysis. We show that free floating and attached apoptotic cells emit light at a low fluorescence, while engulfed apoptotic cells shine brightly, which can be easily detected using either fluorescent microscopy or flow cytometry.
pHrodo-SE-labeled apoptotic cells are responsive to pH change and detectable by FACS and fluorescent microscopy after engulfment
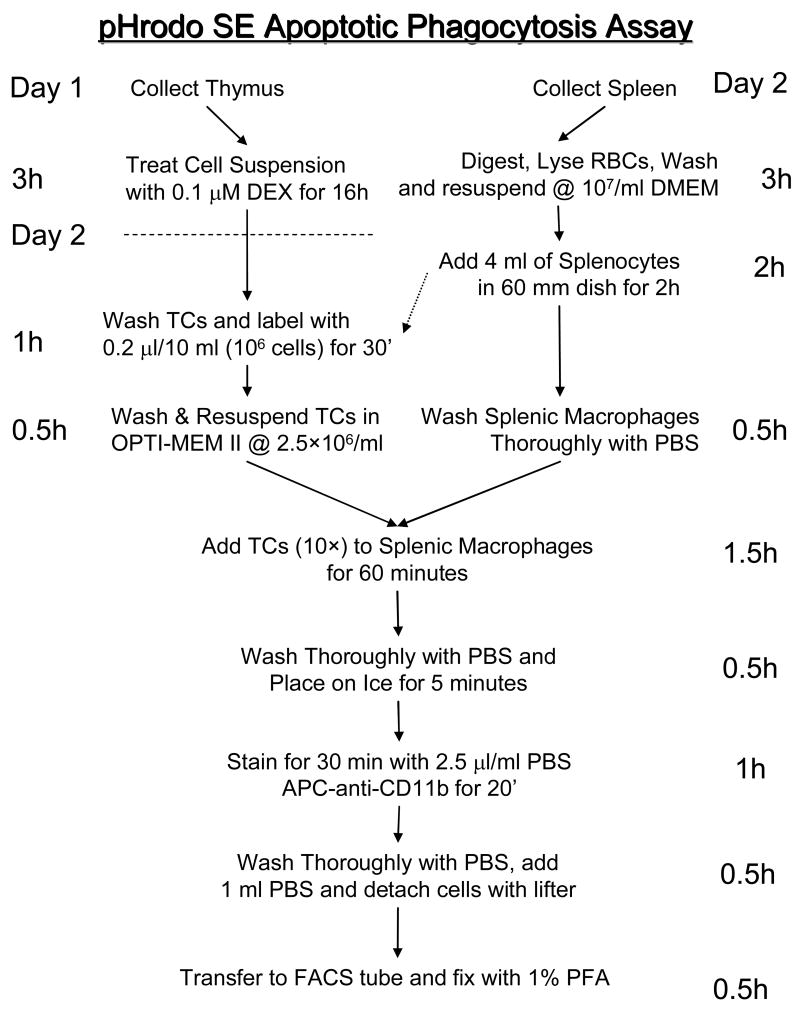
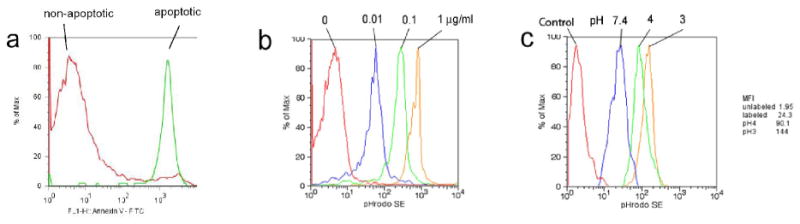
We first induced apoptosis in rat thymocytes, using 0.1 μM dexamethasone for 16h at 37°C. Staining with Annexin V/PI revealed that 5-10% of healthy non-treated thymocytes were apoptotic, on the other hand, over 90% of the dexamethasone-treated thymocytes cells were apoptotic (Fig. 1a). We then stained the apoptotic cells by incubating them in PBS with increasing concentrations of pHrodo-SE (0.01, 0.1, and 1 μg/ml). FACS analysis showed that apoptotic cells consistently and dose-dependently stain with pHrodo-SE and that these cells are detectable by flow cytometry (Fig. 1b). For pH sensitivity analysis, we used a concentration of 0.02 μg/ml pHrodo-SE to determine the pH sensitivity of the dye. Decreasing the pH from an initial 7.4 by adding HCl to either a pH of 4 or even 3, increased the mean fluorescent intensity (MFI) by 3.7-fold and 5.9-fold, respectively (Fig. 1c). A pH below 3 disrupted the cells and a further increase in MFI could not be determined. The addition of NaOH on the other hand quenched the fluorescence of pHrodo-SE-labeled thymocytes (data not shown). To determine whether macrophage-engulfed pHrodo-labeled apoptotic thymocytes also become brighter, we coincubated rat splenic macrophages with pHrodo-SE-labeled apoptotic thymocytes (0.02 μg/ml) for 60 min. We then stained the macrophages with FITC-anti-CD11b/c and fixed them with 1%PFA prior to immunofluorescent microscopy. In Figure 2, the Texas Red filter shows pHrodo-SE-labeled lymphocytes, and the FITC filter (green) shows FITC-CD11b/c-labeled macrophages (Fig. 2, two top left panels). A merged image of the same area shows that weakly fluorescent lymphocytes are free floating or adhered to macrophages, while engulfed lymphocytes shine bright red (single color) or yellow (merged image) (Fig. 2, top right and bottom panels). These results demonstrate that apoptotic lymphocytes can be labeled with pHrodo, which is stable after fixation with 1%PFA and responsive to pH changes. Furthermore, we have shown that phagocytized pHrodo-SE-labeled lymphocytes increase their fluorescent intensity, which could be verified by fluorescent microscopy.
Figure 1.
pHrodo-SE labeling of apoptotic cells and responsiveness to changes in pH. (a) Annexin V staining of non-treated (non-apoptotic) and dexamethasone-treated (apoptotic, 0.1 μM for 16h) rat thymocytes, as analyzed by flow cytometry. (b) Dose-dependent pHrodo-SE staining of apoptotic cells. 107 apoptotic thymocytes were stained with 0.01-1 μg/ml pHrodo-SE, as analyzed by flow cytometry. (c) Responsiveness of pHrodo-SE-labeled apoptotic thymocytes to changes in pH. pHrodo-SE-labeled cells (0.01 μg/ml) were exposed to a pH of 7.4, 4.0 and 3.0 by adding HCl to the cell suspension, as analyzed by flow cytometry. Mean fluorescent intensity (MFI) is shown next to FACS plot.
Figure 2.
Fluorescent intensity of pHrodo-SE-labeled apoptotic thymocytes increases after engulfment by macrophages. Splenic macrophages were labeled with FITC-anti-CD11b/c (OX42) and thymocytes with pHrodo-SE (0.02 μg/ml). Cells were coincubated for 60 min, collected and fixed with 1%PFA prior to fluorescent microscopy. Top three panels show lymphocytes in red (pHrodo-SE+), macrophages in green (CD11b/c) and a merged image, of the same area to the right. Boxes in the merged image indicate the 10× magnification shown below. Arrowheads indicate free floating and attached apoptotic thymocytes and arrows indicate engulfed apoptotic cells. Magnification: 400×.
pHrodo-SE does not affect on phagocytosis of apoptotic cells: comparison of the engulfment of non-apoptotic and apoptotic cells
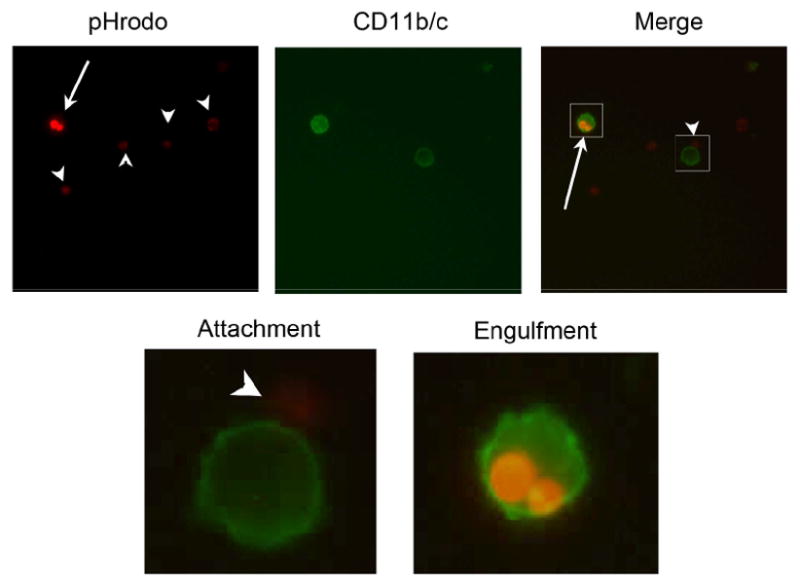
To determine the effect of pHrodo-SE on phagocytosis itself, we performed a phagocytosis assay with pHrodo-SE-labeled non-apoptotic (live) thymocytes (isolated from normal rat and untreated) or pHrodo-SE-labeled apoptotic thymocytes (isolated from normal rats and treated with 0.1μM dexamethasone for 16h at 37°C). We incubated rat peritoneal macrophages with pHrodo-SE-labeled non-apoptotic or apoptotic cells for 60 min and stained the macrophages with FITC-anti-CD11b/c. After fixation with 1% PFA, cells were washed collected and analyzed by flow cytometry as described before. As shown in Figures 3, pHrodo-SE-labeled non-apoptotic cells were engulfed to a significantly lower degree than similar labeled apoptotic cells. We detected 5-10% of apoptotic cells in a normal healthy thymus without any dexamethasone treatment (Fig. 1a), possibly explaining the low grade phagocytosis of non-apoptotic (live) cells in our assay. These results indicate that pHrodo-SE has very minimal, hence negligible, effects on apoptotic cell phagocytosis.
Figure 3.
Effect of pHrodo-SE on phagocytosis. Thymocytes were collected from normal rats and incubated with (apoptotic) or without (non-apoptotic) 0.1μM of dexamethasone for 16h. Cells were labeled with pHrodo-SE, cocultured with peritoneal macrophages for 60min, then stained with FITC-anti-CD11b/c and analyzed by flow cytometry immediately. Representative phagocytosis result shown of CD11b/c and pHrodo-SE (gated on CD11b/c+) of (a) non-apoptotic thymocytes, and (b) apoptotic thymocytes. (c) Average percent phagocytosis index ± SEM of CD11b/c+pHrodo+ cells. (d) Average mean fluorescent intensity (MFI) ± SEM of CD11b/c+pHrodo+ cells. *P<0.05 vs. non-apoptotic, Student's t-test, n=3.
Validation of methodology using MFGE8-deficient splenic macrophages
To show that this methodology is useful in determining the phagocytic capability of macrophages, we used splenic macrophages from MFGE8-/- mice that are known to be deficient in phagocytosing apoptotic cells. We performed a phagocytosis assay by staining autologous apoptotic lymphocytes with 0.02 μg/ml pHrodo-SE and adding these to the isolated macrophages for increasing durations ranging from 5 min to 3 h. After the incubation we washed and labeled the macrophages with APC-anti-CD11b mAb and performed flow cytometric analysis. The cells were gated on CD11b+ macrophages and analyzed against the pHrodo-SE staining. As shown in Figure 4, increasing incubation time lead to an increase in phagocytic macrophage percentage (Figs. 4a-b), reaching a plateau of 30.4±1.2% with a T1/2 of 64.5 min. To determine whether recombinant murine MFGE8 (rmMFGE8) could at least in part recover the deficiency in apoptotic cell phagocytosis, we preincubated the lymphocytes with 0.5 μg/ml rmMFGE8 for 30 min prior to the phagocytosis assay. In this case the same macrophages were able to phagocytize apoptotic cells at a higher rate (43.5±2.9%) and at a higher speed (T1/2 = 40.8 min), demonstrating that the pHrodo-staining method is useful to quantify the opsonization effect of MFGE8, required for sufficient clearance of apoptotic cells by splenic macrophages (Figs. 4a-b). Two-way ANOVA showed that the treatment with rmMFGE8 resulted in a significant difference in phagocytosis response. The MFI is an indication of the amount of engulfed pHrodo-SE-labeled lymphocytes although no specificities concerning the number of engulfed cells can be given. Similarly, the MFI was initially higher in CD11b+pHrodo-SE+ cells after the opsonization of apoptotic cells with rmMFGE8, indicating that not only more macrophages phagocytosed but also individual macrophages phagocytosed more efficiently after rmMFGE8 incubation (Fig. 4c).
Figure 4.
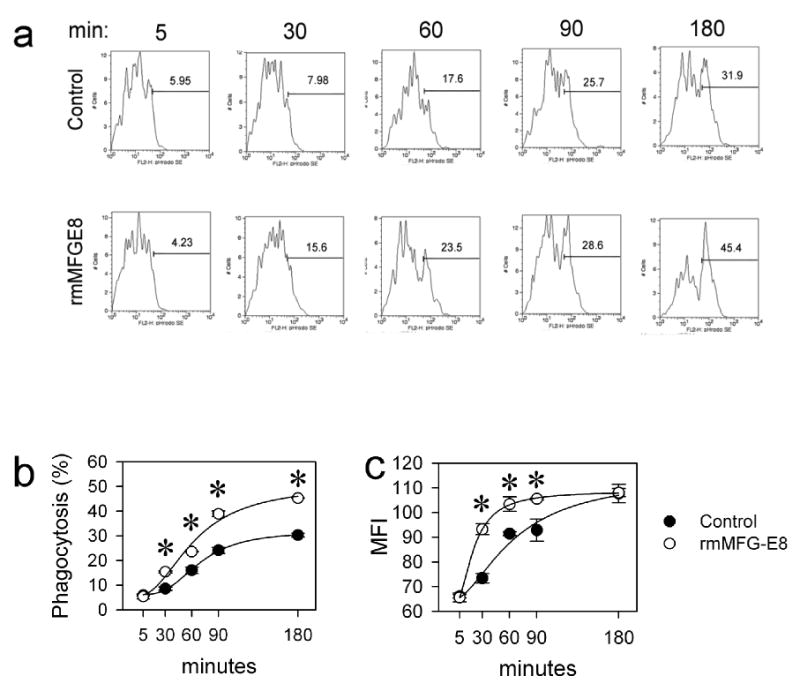
Verification of deficient phagocytosis of apoptotic cells by MFGE8-deficient splenic macrophages. (a) Representative FACS histograms. MFGE8-deficient splenic CD11b+ macrophages were incubated with autologous pHrodo-labeled thymocytes (0.02 μg/ml*106 cells) for 5 to 180 minutes and analyzed by flow cytometry in 3days. (b) Line graph showing means ± SEM (percent phagocytosing macrophages) of triplicates of a representative experiment. *P<0.05 vs. Control, two-way ANOVA and Student Newman Keuls' test, n=3. (c) Average mean fluorescent intensity (MFI) and SEM of CD11b+pHrodo-SE+ cells in one representative experiment performed in triplicate. This represents an estimate for the amount of phagocytized cells by the macrophages. *P<0.05 vs. Control, two-way ANOVA and Student Newman Keuls' test, n=3.
MFGE8-deficient peritoneal macrophages compensate for their delay in apoptotic cell phagocytosis
We were also interested in the responsiveness of resident peritoneal macrophages isolated from MFGE8-/- mice and used the same approach as in splenic macrophages for the phagocytosis assay. To our surprise, we found a different pattern. While we found a similar (dis-)ability to phagocytose apoptotic cells in the peritoneal MFGE8-/- macrophages, the addition of rmMFGE8 merely accelerated phagocytosis without changing the rate at the end of 180 min (Fig. 5). Two way ANOVA revealed that there is an interaction and a time-dependent difference (P<0.001) in rmMFG-E8-mediated phagocytosis (P<0.001) after 60 min. While at 30 min phagocytosis with or without rmMFG-E8 was virtually the same, at 60 minutes the phagocytosis index increased by 39% in the PBS treated cells and by 95% in rmMFG-E8 treated cells. This effect appears to be minimal especially as the untreated macrophages eventually catch up with the rmMFG-E8 treated cells. However, a delay in time needed to clear apoptotic cells by over 50% may be sufficient to lead to the accumulation of apoptotic cells over time under disease conditions. After 90-180 min, the peritoneal macrophages were able to compensate for their insufficient phagocytosis and there was virtually no difference in phagocytic capacity (percent phagocytosis) or efficiency (MFI) after rmMFGE8-treatment (Fig. 5)
Figure 5.
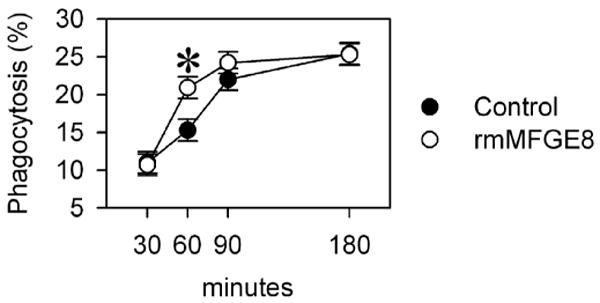
MFGE8-deficient resident peritoneal macrophages compensate for a delay in apoptotic cell clearance. MFGE8-deficient peritoneal CD11b+ macrophages were incubated with autologous pHrodo-SE-labeled thymocytes (0.02 μg/ml*106 cells) for 5 to 180 minutes and analyzed by flow cytometry. Means ± SEM (percent phagocytosing macrophages) of triplicates of a representative experiment. *P<0.05 vs. Control, two-way ANOVA and Student Newman Keuls' test, n=3.
Discussion and Conclusion
Phagocytosis of microorganisms and “altered self” (e.g., apoptotic cells) is important in tissue remodeling and embryogenesis, host-defense and innate immune response, and serves as a nutrient source in unicellular organisms and is required for antigen presentation in mammals. The act of phagocytosis is a sequence of events. It involves the engagement with the object, activation of intracellular signaling pathways, cytoskeleton rearrangement, and internalization. Phagocytized material eventually ends up in phagosomes that fuse with endosomes and lysosomes leading to hydrolytic digestion of their contents. Some microbes have developed mechanisms to avoid their digestion by modifying phagosome maturation, so that this process is also dependent on the cargo it carries (Hornef et al., 2002). As a phylogenetically old phenomenon, phagocytosis has many redundant pathways, which is one of many reasons why it is so difficult to study. Most investigators interested in phagocytosis focus on the study of cell biology, microscopy and developmental biology and many discoveries were made using receptor expression techniques. The receptors found to be required for the cell surface recognition of pathogens and apoptotic cells involve scavenger receptors (e.g. CD14 and CD36), complement-like opsonins (e.g., C3 and α2-macroglobulin), multiple EGF-like domains (e.g., MEGF10 and CD91), and receptors of the immunoglobulin superfamily.
Using pHrodo-SE as a tag for apoptotic cells to be engulfed by macrophages serves as a novel method for apoptotic cell phagocytosis in vitro and ex vivo (Fig. 6). This approach eliminates the possibility of detection of apoptotic cell binding to the surface of the phagocytes and represents an assay equally valid as the use of CAD-/- thymocytes and TUNEL staining (Hanayama et al., 2002). Both methodologies use the fact that engulfed material will be biochemically altered after the incorporation into the phagolysosome within the macrophage. In Hanayama's approach (Hanayama et al., 2002), apoptotic cell DNA is degraded by the phagocyte DNAses while our method uses the pH changes in the endolysosomal microenvironment as an indication for engulfment. The advantage of the present methodology lies in the fact that no knockout animals are needed and this method can be replicated without major difficulties. While this approach has been successfully shown to work in pHrodo-SE-labeled bacteria and latex beads, we show for the first time that it also applicable with apoptotic eukaryotic cells. We have also shown that pHrodo-SE labeled non-apoptotic (live) cells were engulfed by peritoneal macrophages to a significantly lower degree than apoptotic cells. The presence of approximately 5-10% of apoptotic cells in a normal healthy thymus may explain the low-grade phagocytosis in this assay. These results indicate that pHrodo-SE has very minimal effect on phagocytosis. At the same time, we have also addressed the possible limitation of this assay. MFI may have been saturated after a prolonged incubation time (e.g., 180min, Fig. 4c). This could be due to the digestion of engulfed apoptotic cells in the phagosome or the decay of the dye.
Figure 6.
Flow chart of pHrodo-SE-staining and phagocytosis assay protocol.
We have documented the validity of this method by replicating previous results and demonstrating that MFGE8-deficient macrophages show a delayed and insufficient engulfment of apoptotic cells, which can be recovered by simply adding exogenous recombinant MFGE8 protein. Using this methodology, we also discovered that MFGE8-/- peritoneal macrophages show a time-delay in apoptotic cell phagocytosis compared to the rmMFGE8-supplemented group, but that these cells eventually catch up and compensate for their deficiency. MFGE8 is one of several molecules that mediate the binding of apoptotic cells to phagocytes. Other molecules include C3b, oxidized low-density lipoprotein (oxLDL), growth-arrest-specific 6 (Gas6), CD14, T-cell immunoglobulin domain and mucin domain 4 (Tim4), etc. (Savill et al., 2002; Ravichandran and Lorenz, 2007). The deficiency of MFGE8 significantly decreases the phagocytic activity of macrophages, but no complete abrogation. Hence, macrophages are still able to phagocytose apoptotic cells via MFGE8-independent pathways that may compensate for the deficient MFGE8 and the suppressed phagocytic activity in our MFGE8 deficient model of peritoneal macrophages we used. The mechanism of these alternative pathways may involve other opsonizing molecules or complement factors and is of great interest, and this new method will serve for further investigations.
Acknowledgments
We sincerely thank Dr. Asha Varghese for critical reading of the manuscript.
Supported by NIH grants R01 GM057468, R01 GM053008, and R01 AG028352 (P. Wang).
Abbreviations in this paper
- pHrodo-SE
pHrodo succinimidyl ester
- MFGE8
milk fat globule EGF-factor 8
- WT
wild-type
- HBSS
Hank's Balanced Salt Solution
- PBS
Phosphate Buffered Saline
- FBS
Fetal Bovine Serum
- PI
propidium iodine
- MFI
mean fluorescence intensity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Leseche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation. 2007;115:2168–77. doi: 10.1161/CIRCULATIONAHA.106.662080. [DOI] [PubMed] [Google Scholar]
- Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med. 2004;200:459–67. doi: 10.1084/jem.20040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–8. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- Bu HF, Zuo XL, Wang X, Ensslin MA, Koti V, Hsueh W, Raymond AS, Shur BD, Tan XD. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117:3673–83. doi: 10.1172/JCI31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Hornef MW, Wick MJ, Rhen M, Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol. 2002;3:1033–40. doi: 10.1038/ni1102-1033. [DOI] [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invitrogen. pHrodo™ indicators—enlightening phagocytosis. 2008 http://www.invitrogen.com/content.cfm?pageid=12009.
- Klemparskaya NN. 100 years of the phagocytosis doctrine (Ilya Ilyich Mechnikov) J Hyg Epidemiol Microbiol Immunol. 1983;27:249–52. [PubMed] [Google Scholar]
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–74. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Schnitzler N, Haase G, Podbielski A, Lutticken R, Schweizer KG. A co-stimulatory signal through ICAM-beta2 integrin-binding potentiates neutrophil phagocytosis. Nat Med. 1999;5:231–5. doi: 10.1038/5597. [DOI] [PubMed] [Google Scholar]
- Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol. 2008;8:131–41. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- Watts C. Immunology. The bell tolls for phagosome maturation. Science. 2004;304:976–7. doi: 10.1126/science.1098573. [DOI] [PubMed] [Google Scholar]