Abstract
Atopic diseases are complex entities influenced by an array of risk factors, including genetic predisposition, environmental allergens, antenatal exposures, infections and psychosocial factors. One proposed mechanism by which these risk factors contribute to the development of atopic disease is through changes in the production of T helper cell type 1 (Th1) and T helper cell type 2 (Th2) cytokines. The objectives of this review are to discuss antenatal exposures that are associated with paediatric atopic diseases, to discuss the influence of the intrauterine environment on neonatal immune responses, to provide an overview of the Th1 and Th2 pathways and how they relate to atopic disease, and to summarise our current understanding of the association between cytokine responses in cord blood and the development of atopic disease in early childhood.
With the recent rise in atopic disease prevalence, it would be beneficial for clinicians to become familiar with research advances made in the area of paediatric atopic disease pathogenesis. Atopic diseases—asthma, allergic rhinitis, atopic dermatitis and food allergy—are complex entities with an array of risk factors that may be categorised into genetic predisposition, environmental allergens, antenatal exposures, infections and psychosocial factors (fig 1).
Figure 1 Atopic disease risk factors.
Genetic predisposition is central to the development of atopic disease as shown by the increased disease prevalence among first‐degree relatives of affected people and those with a positive family history of atopic disease,1,2,3 by monozygotic versus dizygotic twin studies,4 and by the identification of numerous chromosomal linkages, single‐nucleotide polymorphisms and haplotypes that are associated with an increased risk of atopic disease or biomarkers of atopy, such as serum IgE levels.5,6,7 Atopic diseases are inherited as complex diseases involving the interplay of as many as 20 separate genes. Evidence of gene–environment interactions shows that the environment has a modifying effect on the expression of certain genes in atopic disease.8,9
The environment and infectious diseases affect the development of atopic disease, and have recently received a great deal of attention in view of the recent upsurge in atopic disease prevalence. It is unclear whether infections alter actual disease risk; however, respiratory syncytial virus and rhinovirus infections are associated with an increased likelihood of subsequent wheezing and childhood asthma.10,11 Supporters of the controversial “hygiene hypothesis” attribute the increased prevalence of atopic disease in the Western world to a relative decrease in infectious diseases associated with trends that include, but are not limited to, smaller family size, an increased emphasis on hygiene and the widespread use of antibiotics.12,13 This theory is supported by evidence showing a decreased risk of atopic disease where there is an increased exposure of young children to microorganisms, including an increased exposure to endotoxin in the first several months of life,14 daycare attendance in infancy,15 living with older siblings,12,15 living on a farm16 and early pet exposure.17,18 Additional studies, but not all,19 have shown an association between antibiotic use in early life and an increased risk of asthma or atopy later in childhood.20,21
Paediatricians are generally familiar with genetic predisposition and many of the postnatal exposures associated with atopic disease. However, antenatal exposures associated with the development of atopic disease and recent advances in atopic disease pathogenesis may not be in the purview of the general paediatrician or practitioner. This review focuses on the role of the intrauterine environment and antenatal exposures in the development of atopic disease in early childhood. The objectives of this review are to discuss antenatal exposures that are associated with paediatric atopic diseases, to discuss the influence of the intrauterine environment on neonatal immune responses, to provide an overview of the T helper cell type 1 (Th1) and T helper cell type 2 (Th2) pathways and how they relate to atopic disease, and to summarise our current understanding of the association between cytokine responses in cord blood and the development of atopic disease in early childhood.
Antenatal exposures associated with paediatric atopic disease and cord blood biological assays showing evidence of neonatal antigen‐specific immunity
The importance of the intrauterine environment in atopic disease pathogenesis is supported by data showing a greater influence of maternal over paternal atopy on disease risk in the offspring1,2 and multiple antenatal risk factors for paediatric atopic disease.2,22,23,24 Several maternal health characteristics and behaviours during pregnancy are associated with paediatric atopic disease in the offspring. These include low maternal parity,25 respiratory and genitourinary infections,23,26 cigarette smoking22,27,28,29 and antibiotic use during pregnancy,30 a proxy for maternal infection. At birth, risk factors for atopic disease that may reflect intrauterine exposures include higher gestational age,2 low birth weight and prematurity,22 and delivery by caesarean section.31,32,33
Beyond the epidemiological associations found between certain antenatal exposures and the subsequent development of atopic disease in offspring, there are biological measures that show evidence of neonatal T cell and B cell immune responses to antenatal exposures. Cord blood biological assays among offspring show evidence of neonatal immune responses to antigens.34,35 At birth, in vitro allergen‐stimulated T cell or cord blood mononuclear cell (CBMC) proliferation occurs in response to a variety of environmental allergens, including β‐lactoglobulin, birch pollen, bovine serum albumin, cat fur, cockroach, house dust mite, mouse, ovalbumin and timothy grass pollen.34,36,37,38,39 According to some investigators, these responses indicate that neonatal immune responses, thought to be naive, may in fact be influenced by antenatal exposures,37 but others have challenged the specificity of such studies.40 Antigen‐specific B cell responses to maternal antigen exposures during pregnancy have also been described. After giving tetanus vaccine to pregnant women, investigators have detected antigen‐specific IgM production in cord blood.41 In addition, measurable total and allergen‐specific IgE in cord blood provides evidence that isotype‐class switching occurs in response to in utero allergen exposure.35
Several plausible mechanisms by which in utero sensitisation may occur are seen. One possibility is that the antigen or processed peptide to which the mother is exposed during pregnancy reaches the placenta where antigen presentation occurs. In support of this mechanism, the dust mite antigen, Dermatophagoides pteronyssinus 1, has been detected in amniotic fluid and umbilical cord blood, introducing the possibility of transplacental passage of antigens.42 In addition, antigen presenting cells have been detected in the placenta and have been shown to facilitate antigen‐induced T cell proliferative responses.43
Overview of Th1 and Th2 cytokines and how they relate to atopic disease
In this section, we provide a simplified overview of Th1 and Th2 cytokines and how they relate to atopic disease. In an effort to better understand disease pathogenesis and to identify biological measures to predict atopic disease and potential therapeutic modalities to treat disease, much attention has recently been focused on Th1 and Th2 cytokine production and on their counter‐regulatory actions. A general understanding of Th1 and Th2 cytokines is necessary to understand how cord blood cytokines relate to paediatric atopic disease development.
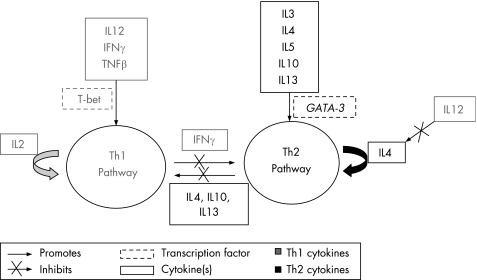
Th1 and Th2 lymphocytes, thought to be the differentiated progeny of a population of naive lymphocytes, are defined by the cytokines that they produce. Table 1 describes the major Th1 and Th2 cytokines. To a large extent, Th1 and Th2 pathways—influenced by cytokines, other immunological cells and transcription factors—are thought to be counter‐regulatory (fig 2). The Th1 pathway is essential for cell‐mediated immunity and occurs in response to some bacterial infections. The Th2 pathway, essential for all humoral immunity, is thought to play a major part in atopic disease (fig 3), with multiple studies showing an association between the atopic phenotype and increases in Th2 cytokines in the sera and the bronchoalveolar lavages of affected people.44,45,46
Table 1 Brief description of major T helper type 1 and 2 (Th1 and Th2) cytokines.
| Cytokine | Description |
|---|---|
| Th1 cytokines | |
| IFNγ | Most reliably produced by Th1 cells |
| Th2 antagonist through activation of T cell transcription factor, T‐bet and subsequent inhibition of Th2 cell differentiation | |
| Activates macrophages | |
| Stimulates production of IgG | |
| Inhibits IL4‐induced IgE synthesis in human PBMCs | |
| TNFα | Stimulates phagocytosis, and production of IL1 and oxidants |
| TNFβ | Involved in cell proliferation, differentiation and apoptosis |
| Cytotoxic to a range of tumour cells | |
| IL12 | Produced by various APCs |
| Induces IFNγ | |
| Inhibits IL4 | |
| Th2 cytokines | |
| IL4 | Reliably Th2 |
| Polarises naive T cells towards the Th2 phenotype, through activation of the transcription factor GATA3 | |
| With IL13 causes isotype switching of B cells to synthesise IgE after exposure to allergen | |
| Activates and stimulates bone marrow production of eosinophils | |
| IL5 | Activates and stimulates bone marrow production of eosinophils |
| IL9 | Involved in eosinophil and mast cell development |
| IL13 | With IL4 causes isotype switching of B cells to synthesise IgE after exposure to allergen in humans |
| Contributes to airway hyper‐reactivity in animals |
APC, antigen‐presenting cell; IL, interleukin; IFN, interferon; PBMC, peripheral blood mononuclear cell; TNF, tumour necrosis factor.
Figure 2 Overview of the counter‐regulatory actions of the T helper type 1 and 2 (Th1 and Th2) pathways. IFN, interferon; IL, interleukin; TNF, tumour necrosis factor.
Figure 3 T helper cell type 2 (Th2) cytokines and atopic disease.
The Th2 dominance seen in people affected by atopic disease has led many researchers to believe that environmental and infectious exposures, including antenatal exposures, may modulate disease risk through changes in the balance between the Th1 and Th2 cytokine pathways. Several exposures associated with an enhanced Th2 response and a reduced Th1 response are also associated with atopic disease. For example, cigarette smoking during pregnancy, a known risk factor for childhood asthma, is associated with increased levels of the Th2 cytokine, interleukin (IL)13, and decreased levels of the Th1 cytokine, interferon (IFN)γ, in cord blood.47 Respiratory syncytial virus infection, a risk factor for wheezing as noted above, is also associated with a Th2 response.48 Similarly, in some studies, early antibiotic use in infancy is associated with an increased risk of atopic disease20,21 and is also associated with a Th2‐dominant response in mice.49 On the other hand, exposures associated with an enhanced Th1 response are associated with a reduced risk of atopic disease. Specifically, Th1‐inducing infections, such as Mycobacterium tuberculosis and hepatitis A virus, are associated with a reduced risk of atopic disease among affected people.50,51
The relationship between Th‐1/Th‐2‐inducing exposures and atopic disease is not always straightforward, and some studies question these associations. Although one study showed that Th1‐inducing immunisation with bacillus Calmette–Guérin was associated with a reduced risk of atopic disease, another study showed that it was not.50,52 Similarly, although Shaheen et al53 showed that people with a history of Th‐1 inducing measles infection had a lower likelihood of atopy than those without measles, a later study showed that measles infection was associated with a higher risk of atopic disease.54 Other studies show that Th1 and Th2 pathways are not always counter‐regulatory. For instance, in mice, Hansen et al55 showed that the production of the Th1 cytokine, IFNγ, was insufficient to counteract the effects of IL4 and IL5. Instead of attenuating Th2 cell‐induced airway hyper‐reactivity and inflammation, Th1 cells actually caused severe airway inflammation.
The intriguing associations between some Th‐1‐inducing exposures and a reduced risk of atopic disease, Th‐2‐inducing exposures and an increased risk of atopic disease, and the counter‐regulatory actions of the Th1 and Th2 pathways for many years have provided an immunological basis for the hygiene hypothesis. In addition, the Th1/Th2 paradigm has prompted a great deal of research that has broadened our understanding of atopic disease pathogenesis. Although the relationship between cytokine responses and atopic disease is complex and not fully understood, we know that cytokines play an important part in atopic disease.
Current understanding of the association between cytokine responses in cord blood and subsequent development of atopic disease
In the search for predictive biological markers for atopic disease, investigators have recently explored the relationship between CBMC and peripheral blood mononuclear cell (PBMC) cytokine responses to mitogen and antigen stimulation and the subsequent development of atopic disease. Table 2 provides a sample of studies from major research groups around the world that have substantially contributed to this body of work. The most consistent finding from these and related studies has been that people who develop atopic disease and those who have a positive family history of atopy even in the absence of disease have lower levels of the Th1 cytokine, IFNγ, at birth when compared with their unaffected counterparts.3,36,39,56,57 IFNγ levels produced in response to allergen‐stimulated CBMCs have been shown to be inversely related to cord blood IgE levels,56 a marker with high specificity but low sensitivity for atopic disease.
Table 2 Research on cytokines and paediatric atopic disease in early childhood from research groups around the world.
| Description of study | Methods and measures | Major findings related to cytokines and atopic disease | ||||
|---|---|---|---|---|---|---|
| Source | Study population | Age: blood sample | Cytokines | Allergens/mitogens | Major clinical outcomes | |
| Contreras et al57 | 112 children all with a parental history of asthma or allergy (54 w/and 58 w/o atopic disease)Birth to age 2 years | Age 2 years: PBMCs | IFNγTNFαIL10IL13 | Bla g 1 (cockroach)Der f 1 (HDM)Feld 1(cat) | Atopic disease:Doctor/nurse diagnosedEczemaHayfeverAllergic rhinitisInhalant allergyRepeated wheeze | Children w/ atopic disease had lower IFNγ (Th1 cytokine) levels in response to HDM and cockroach allergen stimulation of PBMCs than non‐atopic children. This finding appeared to be more pronounced for children w/atopic disease and repeated wheeze. |
| Prescott et al3 | 60 children (44 w/positive family history of atopy; 16 w/o family history)Birth to age 6 years, every 6 monthsAll born by elective caesarean section | Birth: CBMCsAge 6 months: PBMCsAge 12 months: PBMCsAge 18 months: PBMCsAge 24 months: PBMCs | IFNγIL4IL5IL6IL9IL10IL13 | HDMOvalbuminFel d 1 (cat)PHATetanus toxoid | Allergic (atopic) disease:Doctor‐diagnosed asthmaAsthma: recurrent wheezing (⩾3 episodes)Doctor‐diagnosed eczemaSPT at age 6 years | Children w/family history of allergy had lower IFNγ (Th1 cytokine) responses to PHA stimulation of CBMCs (at birth) compared with children w/o a family history.Children with atopic disease at 6 years had an increase in the 1st 2 years of life in IL5 (Th2 cytokine) mRNA in response to HDM, but there was no change in the non‐atopic group.Positive SPT to HDM at 6 years was associated w/ higher IL13 (Th2 cytokine) responses to HDM at 1 year. |
| Neaville et al63 | 285 childrenBirth to age 1 yearNeonates w/at least one parent w/respiratory allergies or asthma | Birth: CMBCsAge 1 year: PBMCs | IFNγIL5IL10IL13 | PHA | Atopic dermatitisFood allergyAntigen‐specific IgE levels | Lower IL10 (Th2 cytokine) production in response to PHA‐stimulated CBMCs (at birth) was a risk factor for egg sensitisation at 1 year of life.For the cohort, IL5 (Th2 cytokine) response increased while IFNγ (Th1 cytokine) decreased over the 1 year of life |
| Kondo et al55 | 21 children (7 w/allergic disorder and 14 w/o)Birth to age 6 yearsFull term, vaginally born infants | Birth: CBMCs | IFNγIL2 | OvalbuminBovine serum albumin | Allergic disorder:Atopic dermatitisBronchial asthmaAllergic rhinitisFood allergy | Children who developed allergic disorder by age 6 years had lower maximal concentrations of IFNγ (Th1 cytokine) in response to ovalbumin or bovine serum albumin stimulation of CBMCs than those w/o allergic disorderMaximal concentrations of IL2 did not differ between children w/ and w/o allergic disorder |
CBMC, cord blood mononuclear cell; HDM, house dust mite; IFN, interferon; IL, interleukin; PBMC, peripheral blood mononuclear cell; PHA, phytohaemagglutinin; SPT, skin‐prick testing; TNF, tumour necrosis factor.
Low levels of IFNγ may represent impaired Th1 pathway function, immature development of the Th1 pathway, early destruction of Th1 cells58 or dominance of Th2 immune responses. Some investigators have speculated that the relative lack of Th1 cytokine expression in newborns at risk of atopic disease may be due to antigen‐presenting cell immaturity and an inability to release IL12, the major induction cytokine for the Th1 pathway. The initially low levels of IFNγ seem to extend beyond the neonatal period, as children who develop atopy also lack the normal Th1 response to bacillus Calmette–Guérin vaccination in infancy.50 Also, children at increased risk of atopic sensitisation have an attenuated production of the Th1 cytokine, IFNγ, in early infancy.59
Levels of Th2 cytokines produced in response to antigen stimulation of CBMCs among children who develop atopic disease, however, are less consistent than the low levels of IFNγ that have been found. Among people at high risk of, or who develop, atopic disease, investigators have shown a Th2 dominant response as shown by increased levels of IL5,60,61 whereas others have shown lower levels of Th2 cytokines such as IL13.62
Some reports suggest that cytokine responses change during early childhood, and that such changes differ for people without atopic disease when compared with people with atopic disease. For people without atopic disease, studies have shown a decline in the Th2 cytokine IL4 and an increase in production of the Th1 cytokine IFNγ in the first 2 years in response to house dust mite antigen‐stimulated mononuclear cells.63 At the same time, children who develop atopic disease show an upregulation of Th2 cytokines, including IL5,62 IL9 and IL13, in response to allergen or mitogen stimulation of PBMCs in the first 2 years of life,64 but the exact timing of this Th2 skewing is unknown.
Some evidence shows that most of the tested newborns, independent of their risk of atopy, have Th2‐skewed responses to common environmental allergens, with cord blood elevations of cytokines, including IL4, IL5 and IL13.64 This Th2 skewing is thought to reflect the Th1/Th2 state of the mother, as women towards the end of pregnancy are believed to be Th2 dominant as a means of maintaining the pregnancy and protecting the fetus against the toxic effects of Th1 cytokines, such as IFNγ.65 Lending strength to this idea, some recent studies have shown a correlation between maternal and neonatal cytokine profiles.66,67 Some think that people without atopic disease shift from being Th2 skewed at birth to being more Th1/Th2 balanced, and that people with atopic disease instead show an upregulation of Th2 and continue to be Th2 skewed thereafter. Whether the ultimate cytokine profiles of an individual are influenced by antenatal or postnatal exposures or a combination of the two is unknown.
This review would be incomplete without mentioning some of the limitations to the current literature on cord blood cytokines and their relationship to paediatric atopic disease. Research in this area is limited by the operational definition of disease, with some investigators comparing cytokine profiles of children with and without atopy. Children with atopy are defined by a positive family history, positive skin‐prick testing or radioallergosorbent testing, or some combination of these, rather than actual symptomatic, clinical disease. Inherent limitations regarding the techniques used for measuring cytokines also exist. For example, certain cytokine levels, including IL4 and IL9, and at times IFNγ, are often below detectable limits in cord blood by standard ELISA methods and have, therefore, been based on semiquantitative reverse‐transcriptase polymerase chain reaction testing for specific mRNA. CBMCs and PBMCs may not show the immunological cells found in the airways or lungs, and cytokine responses from these cells may not reflect those in the target tissues.
Conclusions
Rapid progress has been made in our understanding of how neonatal cytokine production relates to the subsequent development of atopic disease; however, the exact determinants of neonatal cytokine profiles are not well understood. Studies that relate antenatal sociodemographic and health conditions to cord blood cytokine profiles are just beginning. Increasingly, we have begun to not only recognise the importance of antenatal exposures in the subsequent risk of atopic diseases but also postulate the mechanisms by which such relationships occur. In particular, cytokine profiles in cord blood appear to alter in response to antenatal exposures and may predict later development of allergic disease. Although children with atopic disease seem to show a Th2 dominance once they are diagnosed with allergic disease, it is not clear when this Th2 dominance develops; however, it is believed to develop in early childhood. Despite limitations that affect the overall generalisability of work in this area, an increasing prevalence of atopic disease, along with growing evidence that antenatal factors contribute to disease development, calls for further research in the area of neonatal cytokines and their effect on the development of childhood atopic disease.
Abbreviations
CBMC - cord blood mononuclear cell
IFN - interferon
IL - interleukin
PBMC - peripheral blood mononuclear cell
Th1 - T helper cell type 1
Th2 - T helper cell type 2
TNF - tumour necrosis factor
Footnotes
Competing interests: None declared.
References
- 1.Litonjua A A, Carey V J, Burge H A.et al Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med 1998158176–181. [DOI] [PubMed] [Google Scholar]
- 2.Moore M M, Rifas‐Shiman S L, Rich‐Edwards J W.et al Perinatal predictors of atopic dermatitis occurring in the first six months of life. Pediatrics 2004113(Pt 1)468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott S L, King B, Strong T L.et al The value of perinatal immune responses in predicting allergic disease at 6 years of age. Allergy 2003581187–1194. [DOI] [PubMed] [Google Scholar]
- 4.Clarke J R, Jenkins M A, Hopper J L.et al Evidence for genetic associations between asthma, atopy, and bronchial hyperresponsiveness: a study of 8‐ to 18‐yr‐old twins. Am J Respir Crit Care Med 20001622188–2193. [DOI] [PubMed] [Google Scholar]
- 5.Hakonarson H, Bjornsdottir U S, Halapi E.et al A major susceptibility gene for asthma maps to chromosome 14q24. Am J Hum Genet 200271483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niimi T, Munakata M, Keck‐Waggoner C L.et al A polymorphism in the human UGRP1 gene promoter that regulates transcription is associated with an increased risk of asthma. Am J Hum Genet 200270718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ober C, Tsalenko A, Parry R.et al A second‐generation genomewide screen for asthma‐susceptibility alleles in a founder population. Am J Hum Genet 2000671154–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez F D. Gene‐environment interactions in asthma and allergies: a new paradigm to understand disease causation. Immunol Allergy Clin North Am 200525709–721. [DOI] [PubMed] [Google Scholar]
- 9.Eder W, Klimecki W, Yu L.et al Opposite effects of CD 14/‐260 on serum IgE levels in children raised in different environments. J Allergy Clin Immunol 2005116601–607. [DOI] [PubMed] [Google Scholar]
- 10.Lemanske R F., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol 200213(Suppl 15)38–43. [DOI] [PubMed] [Google Scholar]
- 11.Lemanske R F, Jr, Jackson D J, Gangnon R E.et al Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 2005116571–577. [DOI] [PubMed] [Google Scholar]
- 12.Strachan D P. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax 200055(Suppl 1)S2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattes J, Karmaus W. The use of antibiotics in the first year of life and development of asthma: which comes first? Clin Exp Allergy 199929729–732. [DOI] [PubMed] [Google Scholar]
- 14.Phipatanakul W, Celedon J C, Raby B A.et al Endotoxin exposure and eczema in the first year of life. Pediatrics 200411413–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball T M, Castro‐Rodriguez J A, Griffith K A.et al Siblings, day‐care attendance, and the risk of asthma and wheezing during childhood. N Engl J Med 2000343538–543. [DOI] [PubMed] [Google Scholar]
- 16.Riedler J, Eder W, Oberfeld G.et al Austrian children living on a farm have less hay fever, asthma and allergic sensitization. Clin Exp Allergy 200030194–200. [DOI] [PubMed] [Google Scholar]
- 17.Hesselmar B, Aberg N, Aberg B.et al Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy 199929611–617. [DOI] [PubMed] [Google Scholar]
- 18.Remes S T, Castro‐Rodriguez J A, Holberg C J.et al Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J Allergy Clin Immunol 2001108509–515. [DOI] [PubMed] [Google Scholar]
- 19.Celedon J C, Litonjua A A, Ryan L.et al Lack of association between antibiotic use in the first year of life and asthma, allergic rhinitis, or eczema at age 5 years. Am J Respir Crit Care Med 200216672–75. [DOI] [PubMed] [Google Scholar]
- 20.Farooqi I S, Hopkin J M. Early childhood infection and atopic disorder. Thorax 199853927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson C C, Ownby D R, Alford S H.et al Antibiotic exposure in early infancy and risk for childhood atopy. J Allergy Clin Immunol 20051151218–1224. [DOI] [PubMed] [Google Scholar]
- 22.Gold D R, Burge H A, Carey V.et al Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med 1999160227–236. [DOI] [PubMed] [Google Scholar]
- 23.Xu B, Pekkanen J, Jarvelin M R.et al Maternal infections in pregnancy and the development of asthma among offspring. Int J Epidemiol 199928723–727. [DOI] [PubMed] [Google Scholar]
- 24.Litonjua A A, Carey V J, Weiss S T.et al Race, socioeconomic factors, and area of residence are associated with asthma prevalence. Pediatr Pulmonol 199928394–401. [DOI] [PubMed] [Google Scholar]
- 25.Olesen A B, Ellingsen A R, Olesen H.et al Atopic dermatitis and birth factors: historical follow up by record linkage. BMJ 19973141003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes C H, Jones R C, Wright D E.et al A retrospective study of the relationship between childhood asthma and respiratory infection during gestation. Clin Exp Allergy 1999291378–1381. [DOI] [PubMed] [Google Scholar]
- 27.Jaakkola J J, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health 200494136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schafer T, Dirschedl P, Kunz B.et al Maternal smoking during pregnancy and lactation increases the risk for atopic eczema in the offspring. J Am Acad Dermatol 199736550–556. [DOI] [PubMed] [Google Scholar]
- 29.Raherison C, Penard‐Morand C, Moreau D.et al In utero and childhood exposure to parental tobacco smoke, and allergies in schoolchildren. Respir Med 2006 [DOI] [PubMed]
- 30.McKeever T M, Lewis S A, Smith C.et al The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands General Practice Database. Am J Respir Crit Care Med 2002166827–832. [DOI] [PubMed] [Google Scholar]
- 31.Xu B, Pekkanen J, Jarvelin M R. Obstetric complications and asthma in childhood. J Asthma 200037589–594. [DOI] [PubMed] [Google Scholar]
- 32.Negele K, Heinrich J, Borte M.et al Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol 20041548–54. [DOI] [PubMed] [Google Scholar]
- 33.Salam M T, Margolis H G, McConnell R.et al Mode of delivery is associated with asthma and allergy occurrences in children. Ann Epidemiol 200616341–346. [DOI] [PubMed] [Google Scholar]
- 34.Piccinni M P, Mecacci F, Sampognaro S.et al Aeroallergen sensitization can occur during fetal life. Int Arch Allergy Immunol 1993102301–303. [DOI] [PubMed] [Google Scholar]
- 35.Bergmann R L, Edenharter G, Bergmann K E.et al Predictability of early atopy by cord blood‐IgE and parental history. Clin Exp Allergy 199727752–760. [PubMed] [Google Scholar]
- 36.Warner J A, Miles E A, Jones A C.et al Is deficiency of interferon gamma production by allergen triggered cord blood cells a predictor of atopic eczema? Clin Exp Allergy 199424423–430. [DOI] [PubMed] [Google Scholar]
- 37.Devereux G, Barker R N, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy 20023243–50. [DOI] [PubMed] [Google Scholar]
- 38.Szepfalusi Z, Nentwich I, Gerstmayr M.et al Prenatal allergen contact with milk proteins. Clin Exp Allergy 19972728–35. [PubMed] [Google Scholar]
- 39.Prescott S L, Holt P G. Abnormalities in cord blood mononuclear cytokine production as a predictor of later atopic disease in childhood. Clin Exp Allergy 1998281313–1316. [DOI] [PubMed] [Google Scholar]
- 40.Yabuhara A, Macaubas C, Prescott S L.et al TH2‐polarized immunological memory to inhalant allergens in atopics is established during infancy and early childhood. Clin Exp Allergy 1997271261–1269. [PubMed] [Google Scholar]
- 41.Vanderbeeken Y, Sarfati M, Bose R.et al In utero immunization of the fetus to tetanus by maternal vaccination during pregnancy. Am J Reprod Immunol Microbiol 1985839–42. [DOI] [PubMed] [Google Scholar]
- 42.Holloway J A, Warner J O, Vance G H.et al Detection of house‐dust‐mite allergen in amniotic fluid and umbilical‐cord blood. Lancet 20003561900–1902. [DOI] [PubMed] [Google Scholar]
- 43.Mizuno M, Aoki K, Kimbara T. Functions of macrophages in human decidual tissue in early pregnancy. Am J Reprod Immunol 199431180–188. [DOI] [PubMed] [Google Scholar]
- 44.Moverare R, Elfman L, Stalenheim G.et al Study of the Th1/Th2 balance, including IL‐10 production, in cultures of peripheral blood mononuclear cells from birch‐pollen‐allergic patients. Allergy 200055171–175. [DOI] [PubMed] [Google Scholar]
- 45.Grunig G, Warnock M, Wakil A E.et al Requirement for IL‐13 independently of IL‐4 in experimental asthma. Science 19982822261–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker C, Bauer W, Braun R K.et al Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med 19941501038–1048. [DOI] [PubMed] [Google Scholar]
- 47.Noakes P S, Holt P G, Prescott S L. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy 2003581053–1058. [DOI] [PubMed] [Google Scholar]
- 48.Bendelja K, Gagro A, Bace A.et al Predominant type‐2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol 2000121332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oyama N, Sudo N, Sogawa H.et al Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2‐dominant immunity in mice. J Allergy Clin Immunol 2001107153–159. [DOI] [PubMed] [Google Scholar]
- 50.Shirakawa T, Enomoto T, Shimazu S.et al The inverse association between tuberculin responses and atopic disorder. Science 199727577–79. [DOI] [PubMed] [Google Scholar]
- 51.Matricardi P M, Rosmini F, Ferrigno L.et al Cross sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ 1997314999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marks G B, Ng K, Zhou J.et al The effect of neonatal BCG vaccination on atopy and asthma at age 7 to 14 years: an historical cohort study in a community with a very low prevalence of tuberculosis infection and a high prevalence of atopic disease. J Allergy Clin Immunol 2003111541–549. [DOI] [PubMed] [Google Scholar]
- 53.Shaheen S O, Aaby P, Hall A J.et al Measles and atopy in Guinea‐Bissau. Lancet 19963471792–1796. [DOI] [PubMed] [Google Scholar]
- 54.Paunio M, Heinonen O P, Virtanen M.et al Measles history and atopic diseases: a population‐based cross‐sectional study. JAMA 2000283343–346. [DOI] [PubMed] [Google Scholar]
- 55.Hansen G, Berry G, DeKruyff R H.et al Allergen‐specific Th1 cells fail to counterbalance Th2 cell‐induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest 1999103175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kondo N, Kobayashi Y, Shinoda S.et al Reduced interferon gamma production by antigen‐stimulated cord blood mononuclear cells is a risk factor of allergic disorders—6‐year follow‐up study. Clin Exp Allergy 1998281340–1344. [DOI] [PubMed] [Google Scholar]
- 57.Contreras J P, Ly N P, Gold D R.et al Allergen‐induced cytokine production, atopic disease, IgE, and wheeze in children. J Allergy Clin Immunol 20031121072–1077. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Lee H H, Bell J J.et al IL‐4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity 200420429–440. [DOI] [PubMed] [Google Scholar]
- 59.Holt P G, Clough J B, Holt B J.et al Genetic ‘risk' for atopy is associated with delayed postnatal maturation of T‐cell competence. Clin Exp Allergy 1992221093–1099. [DOI] [PubMed] [Google Scholar]
- 60.Prescott S L, Macaubas C, Smallacombe T.et al Development of allergen‐specific T‐cell memory in atopic and normal children. Lancet 1999353196–200. [DOI] [PubMed] [Google Scholar]
- 61.Williams T J, Jones C A, Miles E A.et al Fetal and neonatal IL‐13 production during pregnancy and at birth and subsequent development of atopic symptoms. J Allergy Clin Immunol 2000105951–959. [DOI] [PubMed] [Google Scholar]
- 62.Prescott S L, Macaubas C, Smallacombe T.et al Reciprocal age‐related patterns of allergen‐specific T‐cell immunity in normal vs. atopic infants. Clin Exp Allergy. 1998;28: 39–44; discussion 50–1, (Suppl 5) [DOI] [PubMed]
- 63.Neaville W A, Tisler C, Bhattacharya A.et al Developmental cytokine response profiles and the clinical and immunologic expression of atopy during the first year of life. J Allergy Clin Immunol 2003112740–746. [DOI] [PubMed] [Google Scholar]
- 64.Prescott S L, Macaubas C, Holt B J.et al Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol 19981604730–4737. [PubMed] [Google Scholar]
- 65.Wegmann T G, Lin H, Guilbert L.et al Bidirectional cytokine interactions in the maternal‐fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 199314353–356. [DOI] [PubMed] [Google Scholar]
- 66.Prescott S L, Taylor A, King B.et al Neonatal interleukin‐12 capacity is associated with variations in allergen‐specific immune responses in the neonatal and postnatal periods. Clin Exp Allergy 200333566–572. [DOI] [PubMed] [Google Scholar]
- 67.Chiesa C, Signore F, Assumma M.et al Serial measurements of C‐reactive protein and interleukin‐6 in the immediate postnatal period: reference intervals and analysis of maternal and perinatal confounders. Clin Chem 2001471016–1022. [PubMed] [Google Scholar]