Abstract
Aims
To define the pharmacokinetics of milrinone in very preterm infants and determine an optimal dose regimen to prevent low systemic blood flow in the first 12 h after birth.
Methods
A prospective open‐labelled, dose‐escalation pharmacokinetic study was undertaken in two stages. In stage one, infants received milrinone at 0.25 μg/kg/min (n = 8) and 0.5 μg/kg/min (n = 11) infused from 3 to 24 h of age. Infants contributed 4–5 blood samples for concentration–time data which were analysed using a population modelling approach. A simulation study was used to explore the optimal dosing regimen to achieve target milrinone concentrations (180–300 ng/ml). This milrinone regimen was evaluated in stage two (n = 10).
Results
Infants (n = 29) born before 29 weeks gestation were enrolled. Milrinone pharmacokinetics were described using a one‐compartment model with first‐order elimination rate, with a population mean clearance (CV%) of 35 ml/h (24%) and volume of distribution of 512 ml (21%) and estimated half‐life of 10 h. The 0.25 and 0.5 μg/kg/min dosage regimens did not achieve optimal milrinone concentration‐time profiles to prevent early low systemic blood flow. Simulation studies predicted a loading infusion (0.75 μg/kg/min for 3 h) followed by maintenance infusion (0.2 μg/kg/min until 18 h of age) would provide an optimal milrinone concentration profile. This was confirmed in stage two of the study.
Conclusion
Population pharmacokinetic modelling in the preterm infant has established an optimal dose regimen for milrinone that increases the likelihood of achieving therapeutic aims and highlights the importance of pharmacokinetic studies in neonatal clinical pharmacology.
Keywords: FOCE, first‐order approximation with condition estimation, HPLC, high performance liquid chromatography, P/IVH, peri/intraventricular haemorrhage, SVC, superior vena cava
Low systemic blood flow to the upper body and brain in the early postnatal period, as measured by superior vena cava (SVC) flow is common after preterm birth, affecting 35% of infants born before 30 weeks and 61% of infants less than 27 weeks.1,2 It is strongly associated with lower gestational age and high systemic vascular resistance; it occurs in a predictable time frame in the first 6–12 h after birth and improves over the subsequent 24–48 h.1,3 This pathophysiological state is thought to be a consequence of an immature myocardium adapting to high vascular resistance. Low SVC flow is strongly related to peri/intraventricular haemorrhage (P/IVH),2,4 increased mortality6 and abnormal neurodevelopment at 3 years.5 In 40% of these infants, low SVC flow does not improve when the commonly used inotropes of dobutamine and dopamine are used.6
New cardiovascular strategies are needed in these infants to prevent the low SVC flow from occurring. Agents that lower vascular resistance may improve low systemic blood flow, so our research group has explored the utility of a preventive approach using milrinone from early in the postnatal period.7 Milrinone is a selective phosphodiesterase type III inhibitor, which has positive inotropic effects independent of beta adrenergic receptor stimulation and is a vasodilator (‘inodilator'). It acts by decreasing systemic vascular resistance, increasing cardiac contractility and cardiac output.8 Studies show that milrinone significantly improves cardiac function in children and neonates following cardiac surgery9,10,11,12 and in children with septic shock.13,14 However, milrinone has not been evaluated in the very preterm infant with low cardiac output. Potential adverse effects of milrinone are hypotension, tachyarrhythmia and thrombocytopenia.8,10
Milrinone pharmacokinetic data are available for adult,15 paediatric populations10,11,12,14 and term infants16 but not for very preterm infants. The pharmacokinetics of milrinone have been established to be linear in adults and infants. Early studies in healthy subjects confirmed that milrinone is predominately eliminated by renal excretion with a fraction excreted unchanged of approximately 80%.8 The renal clearance of milrinone was calculated to be approximately 10 times higher than the expected renal clearance by filtration of unbound drug clearly indicating that renal secretion is a major contributor to milrinone renal excretion. The exact renal secretion pathway has not been investigated. Neonates, and specifically the very preterm neonate, have rapid and significant developmental physiological changes leading to variability in drug pharmacokinetics.17,18 The population pharmacokinetic modelling approach has been used to describe pharmacokinetic behaviour of drugs (including inter‐patient variability) and to explore different dosing regimens suitable for specific patient groups.16,18,19,20
The aim of this study was to investigate the pharmacokinetics and safety of milrinone in very preterm infants at high risk of low systemic blood flow and to explore different dose regimens to maintain SVC flow over the first day after birth. This paper focuses on the pharmacokinetics of milrinone in very preterm infants. The clinical and haemodynamic outcomes have been reported elsewhere.7
Methods
Setting
This was a two centre study performed in the Neonatal Intensive Care Units of Royal Prince Alfred Hospital and Royal North Shore Hospital, Sydney, Australia and was approved by the Human Research Ethics Committee and the Drug Committees of both hospitals. Informed parental consent was obtained prior to enrolment.
Study population
Infants born before 29 weeks gestation were eligible. The selection criteria were based on two predictive risk factors for developing low systemic blood flow, gestational age and early SVC flow, which were determined from previous observational cohorts.2,4 Infants born before 27 weeks were enrolled irrespective of SVC flow, whilst infants at 27 and 28 weeks were only enrolled if the SVC flow at 3 h of age was less than 60 ml/kg/min (population median in previous cohorts2,4). Essential for inclusion was invasive arterial blood pressure monitoring. The exit criterion was sustained hypotension less than 24 mm Hg for one hour, unresponsive to volume replacement and inotropes. Infants were excluded from the study if they were outborn; had a structural abnormality of the heart or brain; had P/IVH grade 2 or higher at the initial cranial ultrasound; were not expected to survive; had perinatal asphyxia (defined as base excess less than −12 on the cord gas); were over 6 h of age at the time of study enrolment; had tachyarrhythmia, sustained hypotension less than 24 mm Hg at the time the drug was to be commenced, hyperkalaemia (defined as K+ greater than 6.5 mmol/l) or serum bilirubin concentration greater than 200 mmol/l.
Study design
This was a prospective two‐stage open‐labelled, dose‐escalation pharmacokinetic study. Milrinone lactate injection (Primacor 1 mg/ml; Sanofi –Synthelabo, North Ryde, Australia) was used. A loading bolus dose of milrinone was not used in stage one at the request of the Central Sydney Area Health Service Clinical Trials Committee.
Stage one: involved dose escalation in two patient cohorts. Initially 8 infants received a milrinone infusion at 0.25 μg/kg/min, which is half the ‘recommended dose' for paediatric patients.9,13,14,15 A second cohort of 11 infants received milrinone infused at 0.5 μg/kg/min. Infusions were commenced between 3 and 6 h of age and ceased at 24 h of age.
In stage two: the dose regimen selected from the simulations study (outlined below) was evaluated in a separate cohort of 10 infants. This consisted of a milrinone loading infusion dose of 0.75 μg/kg/min for 3 h followed by a maintenance infusion of 0.2 μg/kg/min until 18 h of age.
Co‐interventions
To ensure adequate intravascular volume, all infants received 15 ml/kg of normal saline infused concurrently with the milrinone infusion during the first hour. Indomethacin (0.1 mg/kg) was administered if the ductus arteriosus at 3 h was unconstricted with a diameter 2 mm or greater. Surfactant was administered to all infants with Respiratory Distress Syndrome. Further cardiovascular support with volume bolus and inotropes was provided on the basis of echocardiographic and invasive blood pressure monitoring, details of which have been reported separately.7 Infants were monitored for haemodynamic and clinical outcomes by serial echocardiography and cranial ultrasound, the results of which have been reported separately.7
Blood sampling
Blood samples (0.6 ml) were collected for milrinone determination from an indwelling arterial line. In stage one of the study, samples were taken 6 h after starting the infusion (9 h of age), at cessation of infusion (24 h of age) and at 2 and 4 h after ceasing the infusion (26 and 30 h of age) (table 1). In stage two, blood samples were collected at 3 h after the loading infusion was commenced (6 h of age), 3 h after the infusion rate was decreased to 0.2 μg/kg/min (9 h of age), at 18 h of age when the infusion was ceased and at 2 and 4 h after ceasing the infusion (20 and 24 h of age) (table 2). The exact timing of each infusion and the blood sample collection was recorded and used in pharmacokinetic assessment. Blood was collected in a clotted tube which was centrifuged, the serum was harvested and stored frozen at −30°C until the assay was performed.
Table 1 Milrinone serum concentration for infusion rates 0.25 and 0.5 µg/kg/min.
| Milrinone dose (µg/kg/min) | n | Age (h) | Mean Time from starting infusion (h) | Milrinone serum concentration (ng/ml) mean ± SD |
|---|---|---|---|---|
| 0.25 | 8 | 9 | 6 | 112±49 |
| 24 | 21 | 336±87 | ||
| 26 | 23 | 245±99 | ||
| 30 | 27 | 216±54 | ||
| 0.5 | 11 | 9 | 6 | 227±56 |
| 24 | 21 | 432±183 | ||
| 26 | 23 | 389±159 | ||
| 30 | 27 | 347±87 |
Table 2 Pharmacokinetic parameter estimates from the final model.
| Parameter | Population mean (95% CI) | Interindividual variability CV% (95%CI) |
|---|---|---|
| CL (ml/h) | 35 (31 to 39) | 24% (0.13 to 0.38) |
| V (ml) | 512 (460 to 580) | 21% (0.11 to 0.31) |
| Residual variability ng/ml (SD) | 36 (19 to 61) | |
Milrinone assay
The assay method was a modification of a previously published method14,22 and was performed at the Department of Clinical Biochemistry, Royal Prince Alfred Hospital. The high performance liquid chromatography (HPLC) system consisted of an automatic injector SIL ‐10AD (Shimadzu, Japan) and pump LC ‐10AT (Shimadzu, Japan) and a laboratory packed column 150 mm×3.9 mm (Alltech Pty Ltd, Australia) with LiChrosorb RP (5 μm particle size, Merck, Germany) and UV detection (Shimadzu, Japan) at 340 nm. The mobile phase consisted of tetrahydrofuran: acetonitrile :phosphate buffer (0.1M, pH 6.0) (1:9:100) pumped at 1 ml/min heated to 30°C. Plasma and QC samples (0.1 ml) were precipitated with methanol (0.4 ml). Samples were vortexed then centrifuged after which the supernatant was removed, evaporated and the residual was reconstituted in mobile phase (0.1 ml ) and injected (0.02 ml). The retention time for milrinone was 9.8 min. The within‐day coefficient of variation was less than 10%.
Population pharmacokinetic analysis
A pharmacokinetic model was developed using the milrinone concentration‐time data obtained from infants in stage one of the study. The pharmacokinetic model was then used as an input‐output model to conduct a series of clinical trial simulations to evaluate different dosing regimens. A nonlinear mixed effect modelling approach was used to analyse the milrinone concentration‐time data which employed the first‐order approximation with condition estimation (FOCE) method implemented in NONMEM (v 5.1.1).23
The population pharmacokinetic model was developed in three steps. In the first step, a basic structural model involving the one‐compartment pharmacokinetic model with zero‐order infusion rate and first‐order elimination was employed (after evaluating different candidate models). Pharmacokinetic parameters were assumed to be log‐normally distributed. Exponential covariance structures of inter‐individual variability were modelled. In the model development process, a decrease in the NONMEM derived objective function value (OFV) by ⩾6.64 (p⩽0.01, df = 1) was used as a model selection criterion. Influential covariates were investigated for their effect on milrinone pharmacokinetic parameters and selected using a stepwise inclusion and backward elimination strategy. A decrease in the objective function by ⩾3.84 (p⩽0.05, df = 1) was used to select covariates for inclusion in the model and a decrease of ⩽6.74 (p⩽0.01, df = 1) was used as exclusion criterion for each covariate. Furthermore, a reduction in inter‐individual variability and improvement in various diagnostic plots were used to assess the model performance. The diagnostic plots were presented using Wings24 for NONMEM with CrossGraphs® (PPD Development, Version 2.3). Graphical displays for model building were based on individual fitting plots; predicted values versus observed values and weighted residuals against predictions.
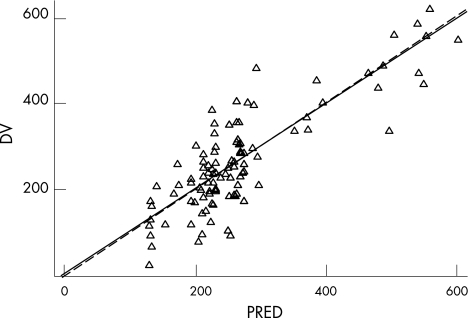
Figure 1 Goodness of fit plot of predicted versus observed concentration of milrinone (ng/ml) (PRED vs DV) (Δ: observed concentration, solid line: trend line, broken line: identity).
Model parameters
Individual values (θi) of clearance (CL) and volume of distribution (V) were modelled as their respective population mean (typical values (θ)) multiplied by the exponentials of their random inter‐individual effect ηi to describe inter‐individual variability, such that:
θi = θ × eηi
in which the inter‐individual ηi values were assumed to be normally distributed with a mean value of zero and a variance of ω2.
The residual variability in milrinone concentration was described as follows:
yij = f + εij
where yij is the observed milrinone concentration in individual ith at time jth, f is the milrinone concentration predicted by the model from the individual parameters, εij is the differences of the jth observed milrinone concentration for the ith individual for the predicted milrinone concentration with a mean value of zero and a variance of σ2.
The 95% confidence intervals (CIs) were obtained and the model evaluated using the bootstrap replicates for each parameter at the 2.5th and 97.5th percentile using Wings for NONMEM. A total of 200 bootstrap runs were conducted.
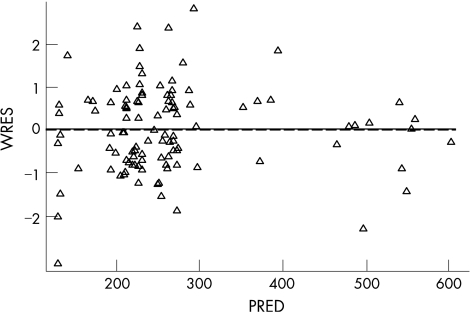
Figure 2 Goodness of fit plot of predicted concentration of milrinone (ng/ml) versus weighted residuals (PRED vs WRES) (Δ: observed concentration, solid line: trend line, broken line: zero line).
Simulations
The simulation of milrinone concentration‐time data to achieve typical target concentrations of between 180 to 300 ng/ml (based on previously published work in adults and children with heart failure, sepsis or post cardiac surgery10,11,14,15) were conducted using NONMEN and were based on the parameters derived from the final validated population pharmacokinetic model. Milrinone concentration‐time profiles for 100 typical infants were simulated using different dose regimens. The aim of the simulations was to select a milrinone infusion regimen to achieve the nominal target drug concentrations by 6–9 h of age and maintain the concentrations to at least 18 h of age. Considering the highest risk time frame for low systemic blood flow is 6 to 12 h after birth,1 our goal was for therapeutic levels by 6 h of age (after 3 h of infusion) and for levels to be falling by about 24 h of age when natural improvement in systemic blood flow is usually occurring. The simulated clinical trial study design was identical to the stage one of this study except for differences in the milrinone dose regimens. The 5th, 50th and 95th centile concentration value at each time point were graphically displayed and tabulated for the dose regimen.
Table 4 Observed and simulated milrinone concentration with 0.75–0.2 μg/kg/min dose regimen.
| Age (h) | Mean Time from starting infusion (h) | Observed milrinone median concentration (range) (ng/ml) | Simulated milrinone mean concentration (5th and 95th percentile) (ng/ml) |
|---|---|---|---|
| 6 | 3 | 231 (97–284) | 197 (144–273) |
| 9 | 6 | 210 (126–354) | 215 (165–289) |
| 18 | 15 | 288 (104–407) | 252 (189–344) |
| 20 | 17 | 247 (95–358) | 257 (193–359) |
| 24 | 21 | 195 (78–257) | 215 (149–317) |
Results
Participants
Twenty nine infants were enrolled in the study. The mean gestational age was 26 weeks (range 23–28 weeks). The mean birth weight was 850 g (range: 520–1258 g). Table 3 shows clinical characteristics of the infants. All babies had normal creatinine levels in the first 24 h while in the second 24 h, 3 babies had creatinine levels just above the normal range with all other babies having normal creatinine.
Table 3 Clinical characteristics of participants.
| Milrinone dose (μg/kg/min) | |||
|---|---|---|---|
| 0.25 | 0.5 | 0.75–0.2 | |
| n | 8 | 11 | 10 |
| Mean GA (weeks) | 26 (25–28) | 25 (23–27) | 26 (24–26) |
| Mean BW (g) | 947 (789–1258) | 849 (562–1114) | 805 (520–960) |
| Any Antenatal steroids | |||
| Any | 3 (38%) | 4 (36%) | 3 (30%) |
| Complete | 5 (62%) | 7 (64%) | 7 (70%) |
| Gender : Male | 4 (50%) | 7 (64%) | 6 (60%) |
| Apgar | |||
| 1 min | 5 | 5 | 6 |
| 5 min | 8 | 7 | 8 |
| Cord pH/BE | 7.31/−6.1 | 7.23/−8.8 | 7.29/−4.5 |
| Singleton | 8 (100%) | 7 (64%) | 7 (70%) |
| SVC flow (>45 ml/kg/min) | 5 (63%) | 7 (64%) | 10 (100%) |
| BP >24 mm Hg | 6 (75%) | 6 (55%) | 7 (70%) |
| Inotropes | 4 (50%) | 6 (55%) | 4 (40%) |
| Indomethacin | 7 (87%) | 7 (63%) | 5 (50%) |
| Creatinine (mmol/l) | 57 (50–80) | 68(55–88) | 61 (50–76) |
| 0–24 h: | |||
| Creatinine (mmol/l) | 76 (60–90) | 78 (66–104) | 79 (54–105) |
| 24–48 h: | |||
| Exit criteria | 0 | 1 | 0 |
Dose escalation study (Infusion rates 0.25 and 0.5 μg/kg/min): Concentration – time data analysis
At both infusion rates, 35% of babies still developed low systemic blood flow. After the 0.25 μg/kg/min infusion, milrinone concentrations were below the target range at 6 h of infusion (9 hrs of age). While the milrinone concentrations were within the target range at 6 h (9 h of age) after the 0.5 μg/kg/min infusion rate this may still have been too slow. For both milrinone dose regimens there was accumulation of the drug towards steady‐state with concentration above the target range by 24 h, table 1. These data suggested that both regimens may have increased milrinone concentrations too slowly to prevent low systemic blood flow (6–12 h) and then concentrations accumulated above the target range at 24 h when systemic blood flow is likely to be improving spontaneously.
Pharmacokinetics of Milrinone
In total there were 58 concentration‐time observations (4–5 per patient). The infusion duration in stage one was 19.6±6.9 h. Four infants had individual data points excluded from the final analysis. Two of these infants had very low concentrations compared to other data for that individual patient and compared to other patients receiving that dose. One other infant receiving the 0.5 μg/kg/min infusion rate had concentrations an order of magnitude lower than the other patients receiving that dose. One infant had a very high value compared to other data from this patient and other patients on this dose. Excluding this data point provided a significantly improved fit of the model. The population pharmacokinetic model provided excellent description of the observed data (figs 1 and 2). The population mean (95%CI) estimate of milrinone clearance was 35 ml/h (31–39) with an inter‐patient variability of 24% (11–31%), 0.64 mls/kg/min. The volume of distribution of milrinone in this patient group was estimated as 512 ml (460–508 ml) with an inter‐patient variability of 21% (11–31%), 576 mls/kg (table 2). The model based estimate of milrinone half‐life in these infants was 10.3 h. Allometric scaling was employed for the effect of body weight as a covariate on CL (0.75) and V (1). However, other patient covariates (such as gestational age, sex, Apgar, and co‐administration with dopamine or dobutamine) did not result significant improvements in model performance and were not included in the final model.
Simulations to achieve target range concentrations in infants
The simulation results for optimised regimens are displayed in table 4</tblref> and fig 3. A milrinone dose regimen involving a fast and slow infusion would optimally achieve the target concentration of milrinone within the range 180–300 ng/ml. The profile which best fits the therapeutic aim, of having target levels by 6 h of age and maintaining this with a waning effect by 24 h, was an infusion 0.75 μg/kg/min for 3 h followed by 0.2 μg/kg/min until 18 h of age.
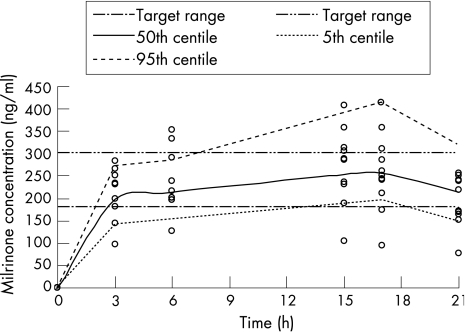
Figure 3 Milrinone concentration‐time profile ○: observed milrinone concentration in 0.75/0.2 μg/kg/min dose regimen, broken line: 5th and 95th percentile from the simulation using 0.75/0.2 μg/kg/min dose regimen) with target range between 180 and 300 ng/ml.
Figure 3 shows the observed milrinone concentration–time data for 10 infants who received the optimised dose regimen of 0.75 μg/kg/min for 3 h followed by 0.2 μg/kg/min until 18 h of age overlayed with the simulated milrinone concentration‐time. The milrinone concentration‐time data demonstrates that optimal therapeutic concentrations have been achieved using this dose regimen. None of the 10 babies in this cohort developed low systemic blood flow.7
Discussion
This study has investigated the pharmacokinetics of milrinone in the very preterm infant and explored optimal dose regimens to prevent low systemic blood flow for the first 24 h after birth. A population pharmacokinetic modelling approach has been employed to describe the pharmacokinetic behaviour (and variability) of milrinone using a limited number of observations per patient. The relatively small amount of data from each patient in this special population was fitted simultaneously to provide a population estimate of the mean and variance of pharmacokinetic parameters. In turn, these data were used to simulate different concentration‐time profiles which were matched against a nominal target concentration to devise an optimal dose regimen. This pharmacokinetic‐guided approach to dose selection was then explored further in a separate cohort of infants.
Of substantial pharmacokinetic importance was the finding in this study that the model‐derived estimate of milrinone half life in this cohort of very preterm infants was more than 10 h which is considerably longer than the estimated 2–4 h in infants and children.10 The clearance of milrinone in the very preterm infant was substantially less than the published data of paediatric populations.10,12,14 Clearance is generally considered a function of body mass,16 so that larger patients tend to have a higher milrinone clearance compared to smaller patients. In the present study the estimated clearance of milrinone was 0.64 ml/kg/min which is significantly lower than reported in children, ranging from 2.5 ml/kg/min to 10.6 ml/kg/min.10,12,14 Interestingly, the estimated mean volume of distribution of 576 ml/kg is less than that reported in other paediatric populations 700–900 ml/kg,10,12 yet still significantly larger than that described in adults at 300 ml/kg.24 The implication of this is unclear and it should be noted that these estimates have been derived using different pharmacokinetic models.
Bailey et al16 recently reported the findings of a population pharmacokinetics study of milrinone conducted in children as part of the multi‐centred PRIMACOR trial. In the neonatal subgroup (n = 48), the milrinone population mean (+/− SEM) clearance was reported as 1.64 +/− 0.37 ml/min/kg and volume of distribution as 523 +/− 28 ml/kg which were in agreement with the results from the present study in very preterm infants (0.64 ml/kg/min and 576 ml/kg respectively). The present study did not identify patient specific characteristics that influence milrinone pharmacokinetics, including gestational age, sex, Apgar, and co‐administration with dopamine or dobutamine. In contrast, Bailey et al16 concluded that milrinone clearance in this cohort of children was significantly influenced by both weight and age. These researchers confirmed the relatively longer half‐life of milrinone in this patient population (3.7 h in the neonatal subgroup) and supported the need for a loading infusion to rapidly achieve milrinone concentrations.
In the adult, milrinone is predominately excreted by the kidneys and approximately 70% is bound to plasma proteins.8 The pharmacokinetic changes of decreased clearance, prolonged half life and larger volume of distribution observed in our study are consistent with the physiological differences noted in preterm infants and can be explained by immature renal function and higher total body water compartment seen in preterm infants.17,18,26 Taken together these findings have implications for dose selection and the pitfalls of scaling doses in mg/kg from infants and children for preterm infants. A significant proportion of the babies in this study were treated with early indomethacin for ductus arteriosus that were failing to constrict. The presence of PDA and co‐administration of indomethacin has been noted to alter the volume of distribution and clearance of some aminoglycosides.27,28 The possible impact of PDA and indomethacin will depend on the pharmacokinetics of the drug in question. The exact effect on milrinone pharmacokinetics is difficult to predict. Milrinone is cleared by renal excretion and has a volume of distribution that is restricted to extracellular space (approx 0.4 to 0.6 l/kg) which suggests that the fluid overload and haemodynamic changes associated with PDA may have an effect on its distribution and excretion.29 The impact of indomethacin on milrinone pharmacokinetics has not been reported. Indomethacin is expected to reduce glomerular filtration rate (GFR). However, milrinone mainly undergoes active secretion in the renal tubules suggesting the GFR alone may not be a major determinant of its clearance. The creatinine levels were normal in all babies within the first 24 h when milrinone was being infused. They were higher in the second 24 h as has been described previously in this population. While no baby had frank renal failure, we cannot exclude an affect of transitional renal adaptation on the pharmacokinetics however this is consistent with our goal to establish the pharmacokinetics within the transitional period.
Details of the clinical and haemodynamic outcomes of this study are reported elsewhere,7 but in the first dose escalation stage, 36% of babies in each cohort still developed low systemic blood flow. We did not take this as strong evidence of preventative efficacy. However analysis of the drug blood levels suggested they may be taking too long to reach target range to effectively prevent the nadir in systemic blood flow between 6 and 12 h of age while accumulating above the target range at 24 h when systemic blood flow will usually be improving spontaneously. A primary reason for undertaking pharmacokinetic analysis is to determine an effective dose regimen in a target population and to accomplish this aim, therapeutic plasma concentrations need to be identified.19,20 The population pharmacokinetics allowed us to simulate a dosage regimen that would increase the likelihood of milrinone fulfilling our therapeutic aim. The value of the simulation approach is highlighted by the close agreement in the model of predicted and observed milrinone concentrations presented in table 2. Furthermore, the data from the last cohort of infants points to a positive haemodynamic effect of milrinone using a dose regimen of loading infusion with 0.75 μg/kg/min for 3 h followed by a maintenance infusion of 0.2 μg/kg/min with no infant developing low systemic blood flow. This was achieved without significant side effects. While this is encouraging, the lack of a control group means that it is not proof of efficacy. We are currently undertaking a double blind placebo controlled randomised trial to confirm efficacy and safety of this dosage regimen.
What is already known on this topic
Milrinone appears effective in treating and preventing post cardiac surgery low cardiac output syndrome in term neonates and infants.
The pharmacokinetics of milrinone have been studied in these populations but not in preterm infants.
What this study adds
That the pharmacokinetics of milrinone in preterm infants during the period of postnatal transition are different from older populations, particularly with a significantly longer half life.
That the resultant optimal dosage regimen is different in preterm babies.
These data highlighted to the clinicians within our group the importance of age appropriate pharmacokinetics in the study of preterm therapeutics. Had we not included a pharmacokinetic analysis, we would probably have discontinued this study after the first stage as not showing convincing evidence of efficacy. Too often in neonatology, drug dosage regimens are adopted from populations of different ages (as we did in the first stage of this study) or because an, often, arbitrary dose was used in the early publications about a drug. These regimens then stick all the way up to large multicentre randomised trials without rigorous assessment of the relationship between the therapeutic aims and the age appropriate pharmacokinetics. In such circumstances, a potentially useful drug may be discarded simply because the dosage is incorrectly structured.
In conclusion, this study has proved a valuable exercise in collaboration of clinical medicine and pharmacokinetics. The natural history of the low systemic blood flow state in the very preterm infant is known1 and population pharmacokinetic modelling in the preterm infant has been able to establish an optimal dose regimen for milrinone that increase the likelihood of fulfilling therapeutic aims. The data highlight the importance of age‐appropriate pharmacokinetic analysis early in the introduction of a new drug to neonatology. The sample size and the lack of control group mean that these data have not yet confirmed the efficacy or safety of milrinone in this population and we would not recommend the use of milrinone in this population outside the context of a trial.
Acknowledgements
The authors gratefully acknowledge the support of Mr David Yeo, from Department of Clinical Biochemistry, Royal Prince Alfred Hospital for conducting the HPLC analysis.
Abbreviations
FOCE - first‐order approximation with condition estimation
SVC - superior vena cava
Footnotes
Funding: North Shore Heart Research Foundation
Competing interests: None.
References
- 1.Kluckow M, Evans N. Superior vena cava flow in newborn infants: a novel marker for systemic blood flow. Arch Dis Child Fetal Neonatal Ed 200082F182–F187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborn D A, Evans N, Kluckow M. Haemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 200311233–39. [DOI] [PubMed] [Google Scholar]
- 3.Kluckow M, Evans N. The relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J Pediatr 1996129506–512. [DOI] [PubMed] [Google Scholar]
- 4.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed 2000b82F188–F194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt R W, Evans N, Rieger I.et al Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr 2004145588–592. [DOI] [PubMed] [Google Scholar]
- 6.Osborn D A, Kluckow M, Evans N. Randomised trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. J Pediatr 2002140183–191. [DOI] [PubMed] [Google Scholar]
- 7.Paradisis M, Evans N, Kluckow M.et al Pilot Study of Milrinone for Prevention of Low Systemic Blood Flow in Very Preterm Infants. J Pediatr 2006148306–313. [DOI] [PubMed] [Google Scholar]
- 8.Stroshane R M, Koss R F, Biddlecome C E.et al Oral and intravenous pharmacokinetics of milrinone in human volunteers. J Pharm Sci 1984731438–1441. [DOI] [PubMed] [Google Scholar]
- 9.Chang A C, Atz A, Wermovsky G.et al Milrinone: systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit Care Med 1995231907–1914. [DOI] [PubMed] [Google Scholar]
- 10.Ramamoorthy C, Anderson G D, Williams G D.et al Pharmacokinetics and side effects of milrinone in infants and children after open heart surgery. Anesth Analg 199886283–289. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman T M, Wernovsky G, Atz A M.et al Efficacy and safety of Milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003107996–1002. [DOI] [PubMed] [Google Scholar]
- 12.Bailey J M, Miller B E, Lu W.et al The pharmacokinetics of milrinone in pediatric patients after cardiac surgery. Anesthesiology 1999901012–1018. [DOI] [PubMed] [Google Scholar]
- 13.Barton P, Garcia J, Kouatli A.et al Hemodynamic effects of IV Milrinone lactate in pediatric patients with septic shock. A prospective double blinded, randomized, placebo controlled interventional study. Chest 19961091302–1312. [DOI] [PubMed] [Google Scholar]
- 14.Lindsay C A, Barton P, Lawless S.et al Pharmacokinetics and pharmacodynamics of milrinone lactate in pediatric patients with septic shock. J Pediatr 1998132329–334. [DOI] [PubMed] [Google Scholar]
- 15.Bailey J M, Levy J H, Kikura M.et al Pharmacokinetics of intravenous milrinone in patients undergoing cardiac surgery. Anesthesiology 199481616–622. [DOI] [PubMed] [Google Scholar]
- 16.Bailey J M, Hoffman T M, Wessel D L.et al A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. J Pharmacokinet Pharmacodyn 20043143–59. [DOI] [PubMed] [Google Scholar]
- 17.Kearns G L, Abdel‐Rahman S M, Alander S W.et al Developmental pharmacology‐‐drug disposition, action, and therapy in infants and children. N Engl J Med 20033491157–1167. [DOI] [PubMed] [Google Scholar]
- 18.Loebstein R, Koren G. Clinical pharmacology and therapeutic drug monitoring in neonates and children. Ped in Review 199819423–428. [DOI] [PubMed] [Google Scholar]
- 19.Reed M D. Optimal sampling theory: An overview of its application to pharmacokinetic studies in infants and children. Pediatrics 1999104627–632. [PubMed] [Google Scholar]
- 20.Ette E I, Ludden M D. Population Pharmacokinetic Modeling: the Importance of Informative Graphics. Pharm Res 1995121845–1855. [DOI] [PubMed] [Google Scholar]
- 21.Thomson A H, Whiting B. Bayesian parameter estimation and population pharmacokinetics. Clin Pharmacokinet 199222447–467. [DOI] [PubMed] [Google Scholar]
- 22.Edelson J, Koss R F, Baker J F.et al High performance chromatography analysis of milrinone in plasma and urine. Intravenous pharmacokinetics in the dog. J Chromatography 1983276456–462. [DOI] [PubMed] [Google Scholar]
- 23.Beal S L, Sheiner L B. NONMEM user's guide. In San Francisco: University of California at San Francisco, 1994
- 24.WFN Bootstrap Available at http://wfn.sourceforge.net/wfnbs.htm
- 25.Das P A, Skoyles J R, Paecock J E.et al Disposition of milrinone in patients after cardiac surgery. Br J Anesth 199472426–429. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg C, Notterman D A. Pharmacokinetics of cardiovascular drugs in children: Inotropes and vasopressors. Clin Pharmacokinet 199427345–367. [DOI] [PubMed] [Google Scholar]
- 27.de Hoog M, Mouton J W, van den Anker J N. Vancomycin: pharmacokinetics and administration regimens in neonates. Clin Pharmacokinet 200443417–440. [DOI] [PubMed] [Google Scholar]
- 28.Williams B S, Ransom J L, Gal P.et al Gentamicin pharmacokinetics in neonates with patent ductus arteriosus. Crit Care Med 199725273–275. [DOI] [PubMed] [Google Scholar]
- 29.Gal P, Gilman J T. Drug disposition in neonates with patent ductus arteriosus. Ann Pharmacother 1993271383–1388. [DOI] [PubMed] [Google Scholar]