Summary
Clinical research suggests that gender differences exist in cocaine dependence. Similarly, preclinical studies have shown that female rats exhibit higher response rates during cocaine self-administration, early extinction, and cocaine-primed reinstatement of drug-seeking. These effects are also estrous cycle dependent and inversely related to plasma progesterone, in that proestrus females (high progesterone) exhibit less cocaine-seeking, while estrous females (low progesterone) show the greatest cocaine-seeking. Based on these findings, we hypothesized that progesterone would attenuate cocaine-seeking behavior in intact, freely cycling animals. The role of the estrous cycle on cocaine-seeking behavior during early (first acquisition day) versus late (last maintenance day) cocaine self-administration was also examined. Female, Sprague-Dawley rats self-administered cocaine (0.5 mg/kg/infusion, IV) along a FR1 schedule, followed by daily extinction sessions in the absence of cocaine reinforcement. Once responding was extinguished, rats received an injection of cocaine (10 mg/kg, IP) immediately prior to reinstatement testing. Progesterone (2 mg/kg, SC) or vehicle was administered 20 and 2 h prior to the first day of extinction (early cocaine withdrawal) and the reinstatement trials. To determine estrous cycle phase, we assessed vaginal cytology prior to the first acquisition and last maintenance days of cocaine self-administration, the first day of extinction training, and each reinstatement test. During early and late cocaine self-administration, proestrus and estrous females exhibited the greatest levels of active lever responding, respectively. A significant increase in responding also occurred during cocaine-primed reinstatement for estrous versus nonestrous females, an effect that was selectively attenuated by progesterone. However, progesterone was not effective at reducing cocaine-primed reinstatement for females in other phases of the estrous cycle, nor was it effective at reducing cocaine-seeking during early withdrawal. Taken together, these results suggest that progesterone may be a useful therapeutic for preventing relapse in abstinent female cocaine users, especially when the likelihood of relapse is greatest.
Keywords: progesterone, female, estrous cycle, cocaine, self-administration, reinstatement
1. Introduction
An abundance of clinical evidence indicates that significant gender differences exist in psychostimulant addiction. While men are more likely to have a cocaine abuse or dependence disorder (Brady and Randall, 1999), women have higher rates of cocaine use (Griffin et al., 1989) and exhibit more behavioral problems during periods of active cocaine intake (Kosten et al., 1993). Moreover, women begin using cocaine at an earlier age (Weiss et al., 1997) and progress more rapidly from casual use to the development of a dependence disorder (McCance-Katz et al., 1999; Westermeyer and Boedicker, 2000; O'Brien and Anthony, 2005). Differences between women and men are also apparent for relapse following prolonged abstinence, in that women tend to have shorter cocaine-free periods (Griffin et al., 1989; Kosten et al., 1996) and are more likely to relapse following stressful life events or depression (McKay et al., 1996; Elman et al., 2001; Back et al., 2005; Hyman et al., 2008). As for relapse triggered by cocaine-paired environmental cues, various studies have shown that female cocaine addicts are more (Robbins et al., 1999), less (Avants et al., 1995), or equally (Negrete and Emil, 1992) susceptible to cocaine-paired cues relative to males. Physiological differences have also been reported for abstinent men and women following exposure to stress-related imagery (Li et al., 2005) or cocaine-associated cues (Kilts et al., 2004).
Similar to clinical research, sex differences have also been revealed in animal models of cocaine effects. In behavioral sensitization models, female rats display greater locomotor activity and enhanced response to acute cocaine and greater sensitization after repeated cocaine relative to males (Glick and Hinds, 1984; van Haaren and Meyer, 1991; Chin et al., 2001; Hu and Becker, 2003). Female rats also acquire conditioned place preference (CPP) with fewer pairing sessions and with lower doses of cocaine than males (Russo et al., 2003a). These effects cannot be simply attributed to differential absorption of cocaine, in that no sex differences have been found for brain and plasma levels of the drug following acute administration in male and female rats (Bowman et al., 1999). Similar to the findings for behavioral sensitization and CPP, sex differences have also been noted using cocaine self-administration models. Relative to males, female rats show greater propensity to acquire self-administration and exhibit faster rates of acquisition (Lynch and Carroll, 1999; Carroll et al., 2002; Hu et al., 2004; Lynch, 2008). Females also display higher operant responding for cocaine (Fuchs et al., 2005; Kippin et al., 2005), greater cocaine intake during extended daily access (Lynch and Taylor, 2004; Roth and Carroll, 2004), and higher breakpoints on progressive ratio schedules of reinforcement (Roberts et al., 1989; Hecht et al., 1999; Carroll et al., 2002; Mello et al., 2007). Using the extinction/reinstatement model of relapse to cocaine-seeking, cocaine-primed reinstatement has been reported to be greater in females relative to males (Lynch and Carroll, 2000; Kippin et al., 2005). In sum, these data suggest that females may be more vulnerable than males to drug-taking and drug-seeking across all phases of the addiction cycle (Lynch et al., 2002).
In addition to evidence for sex differences in cocaine addiction, drug-motivated behaviors also appear to vary as a function of the menstrual cycle in primates (human and non-human) or the estrous cycle in rodents. While similar to men, the subjective effects of cocaine are enhanced in cocaine-dependent women during the follicular phase, relative to women in the luteal phase (Lukas et al., 1996; Sofuoglu et al., 1999; Evans et al., 2002; Terner and de Wit, 2006). Similar results have been reported for healthy women after d-amphetamine administration (Justice and de Wit, 1999; White et al., 2002). Cycle-dependent differences have also been demonstrated for non-human primates, with higher progressive ratio breakpoints seen in follicular vs. late luteal female monkeys (Mello et al., 2007) and in female rats during estrus that exhibit increased cocaine-seeking during cocaine self-administration (Roberts et al., 1989; Hecht et al., 1999; Lynch et al., 2000; Feltenstein and See, 2007; Lynch, 2008) and reinstatement (Fuchs et al., 2005; Kippin et al., 2005; Feltenstein and See, 2007; Kerstetter et al., 2008).
The rodent estrous phase and human follicular phase share a common feature of relatively low levels of both estrogen and progesterone (Festa and Quinones-Jenab, 2004). A number of studies now indicate that estrogen and progesterone may have opposing effects in mediating cocaine-seeking behavior. For instance, estradiol administration has been shown to reverse ovariectomy-induced deficits in active cocaine self-administration (Grimm and See, 1997; Lynch et al., 2001; Hu et al., 2004; Jackson et al., 2006), with similar results reported for cocaine-primed reinstatement of cocaine-seeking (Larson et al., 2005). On the other hand, acute progesterone pretreatment reverses the effects of estradiol on acquisition (Jackson et al., 2006; Yang et al., 2007) and cocaine-primed reinstatement (Anker et al., 2007) in ovariectomized female rats. Furthermore, plasma progesterone levels in freely cycling female rats are negatively correlated with cocaine-seeking (Feltenstein and See, 2007). In clinical laboratory tests, progesterone pretreatment has been shown to attenuate the subjective effects of cocaine in women (Sofuoglu et al., 2002; Sofuoglu et al., 2004; Evans and Foltin, 2006), but not men (Sofuoglu et al., 2007, but see Sofuoglu et al., 2004), suggesting that exogenous progesterone may be an effective therapeutic agent for reducing craving in abstinent cocaine-dependent women.
While these studies on the role of progesterone have been highly informative, most preclinical studies have utilized ovariectomized females, a procedure that affects more than a single hormone and does not model the hormonal variations that occur over time during chronic cocaine self-administration. Thus, we examined the effects of progesterone pretreatment on cocaine-seeking during both early withdrawal from cocaine and during cocaine-primed reinstatement in intact female rats. The role of the estrous cycle on responding for cocaine during early (first acquisition day) versus late (last maintenance day) cocaine self-administration was also assessed.
2. Methods and Materials
2.1. Subjects
Female, Sprague-Dawley rats (n = 60, initial weight 250-275 g; Charles River, Wilmington, MA, USA) were individually housed in a temperature- and humidity-controlled vivarium on a 12-h light-dark cycle (lights on 6AM-6PM). Animals were given water and standard rat chow (Harlan, Indianapolis, IN, USA) ad libitum for the duration of the experiment, except during the first 2 days of cocaine self-administration, during which animals were maintained on 20 to 25 g of standard rat chow per day to facilitate the acquisition of lever responding (Bongiovanni and See, 2008). Rats were acclimated to handling and allowed to adapt for a minimum of three days prior to the start of the experiment. Housing and care of the rats were carried out in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996).
2.2. Surgery
Rats were anesthetized using a mixture of ketamine hydrochloride and xylazine (33 and 0.665 mg/kg, respectively, IP), followed by equithesin (0.25 ml/kg with a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution, IP) and chronic indwelling catheters were implanted into the right jugular vein using previously described methods (Feltenstein and See, 2007). Catheter patency was maintained by flushing with 0.1 ml of 10 U/ml heparinized saline immediately prior to self-administration sessions with a 0.1 ml antibiotic solution of cefazolin (100 mg/ml; dissolved in 70 U/ml heparinized saline) and 0.1 ml 100 U/ml heparinized saline regimen following each session. Stylets were inserted into the catheters when the rats are not connected to infusion pumps. To verify catheter patency, rats occasionally received a 0.12 ml infusion of methohexital sodium (10 mg/ml; dissolved in 0.9% physiological saline), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
2.3. Cocaine self-administration
Rats lever pressed for cocaine in standard operant conditioning chambers (30 × 20 × 20 cm) linked to a computerized data collection program (MED-PC, Med Associates, Inc., St. Albans, VT, USA). The chambers were equipped with two retractable levers, a white stimulus light above each lever, a tone generator, and a house light on the wall opposite the levers. Each chamber was contained within a sound-attenuating cubicle equipped with a ventilation fan.
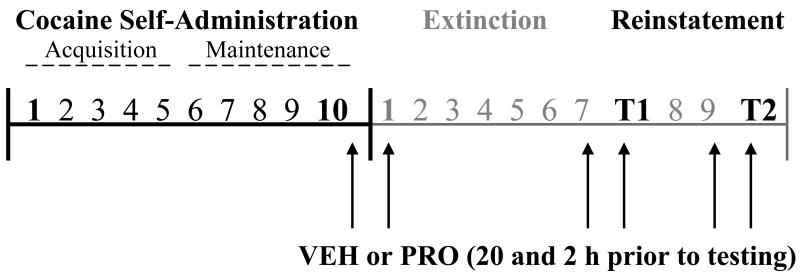
Figure 1 illustrates the phases of cocaine self-administration, extinction, and reinstatement, and the times when progesterone or vehicle pretreatment occurred. Rats self-administered cocaine (cocaine hydrochloride dissolved in 0.9% physiological saline; cocaine provided by the National Institute on Drug Abuse, Research Triangle Park, NC, USA) during daily 2-h sessions according to an FR 1 schedule of reinforcement. At the start of each session, the catheter was connected to a liquid swivel (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One Inc., Roanoke, VA, USA). The house light signaled the initiation of the session and remained illuminated throughout the entire session. Lever presses on the active lever resulted in a 2-s activation of the infusion pump (0.5 mg/kg cocaine per 50 μl infusion) and a 5-s presentation of a stimulus complex, consisting of activation of the white stimulus light above the active lever and the tone generator (78 dB, 4.5 kHz). The 0.5 mg/kg bolus dose of cocaine has been widely used for cocaine self-administration in rats, including our earlier studies in female rats (Fuchs et al., 2005; Kippin et al., 2005; Feltenstein and See, 2007). After each infusion, responses on the active lever had no consequences during a 20-s time-out period. During the sessions, responses on the inactive lever were recorded, but had no programmed consequences. Daily cocaine self-administration continued until each rat had obtained the self-administration criterion of ten sessions with at least ten infusions per session, with “early” and “late” cocaine self-administration being defined as the first (i.e., first acquisition) and last (i.e., last maintenance) days, respectively, that the animal self-administered cocaine to criterion (i.e., ≥ 10 infusions per 2-h session).
Figure 1.
Schematic representing the phases of cocaine self-administration, extinction, and cocaine-primed reinstatement testing (T1 and T2). Arrows indicate administration of progesterone (PRO: 2 mg/kg, SC) or vehicle (VEH). The role of the estrous phase on cocaine-seeking was examined during early cocaine self-administration (i.e., “first acquisition day”), late cocaine self-administration (i.e., “last maintenance day”), the first day of extinction training, and for each reinstatement test (T1 and T2 days).
2.4. Extinction and reinstatement of cocaine-seeking
Following cocaine self-administration and before the first reinstatement test, rats underwent daily 2-h extinction sessions. During each session, responses on both levers were recorded, but had no consequences. Once active lever pressing extinguished to a criterion of a minimum of seven extinction sessions with ≤ 25 active lever responses per session for 2 consecutive days, animal underwent two cocaine-primed reinstatement tests. We have previously found no decrement in responding using three reinstatement trials (Ledford et al., 2003; Kippin et al., 2005; Berglind et al., 2006). Immediately prior to each 2-h reinstatement test, rats received an injection of cocaine hydrochloride (10 mg/kg, IP). The 10 mg/kg dose of cocaine is well established as an optimal priming dose in rats, including our previous studies that demonstrated robust reinstatement of cocaine-seeking in female rats (Kippin et al., 2005; Feltenstein and See, 2007). Further extinction sessions occurred between reinstatement tests until extinction criteria were re-established (i.e. ≤ 25 active lever responses per session for 2 consecutive days). During the reinstatement test session, responses on both levers was recorded, but had no programmed consequences.
2.5. Progesterone pretreatment and estrous cycle monitoring
To determine the effects of progesterone on cocaine-seeking behavior, animals were pretreated with progesterone (2 mg/kg, SC) or vehicle (sesame oil) 20 and 2 h prior to the first day of extinction training and prior to each reinstatement test. The dose of progesterone was selected based on previous studies (Perrotti et al., 2001; Russo et al., 2003b; Niyomchai et al., 2005; Russo et al., 2008) with the short-term treatment regimen aimed at enhancing plasma levels of progesterone and/or its metabolites in rats in the absence of chronic treatment (Anker et al., 2007). Treatment was counterbalanced according to the average number of lever presses across the last three cocaine self-administration sessions.
In order to ascertain the role of estrous cycle on cocaine-seeking behavior, vaginal lumen samples were collected (immediately prior to and following) and scored at the following time points: the first acquisition and last maintenance days of cocaine self-administration (i.e., early and late cocaine self-administration, respectively), the first day of extinction training (i.e., early cocaine withdrawal), and each reinstatement test. However, to habituate females to the vaginal cytology procedure, samples were taken daily across the entire experiment. Vaginal lumen samples were collected by gently flushing 30 μl of double distilled water and extracting the sample using a micropipette and 100 μl pipette tips. Samples were placed on glass slides, stained using Quick-Dip Hematology Stain (Mercedes Medical, FL, USA), examined using a light microscope set to 10× magnification, and classified as diestrus I (also known as metestrus), diestrus II, proestrus, or estrus (note: vaginal estrus as opposed to behavioral estrus) according to previously published criteria (Marcondes et al., 2002). Due to the lower number of animals from which diestrus smears were obtained, the lack of behavioral differences between females in the diestrus I and II states, and similar ovarian hormone profile (i.e., low estrogen and moderate levels of progesterone), females in the two diestrus phases were combined in statistical analyses and are hereafter referred to as diestrus I/II. Representative images of the cytology from each phase of the estrous cycle and classification criterion are presented in Figure 2.
Figure 2.
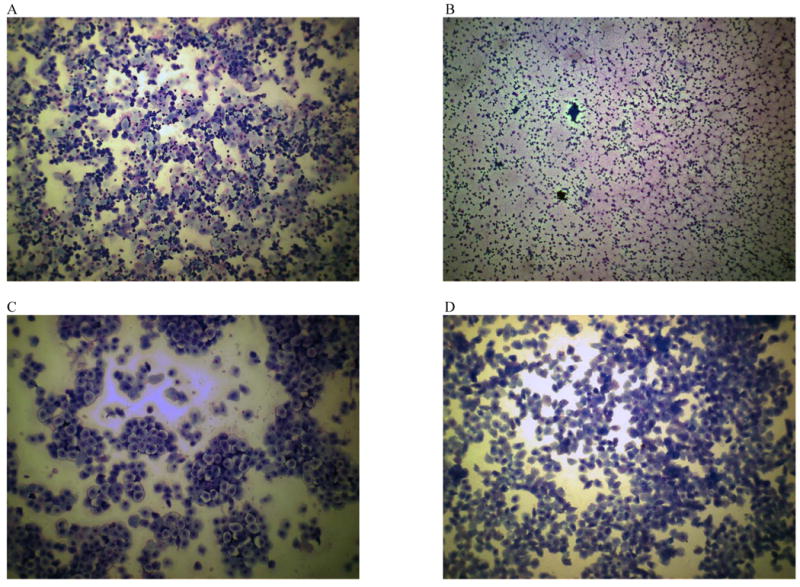
Representative photomicrographs of stained vaginal smear samples from female rats in diestrus I (A), diestrus II (B), proestrus (C), and estrus (D) using a light microscope and 10× magnification. The relative proportion of three types of cells was used to determine estrous cycle phases: small round leukocytes, round and nucleated epithelial cells, and anucleated, irregular cornified cells. The diestrus I (also known as metestrus) phase was defined as the presence of approximately equal proportions of epithelial cells, cornified cells, and leukocytes. The diestrus II phase was defined as a containing a majority of leukocytes and a minimum amount of epithelial and cornified cells. The proestrus phase was defined as the presence of >75% epithelial cells. The estrous phase (note: vaginal estrus as opposed to behavioral estrus) was defined as the presence of >75% cornified cells.
2.6. Radioimmunoassay
For determination of plasma progesterone levels, 0.6 ml of whole blood was collected through the catheter immediately after the first day of extinction training and after each reinstatement test (note: whole blood collection was successful for only a subset of animals). Fluid levels were replaced with an equivalent amount of filtered 0.9% physiological saline with a minimum of three days between each blood sample. Immediately after the whole blood sample was mixed with 0.05 ml of 1000 U/ml heparin, plasma was isolated by centrifugation (10,000 rpm at 4°C for 20 min) and stored at -80°C until assayed. Plasma progesterone (ng/ml) was determined using radioimmunoassay (Diagnostic Systems Laboratories, DSL-3900, Webster, TX, USA) according to the manufacturer's directions.
2.7. Data analysis
For early and late cocaine self-administration, lever responses and number of cocaine infusions were analyzed using one-way analysis of variance (ANOVA) with estrous phase as the between subjects factor. To determine if any preexisting group differences existed, lever responses and number of cocaine infusions during the last 3 days of cocaine self-administration, as well as the number of days of cocaine self-administration and extinction training were analyzed using t-tests, with progesterone pretreatment as the between-subjects factor. For the first day of extinction training and reinstatement testing, lever responses and plasma progesterone levels were analyzed using a two-way ANOVA with progesterone pretreatment and estrous phase as between subject factors, followed by one-way ANOVA or t-test analyses where appropriate. Data points were eliminated if they were 2.5 standard deviations beyond the group mean, the cytology for that particular day was missing, or the animal failed to extinguish prior to testing. All post hoc analyses were conducted using Tukey's with the alpha set at 0.05. Data are expressed as the mean ± SEM.
3. Results
3.1. Cocaine self-administration
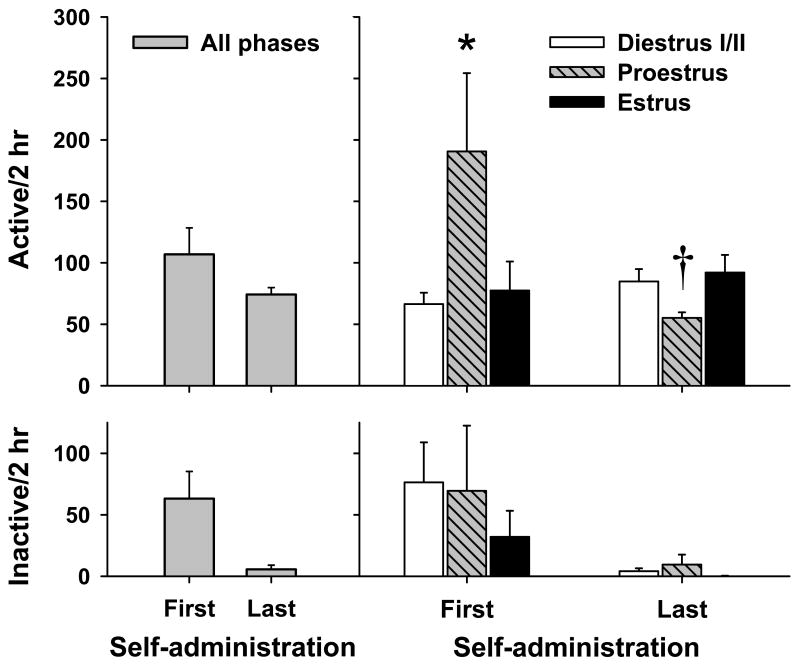
Animals readily acquired cocaine self-administration, responded preferentially on the active lever, and maintained stable lever responding and cocaine intake (i.e., number of cocaine infusions) during the maintenance phase of self-administration. When examined as a function of estrous cycle, an interesting dissociation in drug-seeking behavior became evident, in that proestrus females exhibited the highest and lowest levels of active lever responding during early and late cocaine self-administration, respectively (Figure 3). Statistical analyses of these data confirmed these observations, as a significant estrous cycle main effect for active lever responding was noted for the first [F(2,53) = 3.63, p < 0.05] and last [F(2,57) = 4.53, p < 0.05] days of cocaine self-administration. Post hoc analyses revealed that while females in proestrus exhibited greater responding than diestrus I/II animals during early self-administration, estrus and diestrus I/II females responded greater than proestrus animals on the last day (ps < 0.05). However, there were no estrous cycle main effects for any of the other measures at either time point [Fs(2,53-57) = 0.34-3.12, ps = 0.06-0.72]. The number of cocaine infusions (mean ± SEM) was 28.48±2.85, 40.24±7.35, and 32.29±6.10 during early cocaine self-administration and 47.68±2.16, 40.17±2.40, and 41.27±2.93 during late cocaine self-administration for diestrus I/II, proestrus, and estrous females, respectively.
Figure 3.
Active (top) and inactive (bottom) lever responding (mean + SEM) for the first and last days of cocaine self-administration (left) and as a function of estrous cycle phase for the first (n = 25, 17 and 14 for diestrus I/II, proestrus and estrus, respectively) and last (n = 25, 24 and 11 for diestrus I/II, proestrus and estrus, respectively) days of cocaine self-administration. Proestrus females displayed the greatest and least levels of responding on the first and last days of cocaine self-administration training (* = relative to diestrus I/II, † = relative to diestrus I/II and estrus; p<0.05).
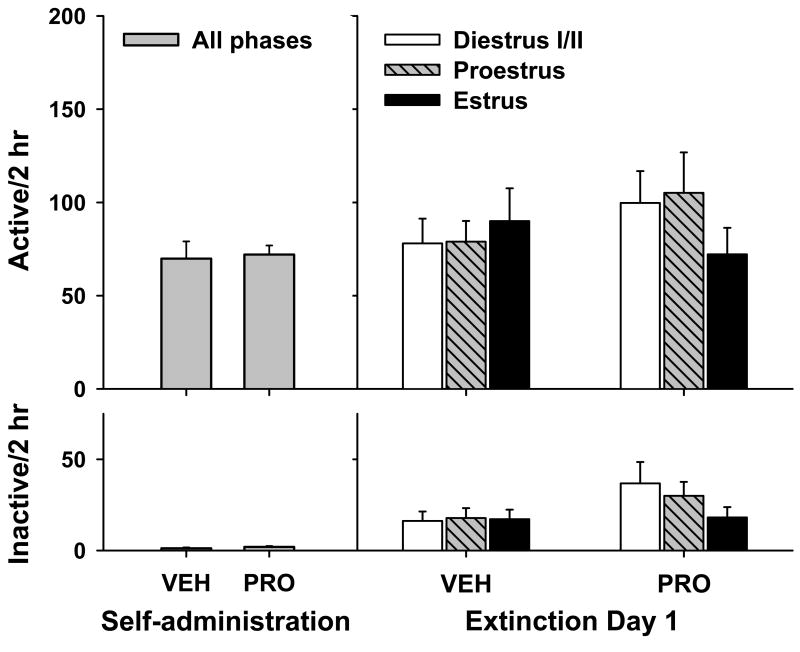
Statistical analyses on active and inactive lever responding and number of cocaine infusions across the last three days of cocaine self-administration failed to reveal any pre-existing differences between the groups subsequently pretreated with vehicle or progesterone [ts(55) = 0.21-1.67, ps = 0.10-0.84; Figure 4]. The number of cocaine infusions (mean ±SEM) was 39.29±2.07 and 43.54±1.46 for vehicle and progesterone pretreated animals, respectively. Moreover, there was no difference in the number of days of cocaine self-administration between the two groups prior to drug treatment and extinction training [t(55) = 0.68, p = 0.50; number of days (mean ± SEM) was 13.86 ± 0.81 (vehicle) and 13.07 ± 0.84 (progesterone)].
Figure 4.
Active (top) and inactive (bottom) lever responding (mean + SEM) during the last three days of cocaine self-administration (left) and as a function of vehicle (VEH) or progesterone (PRO) pretreatment and estrous cycle phase on day 1 of extinction (VEH, n = 16, 7 and 6; PRO, n = 10, 8 and 11 for diestrus I/II, proestrus and estrus, respectively). Progesterone pretreatment had no significant effects on lever responding at this time point.
3.2. Extinction
Following cocaine self-administration, vehicle and progesterone pretreated rats across all phases of the estrous cycle demonstrated similar levels of responding on the first day of extinction training, with no significant effects for progesterone pretreatment [Fs(1,51) = 0.52-3.28, ps = 0.08-0.48], estrous phase [Fs(2,51) = 0.20-0.75, ps = 0.48-0.82], or the progesterone pretreatment X estrous phase interaction [Fs(2,51) = 0.91-0.96, ps = 0.39-0.41]. While progesterone treatment produced an increase in plasma levels (Table 1), there were no significant main effects for progesterone pretreatment [F(1,30) = 2.53, p = 0.12], estrous phase [F(2,30) = 0.72, p = 0.50] or the progesterone pretreatment X estrous phase interaction [F(2,30) = 1.19, p = 0.32]. Finally, neither vehicle nor progesterone pretreatment had any effect on the number of days required for animals to reach the extinction criterion prior to cocaine-primed reinstatement testing [t(55) = 0.23, p = 0.82; number of days (mean ± SEM) was 8.24 ± 0.43 (vehicle) and 8.11 ± 0.40 (progesterone)], nor were there any pre-existing differences in extinction level responding prior to each reinstatement test [ts(115) = 0.60-0.97, ps = 0.43-0.80].
Table 1.
Mean (± SEM) plasma progesterone levels for progesterone (2 mg/kg, SC) and vehicle pretreated animals after the first day of extinction training and each cocaine-primed reinstatement test. Progesterone pretreatment resulted in a significant increase in plasma progesterone levels during reinstatement testing (* = relative to vehicle-pretreated animals, p<0.05).
| Test Phase | Pretreatment | Progesterone (ng/ml) Mean ± SEM | Estrous Cycle Phase | Progesterone (ng/ml) Mean ± SEM |
|---|---|---|---|---|
| Extinction | Vehicle (n = 18) | 12.00 ± 5.07 | Diestrus I/II (n = 9) | 5.85 ± 1.41 |
| Proestrus (n = 6) | 26.15 ± 14.00 | |||
| Estrus (n = 3) | 2.13 ± 0.23 | |||
| Progesterone (n = 18) | 73.89 ± 32.27 | Diestrus I/II (n = 7) | 127.22 ± 78.46 | |
| Proestrus (n = 4) | 27.44 ± 11.59 | |||
| Estrus (n = 7) | 47.10 ± 24.36 | |||
| Reinstatement | Vehicle (n = 24) | 13.41 ± 5.63 | Diestrus I/II (n = 12) | 20.93 ± 10.93 |
| Proestrus (n = 7) | 6.12 ± 1.79 | |||
| Estrus (n = 5) | 5.57 ± 3.41 | |||
| Progesterone (n = 26) | 65.68 ± 18.57 * | Diestrus I/II (n = 12) | 122.77 ± 47.10 | |
| Proestrus (n = 6) | 15.85 ± 2.41 | |||
| Estrus (n = 8) | 58.43 ± 31.38 |
3.3. Reinstatement
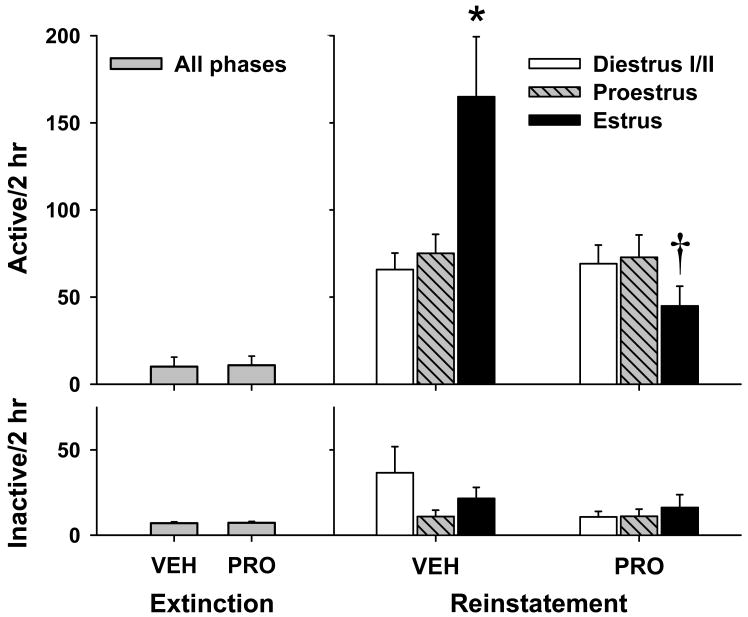
During each reinstatement test, cocaine priming injections produced a robust increase in cocaine-seeking as indexed by responding on the previously cocaine-paired lever (Figure 5), with no order effects being found across the two reinstatement tests [ts(115) = 0.15-0.67, ps = 0.50-0.88]. Following vehicle pretreatment, estrous females exhibited significantly greater cocaine-seeking following the cocaine-priming injection than females in proestrus or diestrus I/II. Although ineffective for proestrus or diestrus I/II females, progesterone pretreatment led to a significant reduction in cocaine-primed reinstatement behavior for females in estrus. A two-way ANOVA of active lever responding revealed a significant main effect for progesterone pretreatment [F(1,111) = 8.42, p < 0.005] as well as a significant progesterone pretreatment X estrous phase interaction [F(2,111) = 7.68, p = 0.001], but not a significant estrous phase main effect [F(1,111) = 2.63, p < 0.08]. Inactive lever responding showed no significant differences [progesterone pretreatment: F(1,111) = 1.84, p = 0.18; estrous phase: F(1,111) = 1.06, p = 0.35; progesterone pretreatment X estrous phase: F(2,111) = 1.29, p = 0.28]. One-way ANOVA of active lever responding for vehicle treated animals revealed a significant main effect for estrous phase [F(2,56) = 7.57, p = 0.001], and post hoc analyses showed that females in estrus responded significantly greater than females in proestrus (p <0.01) or diestrus I/II (p < 0.001). However, the estrous phase main effect for progesterone pretreated animals was not significant [F(2,55) = 1.15, p = 0.33]. When compared to their respective vehicle controls, progesterone pretreatment resulted in a significant reversal of cocaine-seeking behavior during the estrus [t(27) = 2.83, p < 0.01], but not proestrus or diestrus I/II phases [ts(34-50) = 0.14-0.23, ps = 0.82-0.89]. Finally, analyses for plasma progesterone levels (Table 1) revealed a significant main effect for progesterone pretreatment [F(1,44) = 4.08, p = 0.05], but not for the estrous phase main effect [F(2,44) = 2.07, p = 0.14] or the progesterone pretreatment X estrous phase interaction [F(2,44) = 1.12, p = 0.34].
Figure 5.
Active (top) and inactive (bottom) lever responding (mean + SEM) during the last two days of extinction (left) and as a function of vehicle (VEH) or progesterone (PRO) pretreatment and estrous cycle phase during cocaine-primed reinstatement (VEH, n = 25, 17 and 17; PRO, n = 27, 19 and 12 for diestrus I/II, proestrus and estrus, respectively). Estrous females showed greater responding during reinstatement, an effect that was selectively attenuated by progesterone pretreatment (* = relative to diestrus I/II and proestrus, † = relative to VEH; p<0.05).
4. Discussion
The present study demonstrates that progesterone pretreatment can selectively attenuate cocaine-seeking behavior in freely-cycling female rats. Consistent with previous findings (Kippin et al., 2005; Feltenstein and See, 2007; Kerstetter et al., 2008), females in estrus displayed higher reinstatement of cocaine-seeking following a cocaine-priming injection (10 mg/kg) relative to females in other phases of the estrous cycle, with progesterone pretreatment selectively blocking this effect. However, progesterone was not effective at reducing cocaine-primed reinstatement behavior for females in other phases of the estrous cycle, nor was it effective at reducing cocaine-seeking behavior during early cocaine withdrawal. Overall, these results support growing preclinical (Russo et al., 2003b; Jackson et al., 2006; Anker et al., 2007; Russo et al., 2008) and clinical (Sofuoglu et al., 2002; Sofuoglu et al., 2004; Evans and Foltin, 2006) evidence for an inhibitory role of progesterone on the reinforcing effects of cocaine and/or cocaine-seeking behavior.
The inhibitory effects of progesterone on cocaine-primed reinstatement behavior were selective for females in estrus, an effect that appears to be consistent with clinical data suggesting that progesterone inhibits the subjective effects of cocaine in women during the analogous follicular phase (Sofuoglu et al., 2002; Evans and Foltin, 2006). It is interesting to note that progesterone pretreatment has been previously demonstrated to decrease DA activity in the striatum (Fernandez-Ruiz et al., 1990), an area of the brain that has been widely implicated in drug-craving in humans (Volkow et al., 2006; Wong et al., 2006) and drug-seeking behaviors in animals (Fuchs et al., 2006; See et al., 2007). It is unlikely, however, that increased progesterone alone simply inhibits the reinforcing properties of cocaine. For example, while progesterone pretreatment clearly increased plasma levels of the hormone above normal endogenous levels, the highest levels were found for female rats in diestrus I/II, a phase in which cocaine-primed reinstatement was unaffected by such pretreatment. Therefore, while progesterone may attenuate drug-seeking behavior, these effects likely involve multiple processes, including complex interactions with other endogenous hormones (e.g., estrogen) and neurotransmitters. In addition, pharmacokinetic differences in the absorption, distribution, and metabolism of exogenous progesterone may vary across the estrous cycle.
One key mechanism that may account for the effects of progesterone involves the metabolism of progesterone into other bioactive neurosteroids, such as allopregnanolone. In preclinical models, allopregnanolone has been shown to prevent the development of cocaine-induced behavioral sensitization (Kaminski et al., 2003). Allopregnanolone also decreases dopamine concentrations (Laconi et al., 2007) and potentiates GABAA receptor activation (Bitran et al., 1995). It is of note that enhanced GABAergic tone is a putative mechanism by which cocaine craving can be blunted in humans (Brebner et al., 2002). In the context of the current results, endogenous levels of allopregnanolone have been shown to be highest during the estrous phase (Paul and Purdy, 1992). Thus, in addition to the metabolism of the exogenously administered progesterone, plasma concentrations of allopregnanolone would be predicted to be highest in estrous females, leading to a significant reduction in cocaine-seeking behavior. Future studies should examine the role that direct administration of this metabolite may have on reinstatement of drug-seeking.
Unlike cocaine-primed reinstatement testing, progesterone pretreatment did not have a significant effect on cocaine-seeking on the first day of extinction. Several possibilities exist that may explain the lack of effectiveness of progesterone at this time point. Pretreatment and/or dosing parameters of progesterone may need to be altered in order to reduce drug-seeking behavior during early withdrawal relative to reinstatement tests. It is also possible that progesterone is only effective at reducing drug-seeking in the presence of a robust relapsing stimulus (i.e., in the presence of cocaine). In support of this hypothesis, several clinical studies have demonstrated that progesterone attenuates the subjective effects of cocaine in cocaine-dependent subjects (Sofuoglu et al., 2002; Sofuoglu et al., 2004; Evans and Foltin, 2006) and reduces cocaine self-administration in rats (Jackson et al., 2006; Larson et al., 2007; Yang et al., 2007). However, it should be noted that progesterone pretreatment can reduce reward-related learning in the absence of cocaine, such as the expression of a cocaine conditioned place preference (Russo et al., 2003b; Russo et al., 2008). Finally, withdrawal emergent changes in progesterone modulation of drug-seeking may only occur after more prolonged periods of abstinence from the drug. Such time dependent changes have been reported for various measures of cocaine-seeking after withdrawal (Grimm et al., 2003; Fuchs et al., 2006; Kerstetter et al., 2008).
A unique finding from the current study was the contrast in responding for cocaine in females across the estrous cycle during the early versus late phases of cocaine self-administration. Similar to previous research (Roberts et al., 1989; Hecht et al., 1999; Lynch et al., 2000; Feltenstein and See, 2007; Lynch, 2008), females in estrus displayed the greatest level of responding on the cocaine-paired lever during late cocaine self-administration, with proestrus females characteristically exhibiting the lowest level of responding for cocaine at this same time point. However, the opposite was true during early cocaine self-administration, in that females in proestrus exhibited the highest levels of cocaine-seeking. While sex differences in the acquisition of drug self-administration have been explored for different drugs of abuse (Roth et al., 2004), this is the first evidence for divergent patterns of cocaine self-administration during various phases of the estrous cycle. One possible explanation for enhanced drug-seeking during early cocaine self-administration in proestrus females is high levels of estradiol, which peaks during this phase. Estradiol administration has been shown to increase the reinforcing effects of psychostimulants in humans (Justice and de Wit, 2000) and enhance the acquisition (Lynch et al., 2001; Hu et al., 2004; Jackson et al., 2006) and escalation (Larson et al., 2007) of cocaine self-administration in rats, with plasma estradiol levels showing a positive correlation with cocaine-seeking (Lynch, 2008). However, while endogenous plasma estradiol levels are enhanced during proestrus in drug-free females, this estradiol peak is not consistently seen after chronic cocaine self-administration (Feltenstein and See, 2007), due to cocaine's disruption of normal estrous cycle activity (King et al., 1990, 1993; Grimm and See, 1997; Mello et al., 1997). Thus, while elevated plasma estradiol during proestrus may have enhanced the reinforcing effects of cocaine early in self-administration, cocaine's disruption of estradiol following chronic cocaine exposure may reduce the impact of this hormone in enhancing cocaine self-administration. In contrast, progesterone may play a more prominent role at later phases of the addiction cycle.
The current results support the use of exogenous progesterone as an effective therapeutic agent for preventing relapse in abstinent cocaine-dependent women, especially when the likelihood of relapse is greatest. However, because of its selective effects for females in estrus, as well as the complex relationship between fluctuating ovarian hormone levels in intact animals, the exact mechanism by which progesterone reduces drug-seeking behavior remains unknown. Moreover, we do not know whether these selective effects would occur with higher doses of progesterone or following a more prolonged pretreatment regimen. Future studies should examine these issues, as well as the efficacy of progesterone and/or progesterone metabolites for attenuating drug-seeking following exposure to various other relapsing stimuli, such as drug-associated cues or stress, assess similar treatment for reducing relapse behaviors in males, and determine whether progesterone may inhibit relapse for other classes of abused drugs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker JJ, Larson EB, Gliddon LA, Carroll ME. Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol. 2007;15:472–480. doi: 10.1037/1064-1297.15.5.472. [DOI] [PubMed] [Google Scholar]
- Avants SK, Margolin A, Kosten TR, Cooney NL. Differences between responders and nonresponders to cocaine cues in the laboratory. Addict Behav. 1995;20:215–224. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 2005;180:169–176. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE. Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience. 2006;137:699–706. doi: 10.1016/j.neuroscience.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacol Biochem Behav. 2008;89:227–233. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther. 1999;290:1316–1323. [PubMed] [Google Scholar]
- Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- Brebner K, Childress AR, Roberts DC. A potential role for GABA(B) agonists in the treatment of psychostimulant addiction. Alcohol Alcohol. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Campbell UC, Lynch WJ, Dess NK. Influence of estrogen in the acquisition of intravenously self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Fletcher H, Perrotti LI, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral sensitization. Cell Mol Biol (Noisy-le-grand) 2001;47:1089–1095. [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz JJ, de Miguel R, Hernandez ML, Ramos JA. Time-course of the effects of ovarian steroids on the activity of limbic and striatal dopaminergic neurons in female rat brain. Pharmacol Biochem Behav. 1990;36:603–606. doi: 10.1016/0091-3057(90)90262-g. [DOI] [PubMed] [Google Scholar]
- Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;179:662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA. Sex differences in sensitization to cocaine-induced rotation. Eur J Pharmacol. 1984;99:119–121. doi: 10.1016/0014-2999(84)90442-4. [DOI] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 1997;61:755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–145. [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000;71:51–59. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Gasior M, Carter RB, Witkin JM. Protective efficacy of neuroactive steroids against cocaine kindled-seizures in mice. Eur J Pharmacol. 2003;474:217–222. doi: 10.1016/s0014-2999(03)02086-7. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- King TS, Schenken RS, Kang IS, Javors MA, Riehl RM. Cocaine disrupts estrous cyclicity and alters the reproductive neuroendocrine axis in the rat. Neuroendocrinology. 1990;51:15–22. doi: 10.1159/000125310. [DOI] [PubMed] [Google Scholar]
- King TS, Canez MS, Gaskill S, Javors MA, Schenken RS. Chronic cocaine disruption of estrous cyclicity in the rat: dose-dependent effects. J Pharmacol Exp Ther. 1993;264:29–34. [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–252. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Laconi MR, Reggiani PC, Penissi A, Yunes R, Cabrera RJ. Allopregnanolone modulates striatal dopamingergic activity of rats under different gonadal hormones conditions. Neurol Res. 2007;29:622–627. doi: 10.1179/016164107X166281. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15:461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- Ledford CC, Fuchs RA, See RE. Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology. 2003;28:1721–1729. doi: 10.1038/sj.npp.1300249. [DOI] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:487–494. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology (Berl) 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI. Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis. 1996;184:616–622. doi: 10.1097/00005053-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kelly M, Diaz-Migoyo N, Sholar JW. The effects of chronic cocaine self-administration on the menstrual cycle in rhesus monkeys. J Pharmacol Exp Ther. 1997;281:70–83. [PubMed] [Google Scholar]
- Negrete JC, Emil S. Cue-evoked arousal in cocaine users: a study of variance and predictive value. Drug Alcohol Depend. 1992;30:187–192. doi: 10.1016/0376-8716(92)90025-8. [DOI] [PubMed] [Google Scholar]
- Niyomchai T, Russo SJ, Festa ED, Akhavan A, Jenab S, Quinones-Jenab V. Progesterone inhibits behavioral responses and estrogen increases corticosterone levels after acute cocaine administration. Pharmacol Biochem Behav. 2005;80:603–610. doi: 10.1016/j.pbb.2005.01.010. [DOI] [PubMed] [Google Scholar]
- O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000-2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. Faseb J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Perrotti LI, Russo SJ, Fletcher H, Chin J, Webb T, Jenab S, Quinones-Jenab V. Ovarian hormones modulate cocaine-induced locomotor and stereotypic activity. Ann N Y Acad Sci. 2001;937:202–216. doi: 10.1111/j.1749-6632.2001.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003a;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003b;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Sun WL, Minerly AC, Weierstall K, Nazarian A, Festa ED, Niyomchai T, Akhavan A, Luine V, Jenab S, Quinones-Jenab V. Progesterone attenuates cocaine-induced conditioned place preference in female rats. Brain Res. 2008;1189:229–235. doi: 10.1016/j.brainres.2007.10.057. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Gonzalez G, Gonsai K, Oliveto A, Kosten TR. Progesterone effects on cocaine use in male cocaine users maintained on methadone: a randomized, double-blind, pilot study. Exp Clin Psychopharmacol. 2007;15:453–460. doi: 10.1037/1064-1297.15.5.453. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend. 1997;44:35–40. doi: 10.1016/s0376-8716(96)01319-1. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasic JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184:174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]