Abstract
Spontaneous neonatal arterial thrombosis is rare in the neonatal period. Four cases of neonatal arterial thrombosis presenting with suspected congenital heart disease are reported. The urgency for a correct diagnosis in this setting and the need for active treatment for a remediable condition are emphasised. These treatment options are discussed.
Neonatal arterial thrombosis presenting as suspected congenital heart disease, particularly coarctation of the aorta, has been reported previously.1,2,3,4 The incidence is not known; however, the incidence of symptomatic neonatal arterial thromboembolic disease is reported to be approximately 0.25/10 000 live births, with 90% of these associated with arterial access devices.5 Mortality is high, irrespective of treatment, and has been quoted as high as 33% in infants with aortic thrombosis.6
We conducted a retrospective review of our database, looking at cases of arterial thrombosis presenting to our tertiary referral unit from January 2000 over a 5‐year period. The medical notes were reviewed, and four cases presenting to our institution with suspected congenital heart disease were found to have spontaneous neonatal arterial thrombosis. We present these cases, with a discussion of the existing diagnostic and therapeutic difficulties.
Case 1
A term male infant presented acutely at the age of 4 days with a blue, cold left leg associated with weak femoral pulses. Coarctation of the aorta was suspected clinically, and he was started on a continuous prostaglandin E1 (PGE1) infusion and transferred to a tertiary paediatric cardiology centre. He was normotensive, with normal brachial pulses but reduced right femoral pulses and a virtually absent left femoral pulse. A clear demarcation line was noted at the time of presentation along the mid‐tibial portion of the left leg, but there was no murmur. Echocardiography showed a structurally normal heart with a normal aortic arch. Subsequent abdominal ultrasound showed complete occlusion of the descending aorta, with a 15‐mm clot protruding into the mid‐portion of the aorta below the renal arteries.
He had a full thrombophilia screen and was started on a continuous intravenous heparin infusion, together with an infusion of tissue plasminogen activator (tPA). Over the subsequent 48 h, perfusion of his left leg improved and a repeat abdominal ultrasound scan showed some resolution of the thrombus. The tPA was discontinued, but he remained on intravenous heparin. His thrombophilia screen showed low levels of anti‐thrombin III at 21% (normal range 41–93%), and he was also heterozygotic for factor V Leiden mutation. The maternal levels of anti‐thrombin III were also low, and paternal screening showed a heterozygotic factor V Leiden mutation. He was discharged home on low‐molecular‐weight heparin (LMWH), which was subsequently changed to oral aspirin. He has been discharged from cardiology follow‐up, with normal blood pressure and normal femoral pulses, but continues to be seen by a haematologist.
Case 2
A term male infant presented with an acute collapse at day 12 of life. He was small for dates, with a birth weight of 2.16 kg, owing to maternal pre‐eclampsia. On admission, he was desaturated with oxygen saturations of 89% in air. No murmurs were heard, and the peripheral pulses were normal. However, signs of reduced cardiac output were observed, and he was therefore started on a PGE1 infusion. He was suspected of having congenital heart disease.
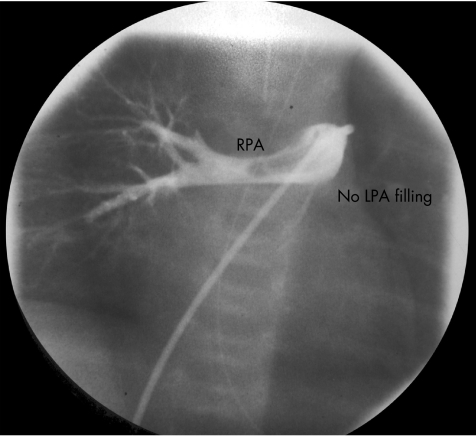
Echocardiography findings were consistent with pulmonary hypertension, with right‐to‐left shunting at the atrial level. The left pulmonary artery (LPA) was not visualised, and he was started on inhaled nitric oxide without improvement. Subsequent echocardiography raised the possibility of an absent or aberrant LPA, and the patient underwent cardiac catheterisation to investigate this more fully. This confirmed marked pulmonary hypertension, and angiography showed a small, hypoplastic LPA (fig 1), which at the time was interpreted as severe congenital LPA stenosis. A coronary artery stent was implanted, which led to a decrease in the right ventricular‐to‐systemic pressure ratio by 40%. Subsequent angiography showed good flow across the stent, but irregular filling of the LPA.
Figure 1 Injection of contrast dye into the main pulmonary artery showing filling of the right pulmonary artery (RPA), but no filling of the left pulmonary artery (LPA) as indicated. There is also a filling defect in the lumen of the RPA.
Retrospective review of the angiograms showed that the filling defect presumed to be LPA stenosis was in fact a large thrombus in the main pulmonary artery and extending into the LPA. Repeat cardiac catheterisation 6 days later showed a marked improvement in flow to the LPA, with a maintained reduction in the right ventricular pressure and no pronounced residual clot in the main pulmonary artery. A subsequent thrombophilia screen was normal. The patient was continued on subcutaneous LMWH for 3 months, which was later changed to oral aspirin. He went on to develop progressive LPA stenosis and required further angioplasty and subsequent surgical augmentation of his LPA. He is clinically asymptomatic, but has persisting LPA stenosis and may need further intervention for this.
Case 3
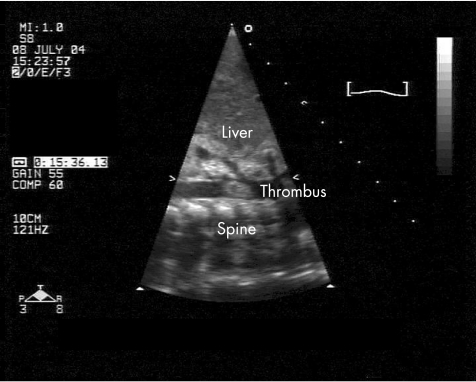
A term baby girl presented at 2 weeks of age with a 24‐h history of poor feeding. She was delivered by uncomplicated ventouse extraction and was discharged home well within the first day of life, with an unremarkable antenatal history. Examination on presentation showed poor peripheral perfusion, and she required bolus fluid resuscitation. Her peripheral pulses remained difficult to feel, but no murmurs were heard. Echocardiography at the time suggested moderately impaired left‐ventricular function, with coarctation of the aorta, and she was transferred to the regional paediatric cardiac surgery unit on a PGE1 infusion for further assessment. Subsequent echocardiography showed a thrombus in the left atrium, left ventricle and descending aorta at the origin of the superior mesenteric artery (fig 2) but no coarctation. She was started on inotropes to support her left ventricle, and on intravenous heparin, in addition to tPA, via an arterial catheter placed directly in the aorta. She remained on low‐dose tPA infusion for 4 days, which was subsequently changed to intravenous heparin infusion owing to considerable improvement in the size of the thrombus and the development of staphylococcal sepsis, necessitating removal of her central lines. She was initially hypertensive and treated with an oral calcium channel antagonist, but her blood pressure settled, with resolution of the thrombus. A thrombophilia screen was negative and the patient was discharged home on LMWH, which was discontinued within 3 months of discharge. She has since been discharged from cardiology follow‐up, with good cardiac function and a normal aortic arch.
Figure 2 Sagittal view of the descending aorta on two‐dimensional echocardiography showing the clot in the lumen of the aorta as labelled.
Case 4
A male infant, born at 42 weeks after postmenstrual age was noted at day 1 to have decreased femoral pulses. Upper limb pulses were palpable, and the preductal oxygen saturations were 95%, with postductal saturations of 91%. No murmurs were heard on auscultation. Echocardiography showed considerably pulmonary hypertension, with an echo‐dense mass in the region of the aortic isthmus. There was little flow around this mass, with a tiny PDA with bidirectional flow. Thrombus was strongly suspected and the patient was started on an intravenous infusion of PGE1 and streptokinase. Subsequent echocardiography 12 h later showed no improvement and streptokinase was substituted for intravenous heparin infusion.
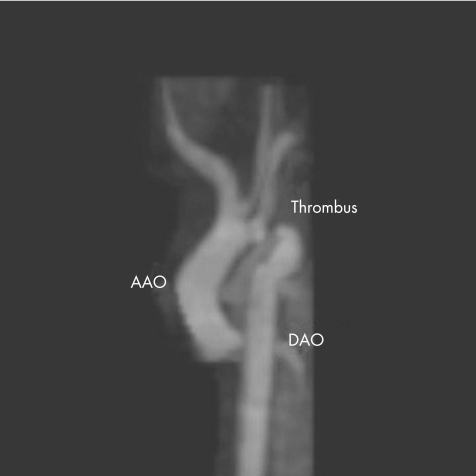
A cranial ultrasound scan at that time showed a bright area in the left frontal region. A subsequent magnetic resonance image of the brain showed an extensive cerebral infarction in the territory of the left middle cerebral artery thought to be of prenatal origin. Magnetic resonance imaging of the chest was also requested, which confirmed the presence of thrombus in the aortic arch (fig 3). The infant was subsequently transferred to the regional paediatric cardiac surgical unit and underwent an aortic thrombectomy, with subclavian flap repair of his aorta. He recovered well. Intravenous heparin was changed to LMWH before he was discharged and this was discontinued after 3 months. A thrombophilia screen was normal. Follow‐up has shown considerable developmental delay, with persisting generalised seizures despite treatment. He is normotensive and has normal femoral pulses.
Figure 3 Magnetic resonance imaging scan of the aorta showing a thrombus in the arch as labelled. The appearance of the arch mimics coarctation of the aorta.18 AAO, ascending aorta; DAO, descending aorta.
Discussion
Although rare, neonatal arterial thrombosis does occur and may mimic congenital heart disease. This may be difficult to diagnose even in a specialist centre, as is highlighted by two of the cases discussed. The importance of acting on clinical suspicion, particularly in the case of diminished femoral pulses, and requesting urgent specialist input cannot be emphasised enough. This is highlighted by the high rate of false‐negative results with ultrasound imaging, with a study by Vailas et al7 reporting four cases of false negatives using ultrasonography in a series of 20 neonates with clinical signs of aortic thrombosis.
Management of arterial thrombosis in the neonatal period remains controversial. A recent systematic review provided no conclusions about the most appropriate treatment, as no eligible studies were found.8 It is generally acknowledged that there is urgent need for large multicentre studies on which to base recommendations. Treatment options include anticoagulant treatment with heparin or LMWH, thrombolytic treatment or surgery. Warfarin is not indicated in the neonatal period because of the difficulties in establishing consistent levels of anticoagulation.9
The optimal treatment depends on the availability of surgical expertise, the associated risk factors for bleeding and the degree of organ ischaemia. Recent recommendations from the Seventh American College of Chest Physicians Conference on Antithrombotic and Thrombolytic Therapy10 suggest “the urgent, aggressive use of thrombolytic or surgical therapy supported by anticoagulation with heparin or LMWH (Grade 2C)” for children experiencing spontaneous aortic thrombosis with evidence of renal ischaemia. It is unclear from this which thrombolytic agent is most effective; however, tPA has become the agent of choice for several reasons, including recent Food and Drug Administration warnings regarding urokinase, experimental evidence of improved clot lysis in vitro compared with that using urokinase and streptokinase, fibrin specificity and low immunogenicity.11,12
The support of thrombolysis with concomitant heparin may be synergistic; however, whether LMWH or unfractionated heparin (UFH) is better is still unclear. A case series by Klinger et al13 suggests successful treatment of severe aortic thrombosis in two neonates with LMWH alone. The potential advantages of LMWH compared with UFH include a more predictable pharmacokinetic profile, minimal monitoring, subcutaneous administration and potentially less bleeding and osteopaenia than in UFH.14 Similar to UFH, the optimal dose of LMWH in newborns remains unknown. All of our infants were discharged home on LMWH.
Surgical treatment may be necessary either acutely or to treat the effects of arterial thrombus, as outlined in the second case. There are also no specific guidelines for the use of thrombectomy in neonates and the risk:benefit ratio needs to be considered individually in each case. There are no controlled data to compare the value of conservative treatment, and such data will probably not become available.
The major risk of treatment is bleeding. A review by Zenz et al15 reported intracranial haemorrhage in only 1 of 83 term infants receiving tPA, with another retrospective analysis of 16 newborns who received tPA reporting one death from bleeding.16
Although the risk of bleeding with LMWH is not precisely known, there are studies reporting the risk of bleeding in newborns as part of larger patient populations. In one study by Dix et al,17 4 of 37 infants had major bleeding. The locations were at the site of subcutaneous catheters in two newborns, with little subcutaneous tissue and in pre‐existing abnormalities in the central nervous system in two other newborns. Subcutaneous catheters should be used with caution in newborns with little subcutaneous tissue. If the anticoagulant effect of LMWH needs to be terminated immediately, protamine sulphate will reverse the anti‐thrombin activity, but will only partially affect the anti‐factor Xa activity. Major bleeding was not seen in any of our cases.
In conclusion, newborn infants with arterial thrombosis may present with suspected congenital heart disease. This diagnosis should be considered, particularly with inconsistent clinical and ultrasound findings. Aetiology in most of the cases is not known. Although mortality is high, urgent instigation of active treatment can lead to complete resolution. Long‐term follow‐up is necessary to assess future risk.
Abbreviations
LMWH - low‐molecular‐weight heparin
LPA - left pulmonary artery
PGE1 - prostaglandin E1
tPA - tissue plasminogen activator
UFH - unfractionated heparin
Footnotes
Competing interests: None declared.
References
- 1.Das B B, Sahoo S, Ivy D D. Neonatal aortic arch thrombosis masquerading as coarctation of the aorta. Pediatr Cardiol 20042580–83. [DOI] [PubMed] [Google Scholar]
- 2.Bonhoeffer P, Bonnet D, Sidi D.et al Thrombus in coarctation of the aorta masquerading as an interrupted aortic arch. Heart 199777183–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guenthard J, Wyler F, Nars P W. Neonatal aortic thrombosis mimicking coarctation of the aorta. Eur J Pediatr 1995154163–164. [DOI] [PubMed] [Google Scholar]
- 4.Evans D J, Pizer B L, Moghal N E.et al Neonatal aortic arch thrombosis. Arch Dis Child 199471F125–F127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenway A, Massicotte M P, Monagle P. Neonatal thrombosis and its treatment. Blood Rev 20041875–84. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt B, Andrew M. Neonatal thrombosis: report of a prospective Canadian and international registry. Pediatrics 199596939–943. [PubMed] [Google Scholar]
- 7.Vailas G N, Brouillette R T, Scott J P.et al Neonatal aortic thrombosis: recent experience. J Pediatr 1986109101–108. [DOI] [PubMed] [Google Scholar]
- 8.John C M, Harkensee C. Thrombolytic agents for arterial and venous thromboses in neonates. Cochrane Database Syst Rev 2005(25)CD004342. [DOI] [PMC free article] [PubMed]
- 9.Williams M D, Chalmers E A, Gibson B E. Haemostasis and Thrombosis Task Force, British Committee for Standards in Haematology. The investigation and management of neonatal haemostasis and thrombosis. Br J Haematol 2002119295–309. [DOI] [PubMed] [Google Scholar]
- 10.Monagle P, Chan A, Massicotte P.et al Antithrombotic therapy in children: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004126S645–S687. [DOI] [PubMed] [Google Scholar]
- 11.Food, Drug Administration ( F D A )Important drug warning regarding the use of Abbokinase (urokinase). Rockville, MD: Public Health Service, 1999
- 12.Gupta A A, Leaker M, Andrew M.et al Safety and outcomes of thrombolysis with tissue plasminogen activator for treatment of intravascular thrombosis in children. J Pediatr 2001139682–688. [DOI] [PubMed] [Google Scholar]
- 13.Klinger G, Hellmann J, Daneman A. Severe aortic thrombosis in the neonate—successful treatment with low‐molecular‐weight heparin: two case reports and review of the literature. Am J Perinatol 200017151–158. [DOI] [PubMed] [Google Scholar]
- 14.Andrew M, Monagle P, deVeber G.et al Thromboembolic disease and antithrombotic therapy in newborns. Hemotology 2001358–374. [DOI] [PubMed]
- 15.Zenz W, Muntean W, Gallistl S.et al Recombinant tissue plasminogen activator treatment in two infants with fulminant meningococcemia. Pediatrics 19959644–48. [PubMed] [Google Scholar]
- 16.Farnoux C, Camard O, Pinquier D.et al Recombinant tissue‐type plasminogen activator therapy of thrombosis in 16 neonates. J Pediatr 1998133137–140. [DOI] [PubMed] [Google Scholar]
- 17.Dix D, Andrew M, Marzinotto V.et al The use of low molecular weight heparin in pediatric patients: a prospective cohort study. J Pediatr 2000136439–445. [DOI] [PubMed] [Google Scholar]
- 18.Dua J S, Suresh S, Wilson D G. An unusual case of aortic obstruction. Images Paediatr Cardiol 20042011–14. [PMC free article] [PubMed] [Google Scholar]