Every doctor likes to make a good diagnosis. If the condition in question is rare, so much the better—at least in terms of professional satisfaction. No branch of medicine affords more opportunity for the diagnosis of rare disorders than clinical genetics. In this specialty the individual rarity of specific conditions, combined with the idealistic, ill comprehended nature of “gestalt” (pattern recognition) diagnosis, can create lifelong impressions in young colleagues who witness such diagnostic feats. It is self evident that the dysmorphic patient often reveals the best and the worst of humankind in doctors. Keen to reach a diagnosis, well balanced, confident physicians can be both brilliant in their recognition of the disorder and considerate towards the patient and family, controlling the all too human temptation to show off to colleagues in such situations. Conversely, those of us who daily meet such patients and their families are familiar with tales of doctors having loudly proclaimed a snap diagnosis, demonstrated signs and, as an afterthought, had a discussion or some further dialogue with the patient and family. Experience relates that snap diagnoses are often incorrect, worsening the experience from the patient's perspective.
Paediatricians, who see children with dysmorphic features on a daily basis, are more exposed than most to these pitfalls. After all, most children with syndromes present first to their paediatrician and the identification of specific clinical signs or behaviour patterns that prompt diagnosis of a syndrome may result in an early resolution of the case. Obviously, with an experienced physician and the exercise of due care and sensitivity, much of the anxiety that surrounds syndrome diagnosis can be dissipated by the manner of discussion undertaken at the time of diagnosis. Breaking bad news is rightly an important part of paediatric training. With very rare exceptions, the diagnosis of a specific condition in a dysmorphic patient is a benefit to the patient, the family and the clinicians managing the case but even the correct diagnosis, if badly handled, can have lifelong negative effects on a family.
Increasingly, the dialogue between paediatricians and parents extends to the demonstration of dysmorphic signs to parents, even in cases where the diagnostic significance of such signs is unclear. With the wide availability of internet access, this practice frequently results in parents not only demonstrating dysmorphic signs to geneticists when they assess the patient but even suggesting possible diagnoses. This reliance on electronic media can lead some people to suppose that dysmorphic diagnosis is a matter of “keying some features into a computer”. This review addresses these perceptions, explains the often arcane process of dysmorphic diagnosis, outlines the natural history of how syndromes are identified, described and consolidated as stand‐alone diagnoses and, perhaps in so doing, highlights some pitfalls for the novice dysmorphology enthusiast!
How are syndromes diagnosed?
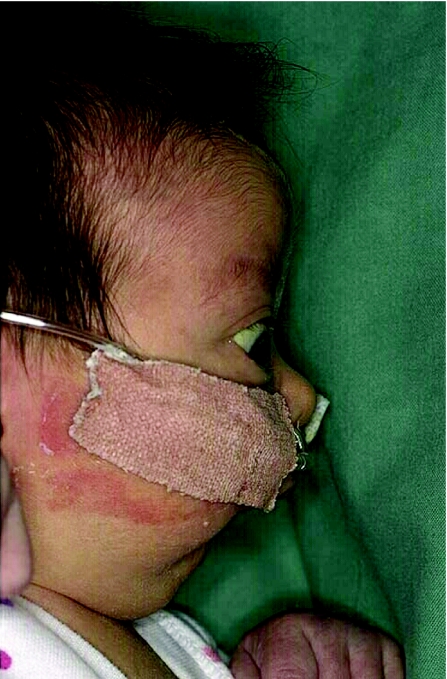
Syndrome diagnosis depends more often upon good basic clinical skills and experience than upon sophisticated and technically complex laboratory investigations. The neonate presenting with micrognathia is an example with which many paediatricians will be familiar. Many such patients will have the accompanying cleft palate and glossoptosis representing the Pierre Robin syndrome (fig 1). A subgroup of such patients will represent examples of Stickler's syndrome. Shprintzen et al1 state that more than half of all patients presenting with Pierre Robin sequence have an underlying diagnosis and their data suggest that up to 25% of such presentations were representative of Stickler's syndrome. A more recent Danish study2 suggests that Stickler's syndrome may account for 12% of all cases of this clinical presentation in the newborn period. Early recognition of Stickler's syndrome as the true cause of Pierre Robin sequence is clinically important so that the affected patient may be offered specialist monitoring. This easily overlooked diagnosis requires monitoring for its devastating ophthalmic complications, including retinal detachment, and auditory evaluation for accompanying deafness.3 Moreover, a detailed history and clinical evaluation of the parents will often establish previously unsuspected cases of Stickler's syndrome in the family. Affected family members may also benefit from appropriate and informed medical management and may appreciate advice regarding genetic risks.
Figure 1 A typical Pierre Robin presentation in a patient with Stickler's syndrome.
While some of the skills of dysmorphology are instinctive, requiring a particular aptitude, many can be learned and taught. The painstaking technnique of obtaining a detailed history, refined over many years of practice, should be a basic skill of every doctor but can be established only with dedication on the part of the trainee and repeated practical demonstration from accomplished trainers. Every paediatrician will be familiar with Williams syndrome and most will have encountered a few cases of this relatively common genetic condition. Clinicians who find the facial features difficult to recognise will often be prompted to request the test for elastin gene deletion on chromosome 7q because of their familiarity with the hypersensitivity to high pitched sound which is almost universal among affected individuals, provided this valuable clue is addressed in history taking.4,5
Many other syndrome diagnoses can be suspected by careful attention to the history, including the family history, and by targeting the subsequent examination and investigations to specifically address the diagnostic considerations provoked by the history. Smith–Magenis syndrome is a good example. The phenotypic changes, which occur with time and increasing age, in the affected child have been well described.6 The cytogenetic and molecular basis of the condition has also been established; there is haploinsufficiency of chromosome 17p11.2 or mutation in the RAI1 locus at this cytogenetic location.7,8 However, the diagnosis can be difficult to identify in the clinic, meaning that the condition is not considered and hence the specific diagnostic tests are not requested. Nevertheless, in addition to the history of developmental delay, temper tantrums and occasional self‐injury, specific questioning with respect to sleep disturbance can prompt the experienced clinician to undertake the appropriate investigations for this condition, not noted for an easily recognised facial gestalt.9 Likewise, the history of hyperventilation in a child with developmental delay and flapping movements of the hands might be the finding that leads to the diagnosis of Pitt–Hopkins syndrome.10 The recent recognition of an increased risk of Beckwith–Wiedemann syndrome and Angelman's syndrome in children conceived by in vitro fertilisation or by intracytoplasmic sperm injection is owed entirely to the attention given to basic history taking by astute clinicians.11,12,13,14
The central role of sophisticated clinical examination by accomplished clinicians is unlikely to ever be replaced in the practice of dysmorphology. However, there is a need for discipline and clarity in the use of terms to describe particular clinical features and there is evidence that such a systematic approach may be lacking . Two relevant examples are worth citing. In the Membership of the Royal College of Physicians of Ireland (Paediatric) Part 2 examination 2 years ago, candidates were asked to “write notes on microtia”. A notable number wrote about micropenis, advocating endocrine studies!15 Within the past few weeks, following a tutorial to a year 3 Specialist Registrar in paediatrics, in which a trident hand was examined in a child with achondroplasia, it emerged that the trainee was unfamiliar with the term and did not understand it. The trainer, an experienced hand, wryly suggested a bit of reading on the subject and addressed the matter a day later to ensure that any omission had been rectified. The response was “I googled it last night but it does not explain what it is, so I am no better off.” Clearly, in tailoring training programmes, we need to keep a weather eye on the sometimes unorthodox sources of information, perhaps intuitively unlikely to a slightly older generation of doctors, to which trainees are apt to turn nowadays. It appears that a principle may need to be re‐established that medical information and explanation is most readily accessed in textbooks and similar resources produced for that express purpose.
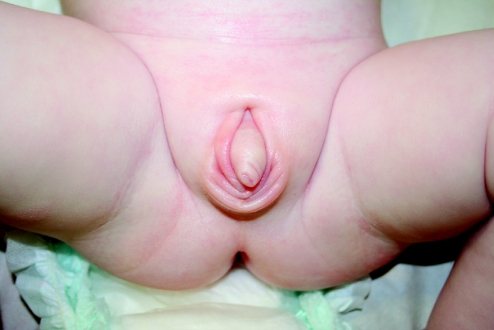
There is no substitute for the repeated observation of important clinical signs and, as the elemental signs of specific diagnoses are sometimes rarely encountered, ready recourse to the literature of dysmorphology is unavoidable. Not every experienced paediatrician or geneticist can feel confident that they have seen an instance of shawl scrotum and could identify this sign with total assurance again in any future boy manifesting the same penile configuration (fig 2). Although these skills are the product of years of experience, they may be attained by the enthusiastic trainee who is prepared to spend time reading old case reports and series of published cases in archived journals, whereas they are unlikely to be the subject of a scholarly explanation found by searching the internet! It is important that the language of dysmorphology, facilitating the unambiguous communication of information often of a recondite nature, should retain its essential character and meaning and be used correctly by all who would contribute to the diagnosis and management of dysmorphic children. In no aspect is language more important than in the description and classification of clinical signs.16 Apart from the recognition and appreciation of clinical signs, it needs to be recognised that these signs can change with time, sometimes facilitating the emergence case of a syndrome diagnosis, which was initially in an individual not forthcoming. Moreover, the emphasis that can be placed on a clinical sign varies somewhat according to normal variation and ethnic background, post‐axial polydactyly being more common in black populations, for instance.
Figure 2 Note the gothic arch shaped fold of skin above the base of the penis, conferring the “shawl” configuration.
The processes by which syndromes become established and the benefits that accrue
There are almost as many ways in which syndromes become suspected, progress through a stage of general acceptance and ultimately find their way into leading textbooks and other sources of information as there are individual conditions. Sometimes, where there is an identifiable extrinsic aetiology, an important element of the process of syndrome emergence is the recognition of a history of exposure. It does not follow, however, that the syndrome is then easily recognisable, as will be attested by anybody who has ever been asked to arbitrate whether developmental delay in a child whose mother was treated with sodium valproate during the pregnancy was caused by the drug exposure. However, although specific answers in individual cases may prove impossible, a general body of experience emerges through case reports, case series and the sharing of clinical observations by interested colleagues. Collective experience does identify the most commonly observed findings in such a condition and thus provides a valuable basis from which colleagues can address similar diagnostic conundrums.17,18,19,20
In the presence of a positive family history, the identification of a syndrome at the clinical level can be eased by the opportunity to assess several affected individuals. Indeed, this extends to the facilitation of research into such Mendelian disorders with a view to identifying their aetiological bases. Such findings are biologically important in terms of enhanced understanding of embryological events that result in dysmorphic conditions. In addition, they have clinical value as a predictive measure for others in the pedigree and as a tool for the positive diagnosis of clinically dubious cases when the same condition is present in other families. The course followed by the severe condition ATR‐X (alpha thalassaemia mental retardation, X‐linked) is a good example. Dating from an initial clinical haematological observation in 1981,21 that some children had unexpectedly been observed in whom Haemoglobin H disease had been identified in a small number of individuals with mental retardation. It was almost a decade later that Wilkie et al,22,23 showed that this observation related to two genetically distinct disorders. One of these disorders was due to deletion of the alpha globin gene complex on chromosome 16p. The other represented an all male cohort of affected individuals who did not have the 16p deletion but in whom a recognisable facial gestalt was often present and several of whom had an identifiable family history that was consistent with X‐linked inheritance. The publication of photographs of affected individuals enabled other workers to identify facially similar individuals (fig 3). A large cohort of affected families led to the localisation of the locus on Xq13 and, subsequently, the characterisation of the causative gene,24 with over 60 mutations now being recorded in 97 families.25 Among dysmorphologists, the facial features of ATR‐X syndrome are now well recognised and the clinical characterisation of the syndrome is well established. Indeed, errors in some of the leading textbooks have been rectified—some cases of this condition had erroneously been published as examples of another, clinically and genetically distinct condition. Moreover, predictive tests for females from affected kindreds are now readily available, and such tests facilitate the identification of clinically unaffected female carriers who are at risk of having affected sons. The positive benefits of this series of clinically driven and laboratory based endeavours have also resulted in the recognition of other conditions, initially thought to be clinically distinct, but are now established as allelic forms of ATR‐X. Such conditions include Juberg–Marsidi syndrome, Chudley–Lowry syndrome, Holmes–Gang syndrome, Carpenter–Waziri syndrome and Smith–Fineman–Myers syndrome.26,27 The literature on all these disorders has now been clarified and sequalised, much to the benefit of affected families. However, for those not familiar with the evolution of the literature relating to these allelic disorders, the potential for confusion is clear. As exemplified by the narrative of how ATRX has moved from a clinical observation 25 years ago to a clearly defined, clinically identifiable disorder for which predictive investigation is nowadays available one of the primary strengths of experienced dysmorphologists is sustained familiarity with a constantly changing literature, which confers an incalculable advantage to such individuals in considering the issues of choosing laboratory investigations germane to specific cases.
Figure 3 The facial features of a patient with ATR‐X syndrome are demonstrated. Note the frontal upsweep of the hair, [Au: ok?] hypertelorism, broad base to the nose, small teeth and everted lower lip, all frequent observations in this condition.
There are always cases that remain insoluble but even in such circumstances experience is an advantage to dysmorphologists. Cohen28 describes the phenomenon of the “provisionally unique pattern syndrome”—in other words, the case whose diagnosis is not achievable but whose constellation of clinical features prompt the clinician to observe that they would recognise another such case in the future. Publication of such single, memorable cases can lead to further examples being published by like‐minded colleagues, as was the case with Costello syndrome.29 Over time, we have thus seen the emergence of a recognisable phenotype and a growing body of knowledge of the paediatric management issues of this condition in terms of feeding difficulties, propensity to childhood cancer and cardiac problems.30 This publication process has also led to the investigation of the clinical overlaps between Costello, Noonan and cardiofaciocutaneous syndromes, with the recent identification of several related genes in the developmental pathway.31,32,33 An important, though less well recognised, by‐product of these processes is the formation of family support groups, often of international nature, which can promote the awareness among the medical profession of management strategies that are found to be useful in some individuals and that may be of wider clinical benefit. Thus, from single case reports, syndromes may move from unique pattern cases to recurrent pattern syndromes that have an established genetic basis and a laboratory test, which may help diagnose individuals with atypical clinical presentations. These developments are all the more welcome and clinically applicable in disorders that occur sporadically.
The value of expert systems in dysmorphology
Databases have become an integral aspect of practice in clinical genetics and dysmorphology. Some resources such as Online Mendelian Inheritance in Man™ (OMIM™, Johns Hopkins University, Baltimore, MD, USA),34 have free access and are routinely consulted by both specialist and nonspecialist readers. OMIM™ is considered less valuable than other databases by geneticists as a diagnostic resource. It is, however, an excellent tool for the identification of key journal references, for summaries of clinical features of classical cases and family studies of Mendelian disorders, and for obtaining an indication of the research progress in terms of gene localisation, errors therein and locus identification. For loci that have been cloned and a “cause and effect” established in relation to phenotypes, OMIM™ does not attempt to embrace all mutational data so such information must be sought elsewhere. Many conditions and disease genes now have dedicated databases in which all recorded mutations that cause disease are available for comparison purposes. The existence of such databases reflects the fact that the identification of a variation in DNA sequence in an affected individual does not always translate into a disease‐causing mutation. The clinician faced with ambiguous laboratory findings can usually seek expert assistance from molecular genetics specialists rather than having to decide for themselves whether a DNA variation represents a “mutation”. However, locus‐specific databases are valuable resources of which it is useful to be aware. The European resource for information on rare disorders, Orphanet®35(Institut National de la Santé et de la Recherche médicale, Paris, France), is accessible to patients and professionals. In addition to synopses and fuller texts on many thousands of conditions, this resource provides country‐specific information on specialist clinics, diagnostic laboratories and patient support groups. The US resource GeneTests36 also contains information on testing centres as well as an encyclopaedia of information on many dysmorphic syndromes. A drawback of these resources, however, is that there are no illustrations of the syndromes described in the databases.
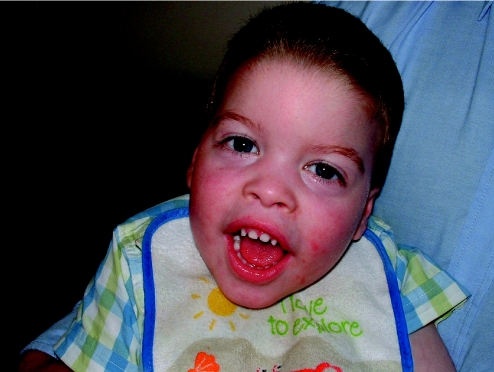
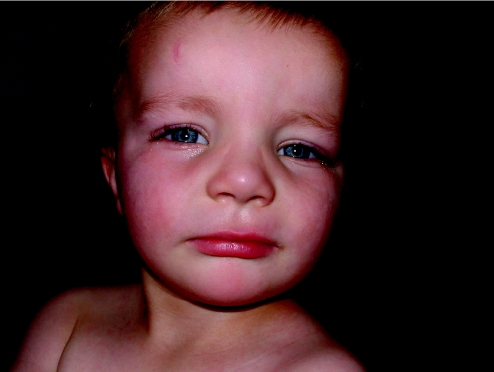
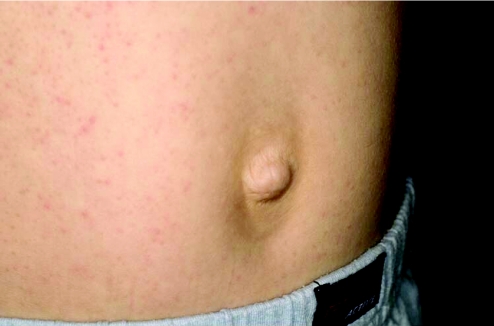
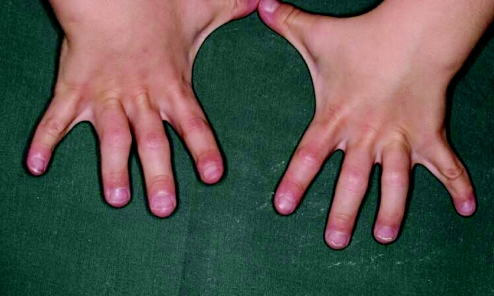
More in keeping with the idea of “keying in a few features and getting a syndrome diagnosis” are some of the commercially available databases, which are maintained by established, far‐sighted experts in the field. The contributions of these resources have been seminal to the development of dysmorphology and other subspecialist practices as independent disciplines. Foremost is the Winter–Baraitser Dysmorphology Database,37 which also offers specialist databases for genetic eye disease and genetic neurological disease. Enormous effort has been made to make available photographs of the conditions and syndromes described. The diagnostic value of these resources is immense but an essential prerequisite is accurate recognition of the clinical signs in a given case. Even with the accurate and practised use of the clinical terms, these databases rely upon the user's extensive and detailed familiarity with the literature, which they summarise and reference in order to sift likely diagnoses from much less likely possibilities but to which the same clinical terms apply in a given instance. Take for example a boy with short stature, whose facial features are suggestive of hypertelorism. Both of these features are “hard” and not open to argument and therefore represent reliable search tools for a database. A search using this combination of features yields in excess of 140 possible syndromes. However, these are the essential clinical features of Aarskog syndrome, which a paediatrician might reasonably expect to identify in a clinical setting without reference to a database (fig 4). The difficulty of sifting this condition from the “background noise” of other syndromes bearing the same clinical findings is enormous without the benefit of having seen affected patients previously and recognising the “gestalt”. Only then, aided by the database search, can the diagnosis be secured, through a reminder of other clinical features that are not so well known or are easily missed on initial inspection. Examples are the periumbilical depression that characterises the condition or the digital syndactyly sometimes observed (figs 5 and 6). In the context of such prior experience, a database can assist a clinician to recall a half‐forgotten condition that they had previously encountered. What the database cannot do is differentiate for the inexperienced clinician between the hyperteloric, short boy with Aarskog syndrome and the hyperteloric, short boy with Robinow syndrome. Databases are immensely valuable, to both specialist and nonspecialist, but do not replace the need for taking a careful history, astute clinical examination, experience, accurate use of descriptive terms and extensive reading. Their value is largely dependent upon the expertise of the user, although this limitation in no way diminishes their parallel value as a teaching and learning resource. Among clinicians concerned with the diagnosis of malformations and the biological basis thereof, other database systems in widespread use include specialist databases in radiology and biochemical diseases. As with the dysmorphology resources, the need for accurate use of language, especially in radiological matters, places a considerable responsibility upon the user to be able to operate comfortably with terms that might reasonably be expected to be outwith the compass of most clinicians. After all, how accurately can most nonradiologists describe a skeletal survey? Moreover, many clinical colleagues admit to bemusement at the basic descriptive terms by which dysmorphologists describe abnormalities (Box 1). Yet it is important to understand such terms in order to take a rational approach to clinical situations involving birth defects.
Figure 4 Many patients with Aarskog syndrome present to the endocrine clinic with short stature, their facial features being relatively unremarkable, apart from a mild degree of hypertelorism, as in this 3‐year‐old boy.
Figure 5 Typical lozenge shaped periumbilical depression seen in Aarskog syndrome. Same patient as Figures 4 and 6.
Figure 6 Mild cutaneous syndactyly of the fingers, coupled with hyperextension of the joints with a compensatory flexion of the distal small joints are frequent findings in Aarskog syndrome.
Box 1 Definition of terms routinely used in the description of birth defects
Malformation: a morphologic abnormality that arises because of an abnormal developmental process. A primary error in morphogenesis—for example, cleft lip.
Malformation sequence: a pattern of multiple defects resulting from a single primary malformation—for example, talipes and hydrocephalus can result from a lumbar neural tube defect.
Malformation syndrome: a pattern of features, often with a unifying underlying cause, that arises from several different errors in morphogenesis (“syndrome” from the Greek “running together”).
Deformation: distortion by a physical force of an otherwise normal structure.
Disruption: destruction of a tissue that was previously normal.
Dysplasia: abnormal cellular organisation within a tissue resulting in structural changes—for example, within cartilage and bone in skeletal dysplasias.
Intuition—the unteachable and the indefinable
Irrespective of their experience, training, extent of reading, clinical ability and observational skills, there exists a small group of individuals whose instinctive ability gives them better diagnostic expertise than their counterparts. No matter how homogenised training becomes, in dysmorphology more than in any other branch of medicine these rare individuals stand out. They possess an ability to make a diagnosis intuitively. It may be their “eye” to recognise a known syndrome with an unfamiliar presentation or their recall after several years of an obscure case that informs their assessment of a current individual, enabling a diagnosis to be reached. This ability could be described as a sixth sense which, combined with relentless reading and enviable powers of recall, enables them to succeed diagnostically where their peers fail. Most dysmorphologists cannot aspire to such heights. However, by careful attention to our specialist training in taking a history, clinical examination, correct use of clinical terms and familiarity with the literature, we can all hope to contribute to the identification of known syndromes and the emergence of new conditions, to counsel and assist families, and to educate and support our colleagues.
Footnotes
Competing interests: None.
Informed consent was obtained for publication of figures 1–6.
References
- 1.Shprintzen R J, Siegel‐Sadewitz V L, Amato J.et al Anomalies associated with cleft lip, cleft palate, or both. Am J Med Genet 198520585–595. [DOI] [PubMed] [Google Scholar]
- 2.Printzlau A, Andersen M. Pierre Robin sequence in Denmark: a retrospective population‐based epidemiological study. Cleft Palate Craniofac J 20044147–52. [DOI] [PubMed] [Google Scholar]
- 3.Snead M P, Yates J R. Clinical and molecular genetics of Stickler syndrome. J Med Genet 199936353–359. [PMC free article] [PubMed] [Google Scholar]
- 4.Klein A J, Armstrong B L, Greer M K.et al Hyperacusis and otitis media in individuals with Williams syndrome. J Speech Hear Disord 199055339–344. [DOI] [PubMed] [Google Scholar]
- 5.Van Borsel J, Curfs L M, Fryns J P. Hyperacusis in Williams syndrome: a sample survey study. Genet Couns 19978121–126. [PubMed] [Google Scholar]
- 6.Hammond P, Hutton T J, Allanson J E.et al Discriminating power of localized three‐dimensional facial morphology. Am J Hum Genet 200577999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slager R E, Newton T L, Vlangos C N.et al Mutations in RAI1 associated with Smith‐Magenis syndrome. Nat Genet 200333466–468. [DOI] [PubMed] [Google Scholar]
- 8.Girirajan S, Elsas L J, 2nd, Devriendt K.et al RAI1 variations in Smith‐Magenis syndrome patients without 17p11.2 deletions. J Med Genet 200542820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Leersnyder H, Bresson J L, de Blois M C.et al Beta 1‐adrenergic antagonists and melatonin reset the clock and restore sleep in a circadian disorder, Smith‐Magenis syndrome. J Med Genet 20034074–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peippo M M, Simola K O, Valanne L K.et al Pitt‐Hopkins syndrome in two patients and further definition of the phenotype. Clin Dysmorphol 20061547–54. [DOI] [PubMed] [Google Scholar]
- 11.Cox G F, Burger J, Lip V.et al Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet 200271162–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orstavik K H, Eiklid K, Van Der Hagen C B.et al Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic semen injection. Am J Hum Genet 200372218–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maher E R, Brueton L A, Bowdin S C.et al Beckwith‐Wiedemann syndrome and assisted reproduction technology (ART). J Med Genet 20034062–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBaun M R, Niemitz E L, Feinberg A P. Association of in vitro fertilization with Beckwith‐Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 200372156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy J F A. Personal communication 2006
- 16.Merks J H, van Karnebeek C D, Caron H N.et al Phenotypic abnormalities: terminology and classification. Am J Med Genet A 2003123211–230. [DOI] [PubMed] [Google Scholar]
- 17.DiLiberti J H, Farndon P A, Dennis N R.et al The fetal valproate syndrome. Am J Med Genet 198419473–481. [DOI] [PubMed] [Google Scholar]
- 18.Ardinger H H, Atkin J F, Blackston R D.et al Verification of the fetal valproate syndrome phenotype. Am J Med Genet 198829171–185. [DOI] [PubMed] [Google Scholar]
- 19.Winter R M, Donnai D, Burn J.et al Fetal valproate syndrome: is there a recognisable phenotype? J Med Genet 198724692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clayton‐Smith J, Donnai D. Fetal valproate syndrome. J Med Genet 199532724–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weatherall D J, Higgs D R, Bunch C.et al Hemoglobin H disease and mental retardation: a new syndrome or a remarkable coincidence? N Engl J Med 1981305607–612. [DOI] [PubMed] [Google Scholar]
- 22.Wilkie A O, Buckle V J, Harris P C.et al Clinical features and molecular analysis of the alpha thalassemia/mental retardation syndromes. I. Cases due to deletions involving chromosome band 16p13.3. Am J Hum Genet 1990461112–1126. [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkie A O, Zeitlin H C, Lindenbaum R H.et al Clinical features and molecular analysis of the thalassemia/mental retardation syndromes. II. Cases without detectable abnormality of the alpha globin gene complex. Am J Hum Genet 1990461127–1140. [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbons R J, Picketts D J, Villard L.et al Mutations in a putative global transcriptional regulator cause X‐linked mental retardation with alpha‐thalassemia (ATR‐X) syndrome. Cell 199580837–845. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons R J, Wada T.ATRX and X‐linked alpha thalassaemia mental retardation syndrome. In: Epstein CJ, Erickson RP, Wynshaw‐Boris A, eds. Inborn errors of development: the molecular basis of clinical disorders of morphogenesis New York, Oxford: Oxford University Press, 2004, xxx–xxx. [Au: please provide the start and end page numbers of this chapter. ]
- 26.Leahy R T, Philip R K, Gibbons R J.et al Asplenia in ATR‐X syndrome: a second report. Am J Med Genet A 200513937–39. [DOI] [PubMed] [Google Scholar]
- 27.Raymond F L. X linked mental retardation: a clinical guide. J Med Genet 200643193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen M M., JrThe child with multiple birth defects. 2nd rev edn. New York, Oxford: Oxford University Press, 1997
- 29.Costello J M. A new syndrome: mentally subnormality and nasal papillomata. Aust Paediatr J 197713114–118. [DOI] [PubMed] [Google Scholar]
- 30.Kerr B, Eden O B, Dandamudi R.et al Costello syndrome: two cases with embryonal rhabdomyosarcoma. J Med Genet 1998351036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoki Y, Niihori T, Kawame H.et al Germline mutations in HRAS proto‐oncogene cause Costello syndrome. Nat Genet 2005371038–1040. [DOI] [PubMed] [Google Scholar]
- 32.Kerr B, Delrue M A, Sigaudy S.et al Genotype‐phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet 200643401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niihori T, Aoki Y, Narumi Y.et al Germline KRAS and BRAF mutations in cardio‐facio‐cutaneous syndrome. Nat Genet 200638294–296. [DOI] [PubMed] [Google Scholar]
- 34.Johns Hopkins University and National Center for Biotechnology Information Online Mendelian Inheritance in Man™. www.ncbi.nlm.nih.gov/omim (accessed 15 Jan 2007)
- 35.Orphanet® www.orpha.net (accessed 15 Jan 2007)
- 36.GeneTests www.geneclinics.org (accessed 15 Jan 2007)
- 37.London Medical Databases Winter–Baraitser Dysmorphology Database www.lmdatabases.com (accessed 15 Jan 2007)