Abstract
Aim
To identify antenatal and perinatal risk factors for in‐hospital mortality of babies born within the Australian and New Zealand Neonatal Network (ANZNN).
Methods
Data were collected prospectively as part of the ongoing audit of high‐risk infants (birth weight <1500 g or gestation <32 weeks) admitted to all level III neonatal units in Australia and New Zealand. Antenatal and intrapartum factors to 1 min of age were examined in 11 498 infants with gestational age >24 weeks. Risk and protective factors for mortality were derived from logistic regression models fitted to 1998–9 data and validated on 2000–1 data.
Results
For the whole cohort of infants born between 1998 and 2001, prematurity was the dominant risk factor, infants born at 25 weeks having 32 times greater odds of death than infants born at 31 weeks. Low birth weight for gestational age also had a dose–response effect: the more growth restricted the infant the greater the risk of mortality; infants below the 3rd centile had eight times greater odds of death than those between the 25th and 75th centiles. Male sex was also a significant risk factor (odds ratio (OR) 1.55, 95% confidence interval (CI) 1.31 to 1.82). Maternal hypertension in pregnancy was protective (OR 0.46, 95% CI 0.36 to 0.50). The predictive model for mortality had an area under the receiver operating characteristic curve of 0.82.
Conclusions
Risk of mortality can be predicted with good accuracy with factors up to the 1 min Apgar score. By using gestation rather than birth weight as the main indicator of maturity, these data confirm that weight for gestational age is an independent risk factor for mortality.
The Australian and New Zealand Neonatal Network (ANZNN) is a network of all 29 tertiary newborn intensive care units (NICUs) in the two countries. Since 1995, all units have contributed to a minimum dataset for all infants born before 32 weeks or with birth weight <1500 g, and for more mature infants needing assisted ventilation or surgery. It has been recognised that a comparison of mortality and other adverse outcome rates in the preterm population among NICUs offers the potential for quality improvement, whereby NICUs within a network learn from those with the better outcomes.1,2,3,4,5,6 However, differing outcome rates are not only due to variations in clinical practice, but also due to ascertainment bias (diagnoses made differently), case mix differences and anomalies of chance variation that are greater for smaller units.7 Before a quality improvement programme can focus on clinical practice and service delivery issues, it is important to control for these other three sources of variation.
Mortality is not vulnerable to ascertainment bias, but it is vulnerable to case mix difference. Recognition of this has led to the development of several modelled scores for defining mortality risk, most notably the score for neonatal acute physiology (and its developments)4 and the clinical risk index for babies (CRIB) score.1 Both these scores incorporate measures of early neonatal condition, which may introduce confounding effects of different early treatment practices. The aim of this study was to develop a predictive model for hospital‐based mortality for very preterm infants in the ANZNN (1998–2001) using prenatal variables up to and including the 1 min Apgar score.
Methods
Subjects
Data were collected prospectively as part of the ANZNN's ongoing audit of high‐risk infants admitted to neonatal nurseries. In both countries, perinatal care is regionalised so that very premature babies (<29 weeks) will almost always be cared for in a tertiary centre and if born in a level II facility, will be transferred in the early postnatal period to a tertiary centre. Thus, the ANZNN database contains 98.8% of live births before 29 weeks and 92% of live births before 32 weeks. The database uses agreed definitions that have been authorised by the ANZNN advisory committee. There are complete data on sex, gestational age and birth weight for all infants. Gestational age is the number of completed weeks assessed from early obstetric ultrasound, first day of the last menstrual period or clinical assessment of the infant. Weight for gestational age categories were determined from the Australian national birthweight centile charts of Roberts and Lancaster,8 using cut‐off points at the 3rd, 10th, 25th, 75th, 90th and 97th centiles. Mortality was defined as death before the infant's discharge home from its first hospitalisation, with babies followed through all interhospital transfers during that first hospitalisation. Maternal ethnicity was defined by self‐report. Maternal age group compared teenagers and mothers >34 years of age with other mothers. Maternal corticosteroids included any dose given to enhance fetal lung development. Obstetric complications included prelabour and preterm rupture of the membranes (ROM); when ROM had occurred for >24 h it was defined as prolonged. Any form of hypertension in pregnancy was noted, as was any major antepartum haemorrhage after 20 weeks of gestation. Maternal hypertension was defined as a systolic blood pressure ⩾140 mm Hg or a diastolic blood pressure ⩾90 mm Hg, or a rise in systolic blood pressure >25 mm Hg or a rise in diastolic blood pressure >15 mm Hg before conception or in the first trimester, and confirmed by readings 6 h apart. Suspected intrauterine growth restriction was diagnosed by serial obstetric ultrasounds. Fetal distress was defined as any signs of fetal distress that led to obstetric intervention. A baby was defined as being ”outborn” if it was not born in a perinatal centre or was born in a perinatal centre and transferred to another level III nursery by a specialist retrieval team within 4 h after its birth.
Infants with a lethal congenital malformation were excluded, as were those infants born with hydrops fetalis. The mortality analysis was restricted to those infants born after >24 weeks of gestation, as it was considered that most NICUs would admit these infants and so mortality would be less affected by different resuscitation policies at the borderlines of viability. Obtaining death information from the labour ward was not possible.
Statistical methodology
The focus of this modelling was on antenatal and perinatal variables that affect the infants' condition before they reach the NICU, that is, up to and including the 1 min Apgar. Variables identified as significant at the 5% level on univariate analysis were entered into a multivariate logistic regression model and were removed sequentially. Variables were retained in the final model if they were significant at p<0.01, using likelihood ratio χ2 test statistics. To assist the identification of potential collinearity or confounding between the predictors, a process of refitting was performed, with individual covariates deleted and verified at each stage. Estimated coefficients were compared between models and between the univariate and multivariate results. The fit of the model was checked using the Hosmer–Lemeshow goodness of fit statistic,9 with additional verification that models were not overfitted (indicated by very high p values). The discriminatory ability of the model was assessed using the area under the receiver operating characteristic (ROC) curve. The predictive model was developed on the cohort of infants born during the period 1998–9, and then validated by fitting a model with the same predictor variables to data for the infants born during 2000–1.
Because some other models of mortality (CRIB and score for neonatal acute physiology) have included birth weight rather than gestation as the primary measure of maturity, we also examined the effect of substituting birth weight for gestational age or weight for gestational age or both in our final model, and of adding birth weight to the predictor variables. Birth weight was divided into seven categories, as for gestational age, with cut‐off points at 750, 1000, 1250, 1500, 1750 and 2000 g.
All analyses were performed using SAS statistical software V. 8.2 (SAS Institute, Cary, North Carolina, USA), adhering to strict confidentiality guidelines.
Results
Mortality analysis
There were 5713 infants born in 1998–9 who were eligible for inclusion in the study, of whom 390 (6.8%) died while in hospital. The median birth weight of the 5713 infants was 1230 g (interquartile range (IQR) 960–1504) and the median gestation was 29 weeks (IQR 27–30). There were 5898 infants in the validation cohort born in 2000–1, with median birth weight 1240 g (IQR 980–1510), median gestation 29 weeks (IQR 27–30) and 373 deaths (6.3%). The ratio of males to females was also similar in the two periods: 1.25:1 (1998–9) and 1.16:1 (2000–1).
Variables that were not significantly (p<0.05) related to death on univariate analysis of the 1998–9 data (table 1) were previous perinatal death, previous preterm baby, premature pre‐labour ROM, prolonged ROM for >24 h, preterm labour, antepartum haemorrhage, any antenatal corticosteroids, plurality, maternal age group and mode of birth.
Table 1 Univariate analysis: perinatal factors not significantly (p>0.05) associated with death for 5713 infants born after 24 weeks and before 32 weeks in 1998–9.
| Perinatal characteristic | Died/total (%) | Number missing | Unadjusted OR (95% CI) | p Value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Previous perinatal death | 32/329 (10) | 330/4690 (7) | 694 | 1.42 (0.97 to 2.09) | 0.07 |
| Previous preterm baby | 65/822 (8) | 297/4231 (7) | 660 | 1.14 (0.86 to 1.50) | 0.4 |
| Premature ROM | 76/1141 (7) | 291/4151 (7) | 421 | 0.95 (0.73 to 1.23) | 0.7 |
| Prolonged premature ROM | 85/1307 (7) | 305/4406 (7) | 0 | 0.94 (0.73 to 1.20) | 0.6 |
| Preterm labour | 257/3702 (7) | 132/2005 (7) | 6 | 1.06 (0.83 to 1.32] | 0.6 |
| Antepartum haemorrhage | 94/1293 (7) | 276/3950 (7) | 470 | 1.04 [0.82 to 1.33) | 0.7 |
| No antenatal steroids | 310/4636 (7) | 69/858 (8) | 219 | 0.82 (0.62 to 1.08) | 0.2 |
| Singleton | 295/4151 (7) | 95/1562 (6) | 0 | 0.85 (0.67 to 1.08) | 0.2 |
| Maternal age | 0 | 0.7 | |||
| <20 years | 44/593 (7) | 1.09 (0.78 to 1.52) | |||
| 20−35 years | 276/4033 (7) | 1.00 | |||
| >35 years | 70/1087 (6) | 0.94 (0.71 to 1.23) | |||
| Mode of delivery | 161 | 0.2 | |||
| Vaginal/instrument | 159/2198 (7) | 1.00 | |||
| Caesarean, in labour | 82/1402 (6) | 0.80 (0.60 to 1.05) | |||
| Caesarean, no labour | 138/1957 (7) | 0.97 (0.77 to 1.23) | |||
ROM, rupture of membranes.
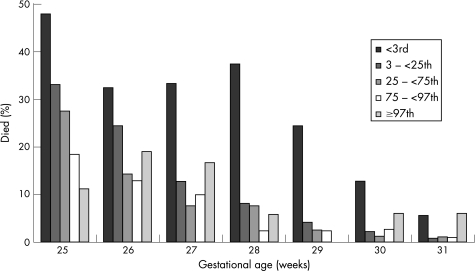
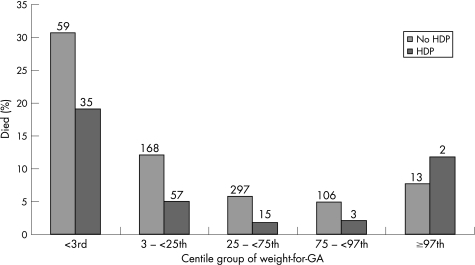
The significantly increased unadjusted risk of death (table 2) was associated with male sex, lower gestational age, low and very high weight for gestational age (fig. 1), Apgar score at 1 min <4, maternal ethnicity, breech presentation, outborn, and fetal distress requiring obstetric intervention. Hypertension in pregnancy (new episode of or existing hypertension) showed a protective association (fig 2).
Table 2 Univariate analysis: perinatal factors significantly (p<0.05) associated with death for 5713 infants born after 24 weeks and before 32 weeks in 1998–9.
| Perinatal characteristic | Died/total (%) | Unadjusted OR (95% CI) | p Value | Missing |
|---|---|---|---|---|
| Sex | <0.0001 | 0 | ||
| Male | 254/3171 (8) | 1.54 (1.24 to 1.91) | ||
| Female | 136/2542 (5) | 1.00 | ||
| GA(weeks) | <0.0001 | 0 | ||
| 31 | 17/1352 (1) | 1.00 | ||
| 30 | 26/1080 (2) | 1.94 (1.05 to 3.59) | ||
| 29 | 31/945 (3) | 2.66 (1.47 to 4.84) | ||
| 28 | 41/770 (5) | 4.41 (2.49 to 7.82) | ||
| 27 | 63/609 (10) | 9.06 (5.25 to 15.61) | ||
| 26 | 97/548 (18) | 16.88 (9.97 to 28.57) | ||
| 25 | 115/409 (28) | 30.70 (18.17 to 51.87) | ||
| Weight for GA (centile) | <0.001 | 0 | ||
| 97+ | 8/92 (9) | 1.58 (0.75 to 3.31) | ||
| 90–<97 | 12/297 (4) | 0.70 (0.38 to 1.27) | ||
| 75–<90 | 37/874 (4) | 0.73 (0.51 to 1.05) | ||
| 25–<75 | 168/2949 (6) | 1.00 | ||
| 10–<25 | 81/900 (9) | 1.64 (1.24 to 2.16) | ||
| 3–<10 | 37/397 (9) | 1.70 (1.17 to 2.47) | ||
| <3 | 47/204 (23) | 4.96 (3.45 to 7.11) | ||
| 1 min Apgar score | <0.0001 | 0 | ||
| 4–10 | 227/4690 (5) | 1.00 | ||
| <4 | 163/1023 (16) | 3.73 (3.01 to 4.62) | ||
| Maternal ethnicity | 0.008 | 503 | ||
| Caucasian/other | 301/4268 (7) | 1.00 | ||
| Asian | 17/329 (5) | 0.72 (0.44 to 1.19) | ||
| Indigenous Australian | 32/257 (12) | 1.88 (1.27 to 2.76) | ||
| Maori | 9/119 (8) | 1.08 (0.54 to 2.15) | ||
| Pacific Islander | 11/237 (5) | 0.64 (0.35 to 1.19) | ||
| Presentation | 0.004 | 642 | ||
| Cephalic | 213/3380 (6) | 1.00 | ||
| Breech | 129/1427 (9) | 1.48 (1.18 to 1.86) | ||
| Other | 18/264 (7) | 1.09 (0.66 to 1.79) | ||
| Transferred after birth (outborn) | 0 | |||
| Yes | 54/584 (9) | 1.45 (1.08 to 1.96) | 0.01 | |
| No | 336/5129 (6) | 1.00 | ||
| Hypertensive disease in pregnancy | ||||
| Yes | 64/1254 (5) | 0.68 (0.52 to 0.90) | 0.006 | 62 |
| No | 333/4397 (7) | 1.00 | ||
| Fetal distress | 0.02 | 499 | ||
| Yes | 92/1061 (9) | 1.34 (1.05 to 1.71) | ||
| No | 275/4153 (7) | 1.00 |
GA, Gestational age.
Figure 1 Percentage dying by gestational age and weight for gestational age. Centile groups for 11 611 infants born after 24 weeks and before 32 weeks in 1998–2001.
Figure 2 Percentage dying with or without maternal hypertension in pregnancy by weight for gestational age for 11 498 infants born after 24 weeks and before 32 weeks in 1998–2001. Numbers above bars show the number of deaths. HDP, Hypertension in pregnancy; GA, gestational age.
Predictive model for death
The final multivariate model contained gestational age, sex, hypertension in pregnancy and weight for gestational age in five categories; categories for the 3rd–10th and 10th–25th centiles were combined, as were the 75th–90th and 90th–97th, because of similarity of their odds ratios (ORs) (table 3). Gestation was the dominant risk factor, the risk of death increasing with decreasing gestational age (trend χ21 = 399, p<0.0001) as indicated by the ORs in table 3. There was a steady fall in risk of mortality for weight for gestational age bands between <3rd centile and 75th–90th centile (table 3). The small number of babies above the 97th centile showed a small increase in risk, which was not significant. The risk for the smallest infants (below 3rd centile) was greater (OR 6.91 (95% confidence interval (CI): 4.52 to 10.55)) than that for the largest infants (97th centile and above) (OR 1.38 (0.63 to 3.04)). Male sex (OR 1.58 (1.25 to 1.99)) remained a strong risk factor for death (χ21 = 15.3, p<0.0001).
Table 3 Multivariate analysis: adjusted ORs for death for 5651* infants born after 24 weeks and before 32 weeks in 1998–9.
| Predictor variable | Died n (% of 386) | Survived n (% of 5265) | Adjusted OR (95% CI) | ||
|---|---|---|---|---|---|
| GA (weeks) | |||||
| 31 | 17 (4) | 1317 (25) | 1.00 | ||
| 30 | 24 (6) | 1042 (20) | 1.97 (1.05 to 3.70) | ||
| 29 | 31 (8) | 904 (17) | 2.75 (1.51 to 5.02) | ||
| 28 | 41 (11) | 724 (14) | 4.81 (2.70 to 8.58) | ||
| 27 | 62 (16) | 538 (10) | 9.18 (5.29 to 15.94) | ||
| 26 | 96 (25) | 448 (9) | 16.22 (9.53 to 27.61) | ||
| 25 | 115 (30) | 292 (6) | 32.04 (18.83 to 54.5)] | ||
| Weight for GA (centile group) | |||||
| 97+ | 8 (2) | 83 (2) | 1.38 (0.63 to 3.04) | ||
| 75–<97 | 48 (12) | 1104 (21) | 0.66 (0.47 to 0.93) | ||
| 25–<75 | 166 (43) | 2746 (52) | 1.00 | ||
| 3–<25 | 118 (31) | 1175 (22) | 2.00 (1.52 to 2.62) | ||
| <3 | 46 (12) | 157 (3) | 6.91 (4.52 to 10.55) | ||
| Male sex | 252 (65) | 2888 (55) | 1.58 (1.25 to 1.99) | ||
| Hypertension in pregnancy | 64 (17) | 1190 (23) | 0.51 (0.37 to 0.71) | ||
*Data for hypertension in pregnancy were missing for 62 mothers.
Area under ROC = 0.82.
Hosmer–Lemeshow goodness of fit = 2.19 on 7 degrees of freedom, p = 0.95.
GA, Gestational age.
The protective effect of hypertension in pregnancy remained highly significant in the multivariate analysis (χ21 = 17.9, p<0.0001). When it was added to the model, the ORs of dying associated with being small for gestational age increased by approximately 20%. In each of the two weight for gestational age groups below the 25th centile, the rate of hypertension was at least 45% compared with <16% in the other three groups.
Excluded variables
In multivariate analysis, the variables outborn, breech presentation and fetal distress did not attain significance. Ethnicity was significant (p = 0.004), but was excluded from the model because of the high percentage of missing data (11%) and the difficulty of separating the effect of ethnicity from that of other measures, such as socioeconomic status, which was not recorded. Low Apgar at 1 min remained significant in multivariate analysis (p<0.0001, OR 2.33 (95% CI 1.85 to 2.95)), but was excluded because of its very strong relationship with gestational age (the proportion with 1 min Apgar <4 increased steadily from 11% at 31 weeks to 37% at 25 weeks) and the subjectivity of its measurement. When 1 min Apgar was included, its only effect on the model was to decrease the ORs for each gestational age category, particularly for the lowest ages. The variable outborn was excluded, despite its apparent clinical relevance, because it became non‐significant at the pre‐determined 1% level in the multivariate model (OR = 1.46, (1.05 to 2.04) p = 0.03).
Model validation
The predictive model for mortality from table 3 yielded an area under the receiver operating characteristic (ROC) curve of 0.82, indicating excellent discrimination. The Hosmer–Lemeshow goodness of fit test statistic was 2.19 at 7 degrees of freedom (p = 0.95), indicating that the model fits well, but perhaps too well. The temporal stability of the model was checked on the 2000–1 dataset of 5847 infants, which yielded an area under the ROC curve of 0.83, and a goodness of fit test statistic of 4.35 at 8 degrees of freedom (p = 0.82). All variables remained significant at the 1% level. Because the variable outborn was close to significant at the 1% level for the 1998–9 cohort, we tried adding it to the model for 2000–1, but it was not significant (OR = 1.23 (0.91 to 1.66), p = 0.19), confirming our decision to exclude it. Table 4 shows the final logistic regression model derived from the whole dataset 1998–2001. The model is dominated by gestation, which alone yields an area under the ROC curve of 0.787.
Table 4 Final logistic regression models for death of infants born in 1998–2001.
| Predictor variable | Model for death (n = 11 498) | ||
|---|---|---|---|
| Parameter estimate | Standard error | Adjusted OR (95% CI) | |
| Intercept | −4.8802 | 0.1920 | |
| GA (weeks) | |||
| 31* | 0* | 1.00 | |
| 30 | 0.6075 | 0.2347 | 1.84 (1.16 to 2.91} |
| 29 | 1.1168 | 0.2193 | 3.06 (1.99 to 4.70) |
| 28 | 1.5269 | 0.2116 | 4.60 (3.04 to 6.97) |
| 27 | 2.2485 | 0.2016 | 9.47 (6.38 to 14.1) |
| 26 | 2.8459 | 0.1948 | 17.22 (11.8 to 25.2) |
| 25 | 3.4715 | 0.1939 | 32.19 (22.0 to 47.1) |
| Weight for GA (centile group) | |||
| 97+ | 0.3283 | 0.2922 | 1.39 (0.78 to 2.46) |
| 75–<97 | −0.1767 | 0.1187 | 0.84 (0.66 to 1.06) |
| 25–<75* | 0* | 1.00 | |
| 3–<25 | 0.7534 | 0.1009 | 2.12 (1.74 to 2.59) |
| <3 | 2.0865 | 0.1565 | 8.06 (5.93 to 10.9) |
| Male sex | 0.4359 | 0.0838 | 1.55 (1.31 to 1.82) |
| Hypertension in pregnancy | −0.7671 | 0.1239 | 0.46 (0.36 to 0.59) |
*Reference group.
GA, Gestational age.
Birth weight as a predictor variable
When birth weight was added to the model with sex, hypertension in pregnancy, gestational age and weight for gestational age, it was significant either as a continuous variable (p = 0.001) or as a seven‐category variable (p = 0.04), but the discriminatory power remained at 0.82. When birth weight as a continuous variable was substituted for either gestational age or weight for gestational age, the fit of the model was poor. When birth weight as a seven‐category variable was substituted for either gestational age or weight for gestational age or both, all terms in the model were highly significant (p<0.0001), but the discriminatory ability of the model either decreased or remained the same.
Discussion
This study has used data from the ANZNN, which is a network of all 29 NICUs in Australia and New Zealand. Because newborn intensive care is regionalised in both countries, this represents essentially population‐based data. The study has developed predictive models for death based on variables up to the 1 min Apgar score. We have shown that these predictive models have good discriminatory ability and stability over time.
The established predictive models for preterm mortality, although more accurate, are potentially limited by their inclusion of measures taken over the first few hours of life such as base excess and mean blood pressure.1,4 Whether this is important is not known. On the one hand, these physiological parameters may reflect intrinsic disease severity that relates to unmeasured antenatal factors such as infection. On the other hand, they may also reflect differences in clinical practice such as resuscitation policy or use of inotropes, which this study aims to explore. To reduce any potential for confounding, the variables included in the models in this study end at the 1 min Apgar score. In using this model to compare NICU outcomes, it will be possible to explore different risk adjustment models that include early postnatal physiological data. If the models show major differences in NICU outcome, it could help identify whether early treatment bias is important.
Maturity is always the dominant predictive variable in any preterm risk model. The previous models have also used birth weight as the primary measure of the maturity of the infant.1,4 The reason for this may be that these models were developed in healthcare systems where accurate gestational assessment was not always available. The advantage of birth weight is that it is always available and should be accurate; the disadvantage is that it combines two parameters, maturity and growth relative to that maturity.10,11 In the healthcare systems in both Australia and New Zealand, gestation is known in most cases either by certain dates or by early ultrasound. In this analysis, there was no improvement in the predictive properties of the model when birth weight was used rather than gestation. The advantage of using gestation is that it measures only maturity. This has given us the power to explore more fully the independent effects of growth relative to maturity in this analysis. The data show not only that growth is a significant predictor of mortality but also that there is an effect across the centiles, with centile weight below the mean being a risk factor and that above the mean being protective for mortality. However, the relationship is not linear in that infants above the 97th centile show an insignificant trend to increased mortality risk.
Several studies have highlighted that growth restriction is a risk factor for mortality and other adverse outcomes.12,13,14,15,16,17,18,19,20,21 In most of these studies, growth restriction is classified as a dichotomous variable with infants above or below the 3rd or 10th centile. These studies consistently show increased mortality, chronic lung disease and retinopathy of prematurity in growth‐restricted infants. Morley et al21 used a birthweight ratio (actual weight divided by the mean for that gestation) in a trial cohort, to show increasing post‐neonatal mortality with decreased birthweight ratio but not neonatal mortality and increased risk of other morbidities. They highlighted the importance of analysing birth weight for gestation as a continuous rather than a dichotomous variable.21 McIntire et al16 looked at birthweight centile bands in infants born before 37 weeks and showed increasing mortality below the 25th centile on univariate analysis. Draper et al22 showed a decrease in mortality risk in higher birthweight bands for each week of gestational age, an approach that was incorporated into the CRIB II score.23 The multivariate analysis in our population‐based analysis of infants born before 32 weeks shows a similar increase in mortality below the 25th centile, but confirms a lower mortality between the 75th and 97th centiles. The mechanistic reason for this relationship remains unclear; however, fetal growth restriction is associated with hypoxaemia, undernutrition and endocrine changes that affect organ growth and development.24 The observation of a trend to increased risk in babies born above the 97th centile is interesting, as this finding is consistent with that of a recent population‐based study from Sweden.20 One possible explanation for this would be hydropic infants. However, these were excluded from our analysis, so the reason for this possible increase in risk for large infants remains unclear and should be interpreted with caution as the numbers are small.
In developing this model of mortality, we excluded some subjects at the borderlines of viability (<25 weeks). Arguably, this exclusion misses an opportunity to compare an important area of performance. It was our view that mortality under 25 weeks reflects a complicated mix of factors, which will include not only NICU performance but also the intensive care philosophy of that NICU and, most importantly, the choices of parents who may request non‐intervention. We also excluded some variables that were significant. Ethnicity is difficult to analyse in the different and distinct multicultural societies of Australia and New Zealand. In particular, we did not have a good measure of socioeconomic background. In addition, the dataset captures a single self‐nominated ethnicity only for the mother but not for the father, and has a high proportion of missing data. We therefore decided to exclude it from the model. Low Apgar at 1 min was excluded because of the subjectivity of its measurement and its very strong relationship with gestational age. We speculate that, within the model, it is a surrogate measure of maturity rather than a direct causative risk factor for death. Hence, the final predictive model includes gestation, male sex, weight centile (weight for gestation) and hypertension in pregnancy. This simple model gives an area under the ROC curve which, although less than that of other previously described scores, is within a similar range of accuracy. Gestation, male sex and birthweight centile have been consistent risk factors in analyses of other outcomes within the ANZNN, specifically chronic lung disease and retinopathy of prematurity.26,27 Interestingly, weight for gestational age was not a major risk factor for the development of major intraventricular haemorrhage.28
What is already known on this topic
Immaturity, whether measured by birth weight or gestation, is the dominant predictor of mortality in preterm infants.
Prediction of mortality is possible with models that include early postnatal physiological variables.
What this paper adds
By using gestation rather than birth weight as the main measure of maturity, this study shows that weight for gestational age is a significant and dose‐dependent predictor of preterm mortality.
Prediction of preterm mortality is possible using variables up to the 1 min Apgar score.
Using this model to control for case mix in comparisons of newborn intensive care unit outcomes should remove the risk of introducing bias from variations in early postnatal management.
In conclusion, this paper has described a simple, stable and accurate model using perinatal variables for prediction of mortality in very preterm infants. By using gestation rather than birth weight as the main indicator of maturity, these data show that weight for gestational age is an independent risk factor for mortality. This model will be a useful tool to control for case mix in the comparison of inter‐NICU outcome.
Abbreviations
ANZNN - Australian and New Zealand Neonatal Network
CRIB - clinical risk index for babies
IQR - interquartile range
NICU - newborn intensive care unit
ROC - receiver operating characteristic
ROM - rupture of membranes
Appendix
The ANZNN advisory committee and executive (*) members.
Australia: Centre for Perinatal Health Services Research, NSW: David Henderson‐Smart*. Flinders Medical Centre, SA: Peter Marshall. John Hunter Hospital, NSW: Chris Wake. King Edward Memorial and Princess Margaret Hospitals, WA: Noel French, Ron Hagan and Karen Simmer. Launceston General Hospital, Tasmania: Chris Bailey. Liverpool Health Service, NSW: Robert Guaran. Mater Mother's Hospital, Qld: David Tudehope. Mercy Hospital for Women, Vic: Andrew Watkins. Monash Medical Centre, Vic: Kaye Bawden*, Andrew Ramsden, Victor Yu. National Perinatal Statistics Unit, NSW: Paul Lancaster*. Nepean Hospital, NSW: Lyn Downe. Newborn Emergency Transport Service (Vic): Michael Stewart. NSW Newborn & Paediatric Emergency Transport Service, NSW: Andrew Berry. Perinatal Research Centre, Qld: Paul Colditz. Royal Children's Hospital, Vic: Linda Johnstone, Peter McDougall. Royal Darwin Hospital, NT: Charles Kilburn. Royal Hobart Hospital, Tasmania: Peter Dargaville. Royal Hospital for Women, NSW: Kei Lui. Royal North Shore Hospital, NSW: Jennifer Bowen. Royal Prince Alfred Hospital, NSW: Nick Evans Royal Women's Hospital, Qld: David Cartwright*. Royal Women's Hospital, Vic: Lex Doyle, Colin Morley, Neil Roy. Sydney Children's Hospital, NSW: Barry Duffy. The Canberra Hospital, ACT: Graham Reynolds. The Children's Hospital at Westmead, NSW: Robert Halliday. The Townsville Hospital, Qld: John Whitehall. Western Australia Neonatal Transport Service, New Plymouth: Jenni Sokol. Westmead Hospital, NSW: William Tarnow‐Mordi. Women's & Children's Hospital, SA: Ross Haslam. Deborah Donoghue is the ANZNN Coordinator.
New Zealand: Christchurch Women's Hospital, Christchurch: Nicola Austin. Christchurch School of Medicine, Christchurch: Brian Darlow*. Dunedin Hospital, Dunedin: Roland Broadbent. Gisborne Hospital, Gisborne: Graeme Lear. Hastings Hospital, Hastings: Jenny Corban. Hutt Hospital, Hutt: Robyn Shaw. Middlemore Hospital, Auckland: Lindsay Mildenhall. National Women's Hospital, Auckland: Carl Kushell. Nelson Hospital, Nelson: Peter McIlroy. Palmerston North Hospital, Palmerston: Jeff Brown. Rotorua Hospital, Rotorua: Stephen Bradley. Southland Hospital, Invercargill: Paul Tomlinson. Taranaki Hospital, New Plymouth: John Doran*. Tauranga Hospital, Tauranga: Hugh Lees. Timaru Hospital, Timaru: Philip Morrison. University of Auckland, Auckland: Jane Harding. Waikato Hospital, Hamilton: David Bourchier. Wairau Hospital, Blenheim: Ken Dawson. Wanganui Hospital, Wanganui: John Goldsmith. Wellington Women's Hospital, Wellington: Vaughan Richardson. Whakatane Hospital, Whakatane: Chris Moyes. Whangarei Hospital, Whangarei: Peter Jankowitz.
Footnotes
Competing interests: None.
References
- 1.Cockburn F, Cooke R W I, Gamsu H R.et al The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet 1993342193–198. [PubMed] [Google Scholar]
- 2.Horbar J D, Onstad L, Wright E. Predicting mortality for infants weighing 501–1500 grams at birth: a National Institutes of Health Research Network report. Crit Care Med 1993212–3. [DOI] [PubMed] [Google Scholar]
- 3.Richarson D K, Phibbs C S, Gray J E.et al Birth weight and illness severity: independent predictors of neonatal mortality. Pediatrics 199391969–975. [PubMed] [Google Scholar]
- 4.Richardson D K, Corcoran J D, Escobar G J.et al SNAP‐II and SNAPPE‐II: simplified newborn illness severity and mortality risk scores. J Pediatr 200113892–100. [DOI] [PubMed] [Google Scholar]
- 5.Pollack M M, Koch M A, Bartel D A.et al A comparison of neonatal mortality risk prediction models in very low birth weight infants. Pediatrics 20001051051–1057. [DOI] [PubMed] [Google Scholar]
- 6.Sankaran K, Chien L Y, Walker R.et al Variation in mortality rates among Canadian neonatal intensive care units. Can Med Asocs J 2002166173–178. [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson J M, Evans N, Gibberd R W.et al Analysing differences in clinical outcomes between hospitals. Qual Safety Health Care 200312257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts C L, Lancaster P A. Australian national birthweight percentiles by gestational age. Med J Aust 1999170114–118. [DOI] [PubMed] [Google Scholar]
- 9.Hosmer D W, Lemeshow S.Applied logistic regression, 2nd edn. New York: John Wiley and Sons 2000
- 10.Arnold C C, Kramer M S, Hobbs C A.et al Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol 1991134604–613. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox A J. On the importance‐and the unimportance‐of birthweight. Int J Epidemiol 2001301233–1241. [DOI] [PubMed] [Google Scholar]
- 12.Reiss I, Landmann E, Heckmann M.et al Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch Gynecol Obstet 200326940–44. [DOI] [PubMed] [Google Scholar]
- 13.Blickstein I, Keith L G. Neonatal mortality rates among growth‐discordant twins, classified according to the birth weight of the smaller twin. Am J Obstet Gynecol 2004190170–174. [DOI] [PubMed] [Google Scholar]
- 14.Regev R H, Lusky A, Dolfin T.et al Israel Neonatal Network. Excess mortality and morbidity among small‐for‐gestational‐age premature infants: a population‐based study, J Pediatr 2003143186–191. [DOI] [PubMed] [Google Scholar]
- 15.Zaw W, Gagnon R, da Silva O. The risks of adverse neonatal outcome among preterm small for gestational age infants according to neonatal versus fetal growth standards. Pediatrics 20031111273–1277. [DOI] [PubMed] [Google Scholar]
- 16.McIntire D D, Bloom S L, Casey B M.et al Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 19993401234–1238. [DOI] [PubMed] [Google Scholar]
- 17.Gortner L, Wauer R R, Stock G J.et al Neonatal outcome in small for gestational age infants: do they really better? J Perinatal Med 199927484–489. [DOI] [PubMed] [Google Scholar]
- 18.Bardin C, Zelkowitz P, Papageorgiou A. Outcome of small‐for‐gestational age and appropriate‐for‐gestational age infants born before 27 weeks of gestation. Pediatrics 1997100E4. [DOI] [PubMed] [Google Scholar]
- 19.Larroque B, Breart G, Kaminski M.et al Survival of very preterm infants: Epigage, a population based cohort study. Arch Dis Child Fetal Neonatal Ed 200489F139–F144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson S, Montgomery S M, Ekborn A.et al Preterm delivery, level of care and infant death in Sweden: a population based study. Pediatrics 20041131230–1235. [DOI] [PubMed] [Google Scholar]
- 21.Morley R, Brooke O G, Cole T J.et al Birthweight ratio and outcome in preterm infants. Arch Dis Child 19906530–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draper E S, Manktelow B, Field D.et al Prediction of survival for preterm births by weight and gestational age: a retrospective population based study. BMJ 19993191093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parry G, Tucker J, Tarnow‐Mordi W. CRIB II: an update of the clinical risk index for babies score. Lancet 20033611789–1801. [DOI] [PubMed] [Google Scholar]
- 24.McMillen I C, Adams M B, Ross J T.et al Fetal growth restriction: adaptation and consequences. Reproduction 2001122195–204. [DOI] [PubMed] [Google Scholar]
- 25.Von Dadelszen P, Magee L A, Taylor E L.et al Maternal hypertension and neonatal outcome among small for gestational age infants. Obstet Gynecol 2005106335–339. [DOI] [PubMed] [Google Scholar]
- 26.Darlow B A, Hutchinson J L, Simpson J M.et al Prenatal risk factors for severe retinopathy of prematurity in very preterm infants of the Australian and New Zealand Neonatal Network (ANZNN). Pediatrics 2005115990–996. [DOI] [PubMed] [Google Scholar]
- 27.Henderson‐Smart D, Hutchinson J L, Donoghue D A, Neonatalet al Prenatal predictors of chronic lung disease in very preterm infants. Arch Dis Child: ed. 10. 1136/adc. 2005. 072264. [DOI] [PMC free article] [PubMed]
- 28.Heuchan A M, Evans N, Henderson Smart D J.et al JM. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995 to 1997. Arch Dis Child 200286F86–F90. [DOI] [PMC free article] [PubMed] [Google Scholar]