Abstract
The present study was designed to dissociate the roles of dorsal CA3, dorsal CA1, ventral CA3, and ventral CA1 in contextual and auditory-cued classical fear conditioning. Rats received excitotoxic lesions of dorsal CA3, dorsal CA1, ventral CA3, or ventral CA3 prior to acquisition of classical fear conditioning. Dorsal CA3 and dorsal CA1, but not ventral CA3 or ventral CA1, lesions caused a deficit for the acquisition of contextual fear. Dorsal CA1, ventral CA3, and ventral CA1, but not dorsal CA3, lesions caused deficits for the retrieval/expression of contextual fear when tested either 24 or 48 h after encoding. Ventral CA3, but not dorsal CA3, dorsal CA1, or ventral CA1, lesions caused a deficit for retrieval of auditory-cued fear when tested either 24 or 48 h after encoding. The data suggest that dorsal CA3 mediates encoding of contextual fear, whereas ventral CA3 mediates retrieval of contextual fear. The data also suggest that dorsal CA1 mediates encoding and retrieval of contextual fear, whereas ventral CA1 mediates only the retrieval of contextual fear.
Keywords: Learning and Memory, Animal Cognition, Context, Classical Conditioning, Fear Learning, CA3, CA1, Encoding and Retrieval, Dorsal Hippocampus, Ventral Hippocampus
Introduction
Classical fear conditioning has been used to assess fear-related learning in the amygdala and hippocampus (Phillips & LeDoux, 1992). In recent years, there has been an increased interest in the role of the anterior and posterior hippocampus (in humans) or ventral and dorsal hippocampus (in rats) for encoding and retrieval processes (Knight, Smith, Cheng, Stein, & Helmstetter, 2004; Lepage, Habib, & Tulving 1998; Maren & Holt, 2004; Rogers, Hunsaker, & Kesner, 2006; Rudy & Matus-Amat, 2005; Zeineh, Engel, Thompson, & Bookmeyer, 2003; cf. Greicius et al., 2003; Yoon and Otto, 2007). Lesions and inactivations within the ventral hippocampus result in deficits for retrieval of both auditory and contextually cued fear (Maren & Holt, 2004; Rogers et al., 2006; Rudy & Matus-Amat, 2005; Yoon & Otto, 2007), whereas lesions and inactivations within the dorsal hippocampus have been shown to attenuate contextual, but not auditory-cued, fear (Lee & Kesner, 2004; Phillips & LeDoux, 1992; Quinn & Fanselow, 2006; Quinn, Loya, Ma, & Fanselow, 2005; Quinn, Oomen, Morrison, & Fanselow, 2002; Rogers et al., 2006; Yoon & Otto, 2007).
In addition to overall hippocampal involvement in fear conditioning, subregional analyses are becoming increasingly prominent and have focused on the role of CA1 for the encoding and retrieval of trace conditioning (eye-blink (fear) conditioning; McEchron, Tseng, & Disterhoft, 2003; Weible, O'Reilly, Weiss, & Disterhoft, 2006). To date, studies have focused on dorsal hippocampal subregions (Lee & Kesner, 2004; McEchron et al., 2003), or on CA1 and CA3 as a whole via NMDAR1 receptor knockout (Huerta, Sun, Wilson, & Tonegawa, 2000; Kashimoto, Nakazawa, Tonegawa, Kirino, & Kano, 2006). Thus far, only a single study has assessed differential effects of ventral CA1 and dorsal CA1 lesions for fear conditioning (trace fear conditioning; Rogers et al., 2006). In addition to dorsal CA1, Lee and Kesner (2004) have shown that all dorsal hippocampal subregions contribute to encoding of contextual information during delay fear conditioning. They also have shown that both dorsal CA1 and the dorsal dentate gyrus, but not dorsal CA3, participate in retrieval of contextual fear.
Lee and Kesner (2004) have shown that dorsal CA3 lesions result in an encoding deficit for delay fear conditioning without a concomitant retrieval deficit. It has also been shown that CA1 can be dissociated across the dorsal-ventral axis for retrieval of contextual and auditory-trace fear conditioning, with ventral CA1 lesions resulting in greater deficits than dorsal CA1 for retrieval of contextual and auditory-trace cued fear (Rogers et al., 2006). The present experiment was designed to evaluate the roles of dorsal CA3, dorsal CA1, ventral CA3, and ventral CA1 for encoding and retrieval of contextual and auditory-cued fear by replicating the experimental methods of Lee and Kesner (2004) using dorsal CA3, dorsal CA1, ventral CA3, and ventral CA1 lesioned animals. The data reveal a double dissociation of dorsal CA3 and ventral CA3 for contextual fear encoding and retrieval wherein dorsal CA3 subserves encoding and ventral CA3 subserves retrieval of contextual fear. The data reveal that dorsal CA1 is involved in both encoding and retrieval of contextual fear, whereas ventral CA1 is recruited during retention of contextual fear.
Materials and Methods
Animals
Thirty-one male Long-Evans rats (Simonsen Laboratories, Inc., Gilroy, CA), approximately six months of age and weighing 350-400 g at the start of experimentation served as subjects. The rats were housed individually in plastic tubs located in a colony with a 12 h light-dark cycle. All testing was conducted during the light portion the cycle. All rats were free-fed and had ad libitum access to water. All experiments were conducted according to the University of Utah Institutional Animal Care and Use Committee (IACUC) guidelines and conformed to all AAALAC protocols. An IACUC veterinarian evaluated the health of animals weekly. All animals had previously been run on an unrelated temporal ordering paradigm prior to the present classical conditioning experiment.
Experimental Apparatus
Two observation chambers were used during three consecutive days of testing. The first chamber was used for encoding of contextual and auditory-cued fear and for the contextual fear retention test. This chamber (28 × 21 × 22 cm; Coulbourn Instruments; Allentown, PA) consisted of two clear transparent Plexiglas walls (rear wall and front door) and two aluminum sidewalls. The chamber floor was made up of 18 steel rods connected to a precision-regulated shock delivery apparatus (Coulbourn Instruments) used to deliver an electric foot-shock stimulus. A speaker was inserted into one of the aluminum walls of the conditioning chamber and a commercial software package (Graphic State v1.013.00; Coulbourn Instruments) controlled the presentation of all stimuli. The chamber was located in an isolated room illuminated by fluorescent and halogen lamps. Numerous visual cues such as toys and posters were located around the conditioning chamber to provide contextual cues and remained undisturbed throughout experimentation. A video camera recorded the animal's behavior, which was viewed and scored by an experimenter in an adjacent room. The camera was camouflaged from the animal's view so as to not act as a retrieval cue during subsequent tests. The chamber was cleaned immediately before conditioning and testing using a weakened cleaning solution.
A second observation chamber tested retention of auditory-cued fear. This chamber (32 × 32 × 32 cm) was constructed entirely of transparent Plexiglas. A speaker was attached to an opening (2.5 cm diameter) made in one of the walls near the floor of the chamber to deliver the auditory cue. The chamber was located in the same room but was surrounded by completely different visual cues, as well as a white curtain to provide a novel visual environment. It was cleaned with the same cleaning solution as the conditioning box immediately after each test, but was cleaned again with unscented water immediately prior to testing to dilute olfactory cues.
Behavioral Methods
Encoding—Day 1
Rats were placed in the conditioning chamber for 2 m prior to the first auditory stimulus as a contextual pre-exposure period to allow animals to efficiently encode the context (cf. Quinn & Fanselow, 2006; Rudy & O'Reilly, 2001) prior to conditioning. After the pre-exposure period, rats received 10 auditory-shock pairings separated by 74 s. An auditory stimulus (10 s duration, 2 kHz, 85-dB) was presented through a speaker to initiate each trial. An electric foot-shock (2 s duration, 0.75 mA) was delivered through 18 floor-rods coterminal with the auditory stimulus (e.g. the last 2 s of the auditory stimulus were in fact the tone+shock pairing). A 74 s intertrial interval (ITI) separated each successive tone+shock pairing. After the ten tone + shock pairings and subsequent ITIs, rats remained in the chamber for an additional 2 m without auditory stimulus or shock. A freezing response (e.g. absence of movement except respiratory; cf. D. Blanchard & Blanchard, 1972; R. Blanchard & Blanchard, 1969) was measured by an observer who scored freezing behavior every 8 s during the pre-exposure period and ITI (during the ITI the 2 s immediately following the shock were discarded (due to the animal still reacting to the aversive shock) and freezing during the subsequent 72 s were recorded) and every 4 s during the tone stimulus; resulting in two auditory observations and nine ITI observations for each trial. ITI freezing was used to assess contextually cued fear. The first 9 trials of overall freezing during each trial (e.g. tone and ITI freezing together) were blocked into three 3-trial blocks for analysis of overall acquisition. Tone and ITI freezing were also analyzed individually in a single 10-trial block to evaluate whether any group differences in freezing were due to differential freezing to the tone or during the ITI.
Contextually cued fear retention test—Day 2 or 3
Each rat was tested for retrieval of contextually cued fear either 24 or 48 h after acquisition (half received the contextual fear retention test on day two and half on day three, counterbalanced with auditory-cued fear retention tests). The rat was placed in the encoding chamber for 8 m in the absence of the auditory cue and aversive stimulus (shock) for eight continuous 1 m trials. Freezing behavior was measured every 8 s. Due to extinction in the control group, only the first 4 m of testing were used for statistical analysis.
Auditory-cued fear retention test—Day 2 or 3
Each rat was tested for retrieval of auditory-cued fear either 24 or 48 h after acquisition (half received the auditory-cue retention test on day two and half on day three, counterbalanced with contextual fear retention tests). The rat was placed in a different chamber from that used during encoding in the presence of the auditory stimulus for eight continuous 1 m trials. Freezing behavior was measured in 8 s intervals. Due to extinction in control animals, only the first 4 m of testing were used for statistical analysis.
Surgical Methods
Prior to conditioning, animals were randomly separated into five groups, ventral CA3 (n=9), ventral CA1 (n=5), dorsal CA3 (n=5), dorsal CA1 (n=6) and vehicle control (n=6). All animals were anesthetized with isoflurane (2-4% (vol/vol) at 1.5-2 L/min) and secured in a stereotaxic frame (David Kopf Instruments; Tujunga, CA) and diazepam (1.5 mL/kg i.p.) was given to animals receiving ventral CA3 and ventral CA1 lesions. At each injection site ibotenic acid (6 mg/mL PBS; Sigma-Aldrich; St. Louis, MO) was infused using a 26-gauge injection cannula (Plastics One; Roanoke, VA) connected to a 10 μL syringe (Hamilton; Reno, NV) with sterile plastic tubing (Cole Parmer; Vernon Hills, IL). The syringe was connected to a micro-infusion pump (Cole-Parmer), and the injection cannula was left in place for one minute after neurotoxin infusion prior to removal. Burr holes were drilled above ventral CA3 at the following coordinates: 1) posterior 4.55 mm from bregma, lateral 3.6 mm from midline, and 7.5 mm ventral to the dura mater (0.05 μL ibotenic acid infused). 2) Posterior 4.55 mm, lateral 4.65 mm, and 4.6 mm ventral (0.05 μL). 3) Posterior 4.9 mm, lateral 3 mm, and 7.1 mm ventral (0.05 μL). 4) Posterior 4.9 mm, lateral 4.8 mm, and 7.1, 5.6, and 5.1 mm ventral (0.05 μL into each site). 5) Posterior 5.25 mm, lateral 4.7 mm, and 5.8 mm ventral (0.05 μL). And 6) posterior 5.25 mm, lateral 5.2 mm, and 6.6 mm ventral (0.05 μL) (injections were always made from the ventral-most to the dorsal-most injection site at the same AP and ML coordinate). Ventral CA1 lesions (n = 5) were also made using ibotenic acid, infused following the same procedures used by Rogers et al. (2006), infusions were made into three sites within the ventral hippocampus located 1) 5.3 mm posterior to bregma, 3.0 mm lateral to the midline, 2.8 mm ventral to dura (0.1 μL infused); 2) 5.3 mm posterior to bregma, 5.2 mm lateral to the midline, 4.0 mm ventral to dura (0.1 μL); 3) 5.3 mm posterior to bregma, 5.8 mm lateral to the midline, 6.2 mm ventral to dura (0.15 μL). For dorsal CA3 surgeries, the drug infusion protocol was identical to Lee and Kesner (2004). Burr holes were drilled at the following coordinates: 1) Posterior 2.8 mm, lateral 3.0 mm, and ventral 3.2 mm (0.05 μL infused). 2) 3.3 mm posterior, 3.4 mm lateral, and 3.2 mm ventral (0.08 μL), and 3) 4.1 mm posterior, 4.2 mm lateral, and 3.3 mm ventral (0.15 μL). Dorsal CA1 lesions were made after the protocol of Lee and Kesner (2004) and Rogers et al. (2006), 1) 3.6 mm posterior to bregma, 1.0 mm lateral to the midline, 2.4 mm ventral to dura (0.1 μL of infused); 2) 3.6 mm posterior to bregma, 2.0 mm lateral to the midline, 2.1 mm ventral to dura (0.1 μL); 3) 3.6 mm posterior to bregma, 3.0 mm lateral to the midline, 2.3 mm ventral to dura (0.15 μL). For vehicle control surgeries of ventral CA3 (all controls in the present study), PBS was injected into each site instead of ibotenic acid using the same parameters as the ventral CA3 lesion. After infusions, each rat was sutured and received medical air for five minutes without isoflurane prior to replacement in their home cage. Animals that received ventral hippocampal subregional lesions and received diazepam reacted to a tail pinch after approximately 2 h and regained movement and began eating approximately 6 h after surgical manipulation. Animals receiving dorsal hippocampal subregional lesions and did not receive diazepam regained movement at approximately 20-30 m post-surgery. No animals exhibited epileptiform activity during or after surgery. Animals received 150 g crushed food mixed with 20-30 g sucrose and were provided acetaminophen (200 mg/100 mL water) in their drinking water as an analgesic for three days after surgery.
Histological Methods
After experimentation, all animals were deeply anesthetized with either pentobarbital or chloral hydrate (both >100 mg/kg) and transcardially perfused with PBS and 10% (wt/vol) formalin. The brains were placed in 30% (wt/vol) sucrose in 10% formalin at 4 degrees Celsius for 72 h prior to sectioning and nissl staining with cresyl violet. All sections were photographed and imported into ImageJ v1.35j (National Institute of Health; Bethesda, MD) to quantify the lesions. In short, the extent of the region of interest was traced using the freehand selection tool and the size of the traced area was calculated (all region of interest extents were taken from the Paxinos and Watson (1997) atlas). Portions of the region of interest remaining after surgery were traced the same way and the area was calculated. Damage to the other subregions (e.g. dorsal and ventral dentate gyrus, CA3, and/or CA1) was also calculated to test for nonspecific damage. The amount of remaining tissue and total region of interest areas were used to calculate percent damage. A similar procedure was used in Lee and Kesner (2004). Extra care was taken to verify that there was never any amygdala damage after dorsal or ventral hippocampal subregional lesions.
Statistical Methods
Freezing scores during encoding and retrieval tests were transformed to percent of observations spent freezing divided by total number of observations and were blocked prior to statistical analysis. For overall acquisition, the first nine trials were blocked into three-block trials for analysis, after Lee and Kesner (2004). This was to evaluate overall acquisition differences between groups as a function of trial block. The acquisition data were then separated into tone and ITI phases of each trial and blocked separately into a single 10-trial block. This procedure and analysis was performed to determine whether any observed acquisition deficits were due to impaired freezing to the tone stimulus or impaired freezing during the ITI. This analysis is important since it has been shown that amygdala lesions, but not hippocampal lesions, disrupt auditory-cued fear, but both amygdala and hippocampal lesions disrupt contextual fear condioning (Phillips & LeDoux, 1992). For retrieval tests, the first four minutes of each test was blocked into a four-trial block for analysis. A two-way repeated measures ANOVA with groups as the between factor and blocks of three trials as the within factor was employed for testing group differences during the overall encoding of delay fear conditioning. One-way ANOVA with groups as the between factor were used to analyze encoding and retention of contextual and auditory-cued fear between groups. All main effects were considered statistically significant at p<0.05. Fisher's least significant difference (LSD) post hoc paired comparison tests were performed upon all significant effects of the post hoc paired comparison area reported as p<0.05, <0.01, or <0.001 (and p>0.1 when appropriate). Overall acquisition of fear conditioning for each group is presented in three blocks of trials, plotted as means +/- standard error (SEM). The tenth trial was not included in this overall analysis since just the first 9 trials were blocked into 3-trial blocks. Contextual and auditory-cued fear encoding were collapsed into a single 10-trial block for analysis. Retention tests are presented as mean percent freezing +/- SEM over single 4 m blocks. The statistical toolbox on MATLAB (v6.5 R13; The MathWorks, Inc.; Natik, MA) was used to analyze all experimental data.
Results
Histology
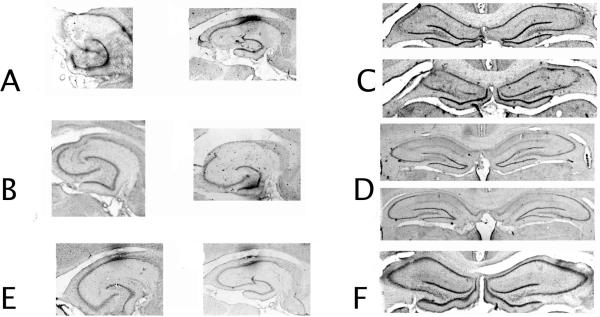
As shown in Figure 1A, ventral CA3 lesions were selective to ventral CA3, sparing ventral CA1 and the ventral dentate gyrus. There appears to be no extrahippocampal cortical damage to perirhinal or entorhinal cortices, nor was there damage to the amygdala. For all photomicrographs in Figure 1, optical artifacts outside the region of interest were removed for aesthetic reasons. This is a similar pattern of damage to that seen after previous hippocampus subregional lesions (Lee & Kesner, 2004; Rogers et al., 2006; cf. Jerman, Kesner, Lee, & Berman, 2005). Data from one animal from the ventral CA3 group had to be excluded from statistical analysis since damage spread to ventral dentate gyrus (~30%). Damage in the remaining animals was as follows: ventral CA3 lesions (Figure 1A) resulted in (mean +/- SEM) 75 +/- 2.5% damage to the ventral CA3a,b pyramidal cell layer and 60 +/- 5% damage to CA3c with 5 +/- 1% damage to ventral dentate gyrus (most pronounced in more ventral sections) and ventral CA1 (most pronounced in dorsal sections near the intermediate portion of the hippocampus). For dorsal CA3 lesions (Figure 1B), there was 85 +/- 10.1% damage to dorsal CA3a,b, and 50 +/- 11% damage to CA3c with 2 +/- 0.5% damage to dorsal dentate gyrus and 5 +/- 1.2% damage to dorsal CA2, and no damage to CA1 both dorsal dentate gyrus and dorsal CA2 damage were located more in rostral than caudal sections. There was no parietal, perirhinal, or postrhinal cortex damage observed, nor was there amygdala damage. Ventral CA1 lesioned animals (Figure 1C) had 72.4 +/- 8.3% damage to the ventral CA1 pyramidal cell layer with 1.5 +/- 0.25% damage to the ventral dentate gyrus and 12.5 +/- 4.6 % damage to the ventral CA3a,b pyramidal cell layer and no damage to ventral CA3c. In no cases was extrahippocampal damage to the parietal, entorhinal, postrhinal, or perirhinal cortices observed, nor was there amygdala damage observed. Dorsal CA1 lesioned animals (Figure 1D) had (mean +/- standard error) 84 +/- 5.2% damage to the pyramidal cell layers of CA1 with 4.5 +/- 0.9% damage to the underlying medial blade of the dentate gyrus and 8.8 +/- 2.1% damage to CA3a,b pyramids and no damage to CA3c pyramids. There was no parietal, perirhinal, or postrhinal cortex damage observed, nor was there amygdala damage. Control lesioned animals (Figure 1E, F) had no damage beyond that caused by the cannula track. Damage was never observed to parietal, entorhinal, postrhinal, or perirhinal cortices, or the amygdala.
Figure 1. Histology.
A. Ventral CA3 lesion photomicrographs. B. Ventral CA1 lesion photomicrographs. C. Dorsal CA3 lesion photomicrographs. D. Dorsal CA1 lesion photomicrographs. E. Ventral hippocampal control lesion photomicrograph. F. Dorsal hippocampal control lesion photomicrograph. In the ventral hippocampal sections in A,B,E optical artifacts outside the lesion area have been removed.
Auditory and Contextual Fear Conditioning
Acquisition
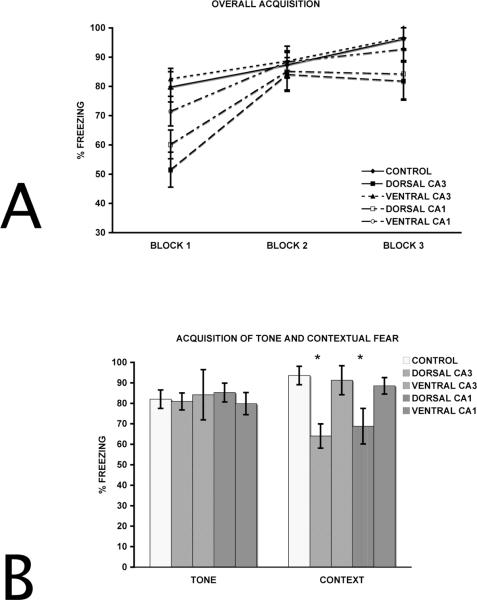
A two-way repeated measures ANOVA with groups (control, dorsal CA3, dorsal CA1, ventral CA3, ventral CA1) as the between factor and blocks of trials as the within factor was performed to analyze acquisition organized into blocks of three auditory stimulus/shock pairings (the tenth trial was removed from analysis). Figure 2A shows overall acquisition for control, dorsal CA3, ventral CA3 animals, dorsal CA1, and ventral CA1 lesioned animals. For the overall encoding of delay fear conditioning, there was an effect of groups (F (4,24) = 11.43, p<0.0001), an effect of block (F (2,48) =78.14, p<0.001), and a group x block interaction (F (8, 48) = 3.84, p=0.001). Subsequent Fisher's LSD post hoc paired comparison tests on the group effect indicated that the dorsal CA3 and dorsal CA1 groups were impaired relative to ventral CA3, ventral CA1, and control groups (p<0.01), but ventral CA3, ventral CA1, and control groups did not differ from each other (p>0.1). Dorsal CA3 lesioned animals did not differ from dorsal CA1 lesioned animals (p>0.1). The dorsal CA3 and dorsal CA1 groups showed the same encoding deficits as those reported by Lee and Kesner (2004). Further Fisher's LSD post hoc paired comparisons on the group x block interaction reveal that the dorsal CA3 and dorsal CA1 deficits relative to the other groups were present only during the first block for dorsal CA1 (p<0.05) and during the first two blocks for dorsal CA3 (ps<0.05). This suggests that, despite the initial acquisition deficit, the dorsal hippocampus subregional lesioned animals were able to acquire the conditioned fear by the end of the acquisition period.
Figure 2. Encoding of delay fear conditioning.
A. Overall acquisition of delay fear conditioning blocked into three, 3-trial blocks (only the first 9 trials were blocked). B. Encoding of tone and contextual fear blocked into single 10-trial blocks. Both dorsal CA1 and dorsal CA3, but not ventral CA1 or ventral CA3, show deficits for contextual fear encoding. * p<0.05 relative to the Control group.
To better characterize the nature of the dorsal CA3 and dorsal CA1 acquisition deficits, tone and ITI freezing were separated and collapsed into single, 10-trial blocks. Figure 2B shows overall averages for auditory-cued fear acquisition separate from contextual fear acquisition for dorsal CA3, ventral CA3, dorsal CA1 and ventral CA1 lesioned animals. The data presented in Figure 2B was analyzed to illustrate that ventral CA3 and ventral CA1 lesioned animals were able to encode both auditory and contextual information individually, but dorsal CA3 and dorsal CA1 lesioned animals could encode the auditory-cued, but not contextually cued, fear.
For encoding of the separate auditory-cued fear and contextual fear, a one-way ANOVA was used with groups as the between factor. As expected, for auditory-cued fear encoding, there was no effect for groups (F(4,28)=0.73, p=0.579). For contextual fear acquisition, there was an effect for groups (F(4,28)=27.15, p<0.0001). A subsequent Fisher's LSD post hoc paired comparison revealed that the dorsal CA3 and dorsal CA1 group were impaired relative to ventral CA3, ventral CA1, and control groups (all ps<0.01), but ventral CA3, ventral CA1, and control groups did not differ (p>0.1). Dorsal CA3 lesioned animals also froze less than dorsal CA1 lesioned animals (p<0.05). These data suggest that the dorsal CA3 and dorsal CA1 acquisition deficits were mediated by an impairment in contextual, but not auditory-cued, fear encoding.
Retention
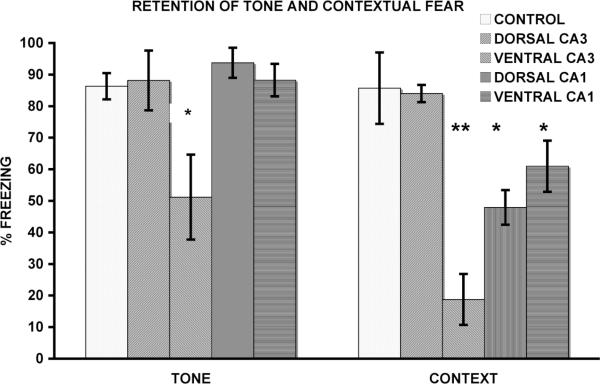
A one-way ANOVA was used to analyze the retention tests. Figure 3 shows the results of auditory-cued fear retention tests for dorsal CA3, ventral CA3, dorsal CA1 and ventral CA1 lesioned animals. Retention test data from animals tested on day two did not differ from data collected from animals tested on day three and were combined for these analyses. For the retention of auditory-cued fear, there was an effect for group (F(4,28)=29.45, p<0.0001). The ventral CA3 group froze less than the dorsal CA3, dorsal CA1, ventral CA1, and control groups (p<0.05). The dorsal CA3, dorsal CA1, and ventral CA1 lesions groups did not differ from each other or controls (ps>0.1). Contextually cued fear retention test results are shown in Figure 3. There was an effect for group (F(4,28)=8.3, p<0.0001). Fisher's LSD post hoc paired comparison test indicated that the dorsal CA1, ventral CA3, and ventral CA1 groups froze less than both control and dorsal CA3 groups (p<0.01), which did not differ (p>0.1). Dorsal CA1, ventral CA3, and ventral CA1 lesion groups did not differ (ps>0.1). These results suggest that although ventral CA3 and ventral CA1 lesioned rats were capable of encoding both contextual and auditory-cued fear, they were unable to retrieve previously encoded contextual fear during subsequent tests of retention. Ventral CA3 lesioned animals were unable to retrieve previously encoded auditory-cued fear. The dorsal CA1 lesioned animals showed similar deficits to those reported by Lee and Kesner (2004).
Figure 3. Retention of delay fear conditioning.
Tone and Contextual fear retention test data grouped into single 4 m blocks. Note that for both context and auditory tests the ventral CA3 lesions group shows deficits, whereas the dorsal CA3 lesion group does not. Note that for the context test, both the dorsal and ventral CA1 lesions groups show deficits. *p<0.01 and **p<0.001 relative to the Control group.
All effects observed in the present study are presented in Table 1. Notice that no ventral hippocampal subregions participate in acquisition or encoding of delay fear conditioning, but are involved for contextual retention. The table shows a double dissociation between dorsal CA3 and ventral CA3, in which dorsal CA3 participates in encoding of contextual fear and ventral CA3 participates in retrieval of contextual fear. The table also shows that dorsal CA1 participates in the encoding of contextual fear and that both dorsal CA1 and ventral CA1 participate in the retrieval of contextual fear. These data suggest that the ventral hippocampal subregions do not participate in the encoding of contextual fear, but they are involved in retrieval of the previously encoded contextual fear.
Table 1.
Impairments of Acquisition and Retention of Delay Fear Conditioning after Dorsal and Ventral Hippocampal Subregional Lesions.
| Lesion Group |
Acquisition |
Retention |
||
|---|---|---|---|---|
| Tone | Context | Tone | Context | |
| Control | -- | -- | -- | -- |
| Dorsal CA3 | none | impaired | none | none |
| Ventral CA3 | none | none | impaired | impaired |
| Dorsal CA1 | none | impaired | none | impaired |
| Ventral CA1 | none | none | none | impaired |
Discussion
The present experiment evaluated the roles of dorsal and ventral hippocampal subregions for the acquisition and retrieval/expression of auditory-cued and contextual classical fear conditioning. Lee and Kesner (2004) have shown both acquisition and retrieval deficits in delay fear conditioning after dorsal hippocampal subregional lesions, using the exact parameters and apparatus as those used presently. Rogers et al. (2006) have also shown differential effects of dorsal CA1 and ventral CA1 during a trace fear conditioning paradigm. The Rogers et al. (2006) report suggested that the ventral CA1 subregion was more critically involved in fear expression than dorsal CA1 (cf. Yoon and Otto, 2007 for the same finding after full dorsal or ventral hippocampal ablations). The present experiment sought to expand upon the findings of these two reports by replicating the Lee and Kesner (2004) experiment with lesion groups similar to Rogers et al. (2006) to better characterize any encoding/retrieval dissociations along the dorsal/ventral axis of the hippocampus.
Contextual Fear Conditioning
Dorsal CA3 and dorsal CA1 lesioned animals showed a significant deficit for the encoding of contextual information during acquisition, and dorsal CA3 lesioned animals showed a greater deficit than dorsal CA1 lesioned animals. The dorsal CA3 lesioned animals did not show concomitant retrieval deficits for the same contextual fear when tested 24 h later. Dorsal CA1 lesioned animals, however, showed similar retrieval deficits as those reported by Lee and Kesner (2004). Ventral CA3 and ventral CA1 lesioned animals did not show encoding deficits for contextual information during acquisition, but did show retrieval deficits when tested 24 or 48 h later. These data suggest that dorsal hippocampal subregions are critical for the encoding of contextual information used during fear conditioning, whereas the ventral hippocampus becomes involved when the information needs to be retrieved. These results suggest that CA3 function, at least for contextual fear encoding and retrieval, can be doubly dissociated across the dorsalventral axis of the hippocampus. The data suggest that dorsal CA3 mediates encoding of contextual fear, whereas ventral CA3 mediates retrieval of contextual fear. CA1 can be dissociated with dorsal CA1 mediating both the encoding and retrieval of contextual fear, whereas ventral CA1 is recruited during retrieval.
Auditory-Cued Fear Conditioning
As has been observed in near innumerable reports, there were no acquisition deficits for auditory-cued fear after any dorsal or ventral hippocampal subregional lesions. Ventral CA3 lesioned animals did not show a deficit for the encoding of auditory-cued fear, but they did show a deficit for retrieval of the auditory-cued fear when tested 24 or 48 h later. These data suggest that the ventral CA3 may play a small role in the retrieval of auditory cued fear. No other hippocampal subregions showed any effects for auditory-cued fear encoding or retrieval.
General Discussion
The results of the present experiment provide compelling evidence that the roles of dorsal and ventral hippocampus are dissociable for auditory-cued and contextual fear encoding and retrieval (cf. Rogers et al., 2006 and Yoon & Otto, 2007 for similar dissociations during auditory-trace fear conditioning). Lee and Kesner (2004) reported that dorsal CA3 and dorsal CA1 lesioned animals were unable to properly encode contextual information during delay fear conditioning, but that the dorsal CA3 lesioned animals could retrieve contextual information during subsequent tests of retrieval, whereas dorsal CA1 animals were unable to retrieve this contextual information. These results suggest that the dorsal CA3 has a unique role for contextual fear encoding, perhaps rapidly generating a configural or conjunctive representation of the context that is sent to the amygdala or to subcortical structures along the Papez circuit, as well as to CA1 for further processing (Gray & McNaughton 1983, 2001; Rudy & O'Reilly, 2001; cf. Quinn & Fanselow, 2006). This representation is later recalled by dorsal CA1 and/or the ventral hippocampus (i.e. ventral CA3 and/or ventral CA1) using contextual cues via reciprocal interconnections between the ventral hippocampus and the amygdala (cf. Huff et al., 2006; Maren & Holt, 2004; Ottersen, 1982; van Groen & Wyss, 1990).
The results of the present experiment suggest that the dorsal-ventral axis of the rodent hippocampus is dissociable for encoding and retrieval as has been suggested for the human hippocampus (posterior-anterior axis; Lepage et al., 1998; Schacter & Wagner, 1999; Zeineh et al., 2003; cf. Greicius et al., 2003). Previous research has suggested that the rodent dorsal hippocampus subserves contextual encoding during fear conditioning, either by rapidly associating a constellation of cues with the shock, or else by forming a conjunctive representation of the context as a whole (similar to Pavlov's dynamic stereotype; cf. Pavlov, 1962; Quinn & Fanselow, 2006) and sending that representation to the amygdala to be associated with the shock (Rudy, Barrientos, & O'Reilly, 2002; Rudy & O'Reilly, 2001), although this result has come under some debate involving lesion methodology and specificity (Maren, Aharonov, & Fanselow, 1997; Maren & Fanselow, 1997; Quinn & Fanselow, 2006). Research has also suggested that the ventral hippocampus is capable of mediating retrieval of both contextual (Rogers et al., 2006; Rudy & Matus-Amat, 2005; Yoon & Otto, 2007), as well as auditory-cued fear (Maren & Holt, 2004; Rogers et al., 2006; Yoon & Otto, 2007), depending upon task parameters. It has been suggested that the ventral hippocampus is important for fear conditioning due to the pronounced reciprocal connectivity between the ventral hippocampus and the amygdala, nucleus accumbens, and septal nuclei (Canteras & Swanson, 1992; Gray & McNaughton, 1983, 2001; Maren, 1999; Ottersen, 1982; Phillips & LeDoux, 1995; Quinn & Fanselow, 2006; Riedel, Harrington, Hall, & Macphail, 1997; van Groen & Wyss, 1990).
The present data suggest that dorsal CA1 is involved in encoding and retrieval of contextual fear, whereas ventral CA1 is recruited only during retrieval of contextual fear. The present experiment was unable to dissociate the specific roles of dorsal CA1 and ventral CA1 for retrieval of contextual information (cf. Rogers et al., 2006). The present experiment doubly dissociated the roles of ventral CA3 and dorsal CA3 for the encoding and retrieval of contextual fear. The data also suggest that ventral CA3 does not mediate encoding of either contextual or auditory-cued fear, similar to results found after lesions to ventral CA1 for auditory-trace and contextual fear acquisition (Rogers et al., 2006). It is possible that dorsal CA3, but not ventral CA3, subserves the formation of a contextual representation that the amygdala would use to associate with the shock, as has been suggested by Rudy and colleagues (Fleshner, Pugh, Tremblay, & Rudy, 1997; Rudy, Barrientos, & O'Reilly, 2002; Rudy & Matus-Amat, 2005; Rudy & O'Reilly, 2001). The data suggest ventral CA3, but not dorsal CA3, may retrieve this representation, perhaps via a pattern completion mechanism (Rudy & O'Reilly, 2001).
Since there is limited connectivity between the dorsal and ventral portions of the hippocampus (for a thorough evaluation of the lamellar hypothesis cf. Risold & Swanson, 1996), it is assumed that ventral CA3 and dorsal CA3 act as relatively independent processing agents to encode and/or retrieve patterns of contextual information, but that neither dorsal nor ventral CA3 appear to provide storage for the acquired contextual representation per se. The data also suggest that ventral CA3, but not dorsal CA3, retrieves representations of the auditory stimulus, either via differential processing, high connectivity with the amygdaloid complex (relative to dorsal hippocampus, the ventral hippocampus maintains a robust, reciprocal connection with the amygdala), or via conjunctive pattern completion that may retrieve representations of context that include a representation of the auditory stimulus (cf. Rudy & O'Reilly, 2001).
Acknowledgments
This research was supported by NSF Grant: IBN-0135273 and NIH Grant: R01MH065314 awarded to R. P. K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threats in rats with amygdaloid lesions. Journal of Comparative and Physiological Psychology. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: A PHAL anterograde trace-tracing study in the rat. Journal of Comparative Neurology. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Pugh CR, Tremblay D, Rudy JW. DHEA-S selectively impairs contextual-fear conditioning: support for the antiglucocorticoid hypothesis. Behavioral Neuroscience. 1997;111:512–517. doi: 10.1037//0735-7044.111.3.512. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neuroscience and Biobehavioral Reviews. 1983;7:119–188. doi: 10.1016/0149-7634(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: an enquiry into the functions of the epto-hippocampal system. Oxford University Press; Oxford, UK: 2001. [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–17. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. Journal of Neuroscience. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman TS, Kesner RP, Lee I, Berman RF. Patterns of cell loss based on subregional lesions of the hippocampus. Brain Research. 2005;1065:1–7. doi: 10.1016/j.brainres.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Kashimoto Y, Nakazawa K, Tonegawa S, Kirono Y, Kano M. Hippocampal CA3 NMDA receptors are crucial for adaptive timing of trace eyeblink conditioned response. Journal of Neuroscience. 2006;26:1562–1570. doi: 10.1523/JNEUROSCI.4142-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cognitive and Affective Behavioral Neuroscience. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential contributions of dorsal hippocampal subregions to memory acquisition and retrieval in contextual fear conditioning. Hippocampus. 2004;14:301–310. doi: 10.1002/hipo.10177. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurotoxic or electrolytic lesions of the ventral subiculum produce deficits in the acquisition and expression of Pavlonian fear conditioning in rats. Behavioral Neuroscience. 1999;113:283–290. doi: 10.1037//0735-7044.113.2.283. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlonian fear conditioning in rats. Behavioural Brain Research. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the dorsal hippocampus, fimbria/fornix, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiology of Learning and Memory. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt WG. Hippocampus and Pavlonian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral Neuroscience. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. Journal of Neuroscience. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. Journal of Comparative Neurology. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Essays in psychology and psychiatry. Citadel; New York: 1962. [Google Scholar]
- Paxinos G, Watson C. The rat brain: In stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. Journal of Neuroscience. 1995;15:5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Fanselow MS. Defenses and memories: Functional neural circuitry of fear and conditional responding. In: Craske MG, Hermans D, Vasteenwegen D, editors. Fear and learning: From basic principles to clinical implications. American Psychological Association; Washington, D.C.: 2006. [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oomen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Riedel G, Harrington NR, Hall G, Macphail EM. Nucleus accumbens lesions impair context, but not cue, conditioning in rats. NeuroReport. 1997;8:2477–2481. doi: 10.1097/00001756-199707280-00013. [DOI] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272:1484–1486. doi: 10.1126/science.272.5267.1484. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Hunsaker MR, Kesner RP. Effects of ventral and dorsal CA1 subregional lesions on trace fear conditioning. Neurobiology of Learning and Memory. 2006;86:72–81. doi: 10.1016/j.nlm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behavioral Neuroscience. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral Neuroscience. 2005;119:154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, Affective, and Behavioral Neuroscience. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Weible AP, O'Reilly JA, Weiss C, Disterhoft JF. Comparisons of dorsal and ventral hippocampus cornu ammonis region 1 pyramidal neuron activity during trace eye-blink conditioning in the rabbit. Neuroscience. 2006;141:1123–1137. doi: 10.1016/j.neuroscience.2006.04.065. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. Journal of Comparative Neurology. 1990;302:515–528. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiology of Learning and Memory. 2007;87:464–475. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Zeineh NM, Engel SA, Thompson PM, Bookmeyer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]