Abstract
Background and objective
It has been suggested that fetal growth restriction (FGR) is associated with fetal maturation so that, compared with appropriately grown preterm infants, mortality and some neonatal morbidities may be reduced. The evidence for this is conflicting, and severe FGR has been shown to be harmful. In addition excessive growth has also been shown to be associated with poorer outcomes. As preterm infants are often also growth restricted, centiles for birth weights are distorted and may conceal the degree of growth restriction in a given infant. This study investigated whether using estimated fetal weights (EFW) might reveal the effects of hidden FGR.
Population and methods
Using a 25‐year database of preterm admissions to a single neonatal unit the ORs for mortality and neonatal morbidities for z scores for birth weight above and below the mean were computed and compared with those computed for z scores for EFW.
Results
In 7898 infants born at less than 35 weeks' gestation, the OR for mortality was lowest for birth weights between 1 SD and 3 SD above the mean, but was lowest for EFW between −2 SD and 0 SD below the mean. For periventricular haemorrhage, increasing FGR below the mean reduced the OR with both birth weight and EFW. Apparent reductions in OR for septicaemia, chronic lung disease, persistent ductus arteriosus and necrotising enterocolitis with birth weights of >1 SD above the mean were not seen with EFW. FGR of >−3 SD was associated with increased OR for necrotising enterocolitis with both birth weight and EFW.
Conclusion
Using fetal growth rather than birth weight standards gives a better indication of the incidence and role of FGR in neonatal disease.
Keywords: birth weight, estimated fetal weight, growth restriction, mortality, morbidity
The association between fetal growth restriction (FGR) and neonatal mortality has been long recognised,1,2,3 together with an association with a reduced incidence of some morbidities such as respiratory distress syndrome1 and cerebral haemorrhage,4 but an increased incidence of other morbidities such as chronic lung disease.1,2,3 Long‐term morbidity such as cerebral palsy is also more common in infants born with FGR, although this seems to be less marked in the preterm.5
More recently it has been shown that although infants with a birth weight below the mean are more vulnerable, the birth weight associated with the lowest mortality is 1 or 2 SD above the mean.6 The same “reversed J‐shaped” distribution in birth weight has been reported for risk of cerebral palsy in infants born at term and preterm.5 Higher risk is associated both with FGR and with a birth weight well above normal, although it is uncertain whether the deviant growth is the cause or result of the disability. It also seems that this effect is more marked in male fetuses. How far this phenomenon applies to the preterm with respect to mortality and morbidity in the neonatal period is unclear.
As very preterm infants probably have greater FGR than more mature infants at birth, birth weight standards for these infants are likely to be distorted by this when compared with estimated growth standards derived from ultrasound measurements on fetuses going on to be born at term.7,8 This study therefore used a large database of neonatal outcomes to explore the relationship of FGR with mortality and morbidity in preterm infants, using both birth weight and estimated fetal weight standards.
Population and methods
Over the past 25 years, a database has been prospectively maintained, derived from the clinical records of all infants born alive at 34 weeks' gestation or less and admitted to the Mersey Regional Neonatal Unit at Liverpool Maternity Hospital and later Liverpool Women's Hospital, Liverpool, UK. The anonymised record includes year of birth, birth weight, gestation, sex, whether from a multiple birth, where booked for delivery, and clinical interventions, mortality and morbidity data. The main function of this database has been for audit and annual reports.
The z score (SD score) for birth weight for each infant in the database was computed using data from the Child Growth Foundation9 for mean (SD) birth weight for each gestation and sex. The infants were then grouped by z score into those 1.99 to 1.00, 2.99 to 2.00 and 3.00 SD or more below the mean, and those 1.00 to 1.99, 2.00 to 2.99 and 3.00 SD or more above the mean. Infants with scores between −0.99 and 0.99 SD formed the comparison group. The odds ratio (OR) and its 95% CI was computed using logistic regression for mortality and each neonatal morbidity in all z score groups compared with the comparison group.
Each infant's z score was again computed for estimated fetal weight rather than actual birth weight using published ultrasonically estimated fetal weights and their standard deviations for each gestation and sex.10 Using logistic regression in a similar way as before, the ORs and 95% CI were again computed for mortality and each neonatal morbidity in all the z score groups, compared with the comparison group. The estimated fetal weight data were obtained from serial fetal ultrasound measurements made in 86 women in four Scandinavian centres. Between 9 and 11 measurements were made on each woman, and only the data from women having an uncomplicated pregnancy and subsequently delivering a normal term infant were included. The measurements used were biparietal diameter, abdominal diameter and femur length. Fetal weight was then calculated using a formula developed by Persson and Weldner.11 The best fit for the data was found using a fourth degree polynomial equation. Separate fitted curves were produced for male and female. The SD was calculated cross‐sectionally for each week of gestation. The data were normally distributed, and in the reference curves a uniform SD of 12% was adopted. These data are currently used for comparing estimated fetal weights on our fetal medicine unit.
Results
Between 1 January 1980 and 31 December 2004, 7898 infants born at less than 35 weeks' gestation were admitted to the Mersey Regional Neonatal Intensive Care and Special Care Units at Liverpool Maternity Hospital (later Liverpool Women's Hospital). Table 1 shows the characteristics of this cohort.
Table 1 Characteristics of the study cohort. Values are n (%).
| Number in cohort | 7898 |
| <28 weeks' gestation | 1634 (20.7) |
| <1000 g birth weight | 1669 (21.1) |
| Male | 4437 (56.2) |
| Multiple pregnancy | 1959 (24.8) |
| Delivered by caesarean section | 3863 (48.9) |
| All deaths | 1133 (14.3) |
| Deaths from lethal malformations | 119 (1.5) |
| Ultrasound scan evidence of periventricular haemorrhage | 1873 (23.7) |
| Sepsis (positive blood culture) | 1873 (23.7) |
| Chronic lung disease (O2 at 28 days) | 1036 (13.1) |
| Persistent arterial duct (clinical diagnosis) | 886 (11.2) |
| Necrotising enterocolitis (radiographic or surgical diagnosis) | 319 (4.0) |
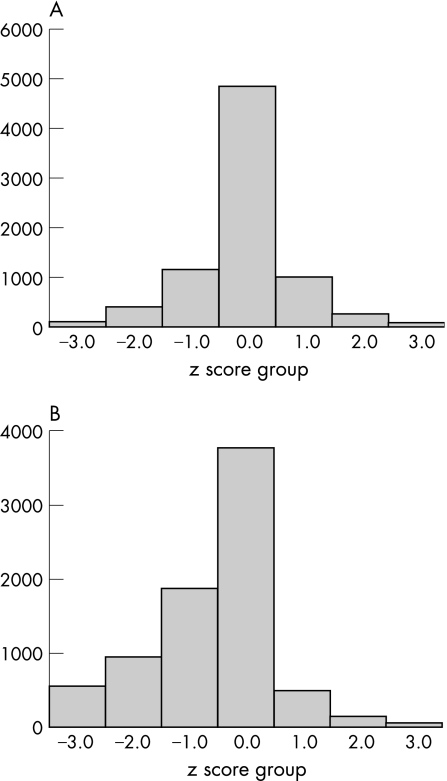
Figure 1 shows the distribution of z scores for birth weight and for estimated fetal weight. The z scores for birth weight were normally distributed (mean 0.1 (SD 0.92)), but those for estimated fetal weight were markedly skewed to the left (mean −0.6 (SD 1.13)) indicating that the birth weight data for preterm infants used contained weights from a large proportion infants with “hidden” FGR. Healthier and better grown infants progressed to term rather than been born preterm. When the cohort was divided by gestation into two groups (below 28 weeks and 28 weeks and above) a similar distribution of z scores was seen, but with higher ORs at the extreme limits for the higher gestation group. Table 2 shows the ORs for mortality and neonatal morbidities by z score groups for birth weight and for estimated fetal weight.
Figure 1 (A) Distribution of birth weights by z scores. (B) Distribution of estimated fetal weights by z scores.
Table 2 Odds ratios (95% CI) for mortality and major morbidities by z score for birth weight (BW) and estimated fetal weight (EFW).
| Z score | < −3.00 | −2.99 to −2.00 | −1.99 to −1.00 | −0.99 to 0.99 | 1.00 to 1.99 | 2.00 to 2.99 | >3.00 | χ2 trend | |
|---|---|---|---|---|---|---|---|---|---|
| No of cases in each Z score category | BW | 114 | 422 | 1174 | 4843 | 1008 | 250 | 87 | |
| EFW | 565 | 950 | 1870 | 3779 | 512 | 153 | 69 | ||
| Mortality | BW | 2.14 (1.40 to 3.28)*** | 1.19 (0.90 to 1.58) | 1.07 0.88 to 1.29) | 1.00 | 0.70 (0.55 to 0.88)** | 0.50 (0.31 to 0.83)** | 0.86 (0.45 to 1.68) | 15.7, p<0.001 |
| EFW | 1.63 (1.30 to 2.04)*** | 0.96 (0.77 to 1.18) | 0.94 (0.80 to 1.11) | 1.00 | 1.37 (1.07 to 1.74)** | 1.07 (0.67 to 1.69) | 2.23 (1.29 to 3.84)** | 0.5, p = 0.8 | |
| Periventricular haemorrhage (all) | BW | 0.54 (0.36 to 0.81)** | 0.58 ( 0.47 to 0.73)*** | 0.78 (0.68 to 0.90)*** | 1.00 | 1.02 (0.88 to 1.17) | 0.93 (0.72 to 1.21) | 1.08 (0.71 to 1.66) | 31.0, p<0.001 |
| EFW | 0.54 (0.44 to 0.65)*** | 0.63 (0.54 to 0.73)*** | 0.79 (0.71 to 0.89)*** | 1.00 | 1.12 (0.93 to 1.35) | 0.92 (0.66 to 1.27) | 0.99 (0.62 to 1.61) | 68.5, p<0.001 | |
| Septicaemia | BW | 1.18 (0.78 to 1.79) | 1.06 (0.84 to 1.33) | 1.07 (0.93 to 1.24) | 1.00 | 0.81 (0.69 to 0.96)* | 0.83 (0.61 to 1.13) | 0.55 (0.31 to 1.00)* | 10.8, p = 0.001 |
| EFW | 1.29 (1.06 to 1.58)** | 1.07 (0.91 to 1.27) | 1.07 (0.94 to 1.22) | 1.00 | 0.98 (0.79 to 1.22) | 1.25 (0.86 to 1.80) | 0.78 (0.43 to 1.44) | 4.8, p = 0.03 | |
| Chronic lung disease | BW | 1.10 (0.66 to 1.83) | 1.05 (0.79 to 1.40) | 0.98 (0.82 to 1.19) | 1.00 | 0.66 (0.53 to 0.83)*** | 0.62 (0.40 to 0.97)* | 0.38 (0.15 to 0.93)* | 19.5, p<0.001 |
| EFW | 1.45 (1.14 to 1.84)** | 1.10 (0.89 to 1.36) | 1.08 (0.89 to 1.27) | 1.00 | 1.24 (0.96 to 1.61) | 1.34 (0.85 to 2.10) | 0.81 (0.37 to 1.78) | 2.6, p = 0.1 | |
| Persistent ductus arteriosus | BW | 0.94 (0.54 to 1.66) | 0.79 (0.56 to 1.10) | 0.82 (0.66 to 1.01) | 1.00 | 0.68 (0.54 to 0.86)** | 0.47 (0.28 to 0.80)** | 0.26 (0.08 to 0.81)* | 2.5, p = 0.1 |
| EFW | 0.97 (0.74 to 1.29) | 0.94 (0.75 to 1.18) | 0.86 (0.72 to 1.03) | 1.00 | 1.04 (0.78 to 1.38) | 1.02 (0.62 to 1.68) | 0.59 (0.24 to 1.48) | 0.2, p = 0.7 | |
| Necrotising enterocolitis | BW | 3.44 (1.97 to 6.02)*** | 1.87 (1.26 to 2.79)** | 0.91 (0.64 to 1.27) | 1.00 | 0.71 (0.48 to 1.06) | 0.50 (0.21 to 1.24) | 0.28 (0.04 to 2.03) | 31.5, p<0.001 |
| EFW | 2.48 (1.77 to 3.47)*** | 1.01 (0.69 to 1.47) | 1.03 (0.77 to 1.38) | 1.00 | 1.05 (0.65 to 1.70) | 0.71 (0.26 to 1.95) | 1.22 (0.38 to 3.91) | 12.5, p<0.001 |
*p<0.05, ** p<0.01, *** p<0.001.
The OR for mortality, whether including lethal malformations or not, was highest for the most growth‐restricted group of infants with birth weight more than 3 SD below the mean. Significantly lower mortality was found in the group with birth weight between 1 SD and 3 SD above the mean. Mortality among those infants with birth weight of more than 3 SD above the mean did not differ significantly from that in the group with birth weight around the mean. The ORs for mortality when estimated fetal weight were used showed a different pattern, with highest mortality at more than 3 SD above or below the mean. Excluding malformations left only a weight of more than 3 SD below the mean associated with a higher mortality.
FGR of more than −1 SD appeared to be significantly protective against periventricular haemorrhage (PVH), with an OR of 0.54 (95% CI 0.36 to 0.81) for more than −3 SD whether birth weight or estimated fetal weight was used. On the other hand, FGR significantly increased the risk of necrotising enterocolitis (NEC) in infants with birth weight of more than −2 SD below the expected mean, or more than −3 SD below estimated fetal weight. For infants with birth weight of more than 1 SD above the expected mean, significantly lower ORs were seen for septicaemia, chronic lung disease and persistent ductus arteriosus, the effects being most marked with the heaviest infants. These reductions in ORs were not seen when estimated fetal weight were used.
Discussion
FGR has long been recognised as increasing the risk of mortality and morbidity in preterm infants but above‐average weight has not usually been considered to convey an advantage. In this study, extreme FGR was associated with a doubling of the likelihood of mortality, with the lowest risk being associated with birth weight between 2 SD and 3 SD above the expected mean. At even higher birth weights, the OR for mortality increased again. This probably reflects the likelihood of abnormality in this group, possibly associated with hydrops fetalis or maternal diabetes. Removal of cases in the category of known lethal malformation (which included some with hydrops fetalis) did not alter this finding. Underestimation of gestational age could have allowed more mature infants to be included, whose higher weights would make them seem to be 3 SD or more. However, it is unlikely to be the case as this category would then have had a lower than expected mortality, as higher gestation infants are less likely to die. Although the mortality of males was much higher than of females 703/4437 (15.8%) vs 430/3461 (12.4%), respectively, the effect of z score of the birth weight was similar in both sexes. Preterm infants of 28 weeks' gestation or more had higher ORs for mortality at the extremes of z score, which probably represents a mortality effect, very preterm fetuses with extremes of growth simply not surviving to be born alive and not appearing in the data. When the z scores for estimated fetal weight were examined a similar pattern of mortality was seen, but with significantly higher mortality only at more than −3 SD and +3 SD. This effect has been described before and is attributed to an adverse effect of excessive weight gain on perinatal mortality.6 Removing deaths due to malformations reduced the OR, suggesting that they play a part in determining this excess mortality.
What is already known on this topic
Fetal growth restriction is associated with increased mortality and morbidity in both term and preterm infants.
Considerably above average fetal growth is also associated with increased mortality in term infants, and increased rates of cerebral palsy in term and preterm infants.
What this study adds
The pattern of mortality and neonatal morbidity associated with weight is different when estimated fetal weight rather than birth weight standards are used in preterm infants.
Use of fetal growth standards rather than birth weight standards in neonatal care could give a better indication of the incidence and role of fetal growth restriction in neonatal disease.
The OR for PVH was reduced significantly with greater degrees of FGR, and this effect was also seen when estimated fetal weight was used. A reduction in PVH with FGR has been described previously12,13 and tentatively ascribed to alterations in prostanoid production and cerebral blood flow caused by in‐utero hypoxia. However, others have found a trend to an increase of PVH in infants with FGR.14 The use of the term PVH can be criticised for being a generic term for several conditions visible on cranial ultrasound scanning including germinal matrix haemorrhage‐intraventricular haemorrhage, parenchymal infarction and probably early periventricular leukomalacia. The data in this study were collected over 25 years, a period during which scanning technology and the classification of the appearances has changed considerably, making more precise definition unreliable.
NEC has been recognised to be associated with FGR for some time, especially when intrauterine Doppler scans show signs of circulatory compromise, such as reversed diastolic flow in umbilical or fetal blood vessels.2,8,15,16 The ORs for NEC in this study ranged from 3.44 (1.97 to 6.02) in infants more than −3 SD below mean birth weight to 0.28 (0.04 to 2.03) in those 3 SD or more above it. The small number of cases meant that the confidence limits were wide, but the trend across the weight groups was highly significant (χ2 for trend 31.5, p<0.001). This seems to indicate that the relationship between z score birth weight and NEC is not just with FGR—being larger than average carries a protective advantage, perhaps through a reduced risk of infection. However, when estimated fetal weight was used, only the OR for weights below −3 SD were significantly associated with NEC.
An increased risk of septicaemia, chronic lung disease and persistent ductus arteriosus has been shown to be associated with FGR in several studies.1,2,3,12,17 However, in the present cohort, rather than an increased risk with FGR, a decreased risk was found with increasing birth weight at a given gestation. As these three outcomes are seen in infants surviving for at least a few days there could have been a mortality effect, that is the smallest infants may have died before they could develop these morbidities. When only survivors were considered, however, the ORs were essentially unchanged, suggesting that this is not the case. These effects may be spurious, as when estimated fetal weight was considered they were no longer significant, and only severe FGR was associated with higher ORs for septicaemia and chronic lung disease.
Most published studies on the epidemiology of neonatal mortality and morbidity have used birth weight rather than estimated fetal weight, despite the latter having been available for many years. As seen in the present study, this can produce apparently spurious associations, due to the cohort of preterm infants having a high proportion of infants with FGR. The range of estimated fetal weight used in this study is derived from a single publication, and others may differ slightly but will probably be in the same direction. Also, the reason for FGR was not considered, and this may have altered some of the findings. FGR in normotensive women has been shown to be associated with a higher perinatal mortality than FGR in hypertensive women.18 Using fetal growth standards in neonatal care for preterm infants could indicate the true incidence of FGR and its role in neonatal disease.
Abbreviations
FGR - fetal growth restriction
NEC - necrotising enterocolitis
OR - odds ratio
PVH - periventricular haemorrhage
Footnotes
Competing interests: none declared.
References
- 1.Sharma P, McKay K, Rosenkrantz E S.et al Comparisons of mortality and pre‐discharge respiratory outcomes in small‐for‐gestational‐age and appropriate‐for‐gestational‐age premature infants. BMC Pediatrics 200449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garite T J, Clark R, Thorp J A. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol 2004191481–487. [DOI] [PubMed] [Google Scholar]
- 3.Lal M K, Manktelow B N, Draper E S.et al Chronic lung disease of prematurity and intrauterine growth retardation: a population‐based study. Pediatrics 2003111483–487. [DOI] [PubMed] [Google Scholar]
- 4.Amato M, Konrad D, Huppi P.et al Impact of prematurity and intrauterine growth retardation on hemorrhagic and ischemic brain damage. Eur Neurol 199333299–303. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis S, Glinianaia S V, Torrioli M ‐ G.et al Cerebral palsy and intrauterine growth in single births: European collaborative study. Lancet 20033621106–1111. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox A J, Russell I. Birth weight and perinatal mortality II: on weight specific mortality. Int J Epidemiol 198312319–325. [DOI] [PubMed] [Google Scholar]
- 7.Lackman F, Capewell V, Richardson B.et al The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol 2001184946–953. [DOI] [PubMed] [Google Scholar]
- 8.Zaw W, Gagnon R, da Silva O. The risks of adverse neonatal outcome among preterm small for gestational age infants according to neonatal versus fetal growth standards. Pediatrics 20031111273–1277. [DOI] [PubMed] [Google Scholar]
- 9.The Child Growth Foundation London
- 10.Marsal K, Persson P ‐ H, Larsen T.et al Intrauterine growth curves based on electronically estimated foetal weights. Acta Paediatr 199685843–848. [DOI] [PubMed] [Google Scholar]
- 11.Persson P ‐ H, Weldner B ‐ M. Intrauterine weight curves obtained by ultrasound. Acta Obstet Gynecol Scand 198665169–173. [DOI] [PubMed] [Google Scholar]
- 12.Bardin C, Zelkowitz P, Papageorgiou A. Outcome of small‐for‐gestational age and appropriate‐for‐gestational age infants born before 27 weeks gestation. Pediatrics 19971004–8. [DOI] [PubMed] [Google Scholar]
- 13.Pena I C, Teberg A J, Finello K M. The premature small‐for‐gestational age infant during the first year of life: comparison by birth weight and gestational age. J Pediatr 19881131066–1073. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein I M, Horbar J D, Badger G J.et al Morbidity and mortality among very‐low‐birth‐weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 2000182198–206. [DOI] [PubMed] [Google Scholar]
- 15.Beeby P J, Jeffery H. Risk factors for necrotising enterocolitis: the influence of gestational age. Arch Dis Child 199267432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craigo S D, Beach M L, Harvey‐Wilkes K B.et al Ultrasound predictors of neonatal outcome in intrauterine growth restriction. Am J Perinatol 199613465–471. [DOI] [PubMed] [Google Scholar]
- 17.Simchen M J, Beiner M E, Straus‐Liviathan N.et al Neonatal outcome in growth‐restricted versus appropriately grown preterm infants. Am J Perinatol 200017187–192. [DOI] [PubMed] [Google Scholar]
- 18.Piper J M, Langer O, Xenakis E M ‐ J.et al Perinatal outcome in growth‐restricted fetuses: do hypertensive and normotensive pregnancies differ? Obstet Gynecol 199688194–199. [DOI] [PubMed] [Google Scholar]