Abstract
Background
Metabolic bone disease of prematurity is characterised by impaired postnatal mineralisation of the rapidly growing infant skeleton.
Objective
To longitudinally evaluate postnatal changes in tibial speed of sound (tSOS; which reflects cortical thickness and bone mineral density) and lower limb length (LLL; a measure of tibial growth) in very low birthweight preterm infants receiving contemporary neonatal care.
Methods
tSOS and LLL were measured using a quantitative ultrasound device and an electronic neonatal knemometer, respectively, in the same limb, weekly, for a median period of four weeks (3–16 weeks) in 84 preterm infants (median gestation 26.8 weeks (range 23–35.2 weeks) and median birth weight 869.5 g (range 418–1481 g)).
Results
Initial tSOS and LLL were correlated with gestation (r = 0.42, p<0.001; r = 0.76, p<0.001, respectively) and birth weight (r = 0.23, p = 0.038; r = 0.93, p<0.001, respectively). Postnatally, tSOS decreased (r = −0.15, p = 0.011) whereas LLL increased (r = 0.96, p<0.001) with age. The rate of postnatal change in LLL, but not in tSOS, was positively influenced by intake of calcium (p = 0.03), phosphorus (p = 0.01) and vitamin D (p = 0.03).
Conclusions
The postnatal decline in tSOS, which is probably due to cortical thinning secondary to endocortical bone loss, and increase in LLL provide new insight into the development of long bones in preterm infants.
Keywords: quantitative ultrasound, speed of sound, lower limb length, knemometry, metabolic bone disease of prematurity
Metabolic bone disease of prematurity (MBDP) is characterised by skeletal demineralisation and fractures that can occur during normal handling.1 As a fetus accrues two‐thirds of its total calcium during the third trimester,2,3 babies who are born as early as 23 weeks are deprived of this mineral accumulation. After birth, it is difficult to maintain a comparable intake of minerals, and medications such as corticosteroids and diuretic therapy lead to resorption of the bone matrix.3,4 It is thought that inadequate provision of nutrients necessary for skeletal mineralisation in utero and increased bone resorption lead to the development of MBDP. More recently, the increased rate of bone resorption following postnatal immobilisation and loss of placental supply of oestrogen have been suggested as important factors in the development of MBDP.5,6
Biochemical features of MBDP include raised serum alkaline phosphatase activity and low serum phosphate concentration.7 MBDP may be diagnosed from x rays taken for routine clinical reasons. However, changes of osteopenia, the washed out appearance of bones with thinning of the cortices, only becomes reproducibly apparent when 30–40% of mineral is lost.8,9,10 Densitometric techniques such as dual energy x ray absorptiometry (DXA) can also be used to estimate bone mineral content (measured in g) and areal bone mineral density (measured in g/cm2) in infants.11,12 However, it is not feasible to measure these variables in sick, very low birthweight infants by conventional DXA machines. Peripheral DXA machines have been used in clinically stable infants.13 Bone status in adults has been assessed with quantitative ultrasound since 1984.14 In older postmenopausal women, quantitative ultrasound variables are known to predict fracture risk independently of bone mineral density, suggesting that they are related to some aspect of bone strength.15 We16 and others17,18 have successfully used the Sunlight Omnisense (Sunlight Medical Ltd, Tel Aviv, Israel) to reproducibly measure tibial speed of sound (tSOS), in the axial transmission mode, in sick preterm infants cared for in their incubator, without the need for sedation. We have published reference values for tSOS for singleton newborn infants of 31–42 weeks' gestation, and our preliminary data suggested that tSOS measurement might allow non‐invasive and radiation‐free assessment of MBDP.16
The present study aimed to measure the longitudinal changes in tSOS and lower limb length in very low birthweight preterm infants. We also studied the relationship between initial measurements of, and subsequent changes in, tSOS and lower limb length with gestation, birthweight standard deviation score (BwSDS) and intake of calcium, phosphorus and vitamin D.
Methods
Participants
The Central Manchester local research ethics committee approved the study, and written informed consent was obtained from parents. We approached parents of all preterm infants weighing <1500 g, admitted to the regional neonatal medical unit at St Mary's Hospital, Manchester, UK, between January 2003 and November 2004. Exclusion criteria included congenital malformations, family history of bone disorders and inborn errors of metabolism. Infants of all ethnic groups (self‐defined) and both sexes were included (table 1).
Table 1 Demographics of study cohort.
| Sex, n | |
| Male | 42 |
| Female | 42 |
| Ethnicity, n (%) | |
| Caucasian | 60 (72) |
| Black African | 10 (12) |
| South Asian | 7 (8) |
| Mixed | 5 (6) |
| Other | 2 (2) |
| Median (range) gestational age (weeks) | 26.8 (23–35.2) |
| Median (range) birth weight (g) | 869.5 (418–1481) |
| Small for gestational age (<10th centile), n (%) | 20 (24) |
| Appropriate for gestational age (10th–90th centile), n (%) | 63 (75) |
| Large for gestational age (>90th centile), n (%) | 1 (1) |
The sample size was calculated at 85 babies based on an 80% power to detect changes in tSOS over time (within subjects) of 30 m/s or more, based on a minimum of three weekly measurements. One infant was subsequently found to have congenital renal anomalies and was excluded; therefore data on 84 infants are presented. Outcome measurements were made weekly by a single investigator (JM) for the duration of the infant's stay on the unit.
Demographic and nutritional data
We reviewed the case notes for details of weight and gestation and calculated the BwSDS using the Child Growth Foundation reference growth data for preterm infants.19 Weekly parenteral and enteral nutritional data were collected prospectively during the study period and from these we estimated the daily intakes of calcium, phosphate and vitamin D.
Quantitative ultrasound parameters
We used the Sunlight Omnisense 7000 Premier to measure SOS in metres per second in the axial transmission mode with a small ultrasound probe (1.4×2.7×11 cm), 900–1000 kHz, along the mid tibia. This site was chosen because it is relatively accessible and has a flat surface covered with a small amount of soft tissue. The midpoint between the medial malleolus and the distal patellar apex was determined with a measurement gauge provided by the manufacturer. Either leg was used for measurements, because previous work has shown no difference between right or left tSOS in infants with normal limb movement.17 Also, at least for the initial measurements, this was often predetermined because of the position of intravenous/arterial lines. The details of the measurement procedure in infants has been previously described.16,17,18,20 Reported precision and instrumental accuracy in term and preterm infants range from 0.32% to 0.8%21 and from 0.25% to 0.5%, respectively,17. In vivo precision in our department was 1.26%, based on two consecutive measurements of 28 stable infants (24–33 weeks' gestation), median age 33 weeks (range 26–37 weeks) when the precision measurement was taken.
Measurement of lower limb length
Lower limb length was measured in the same leg in which tSOS was measured, using a neonatal knemometer (Force Institute, Copenhagan, Denmark). This electronic caliper consists of a fixed arm and a moveable arm with a measurement resolution of 0.01 mm.22 We measured the limb length with the infant lying in the supine position, with 90° flexion at the hip and knee. The knee was placed in the fixed arm of the knemometer and the sliding arm was gently brought into apposition against the sole of the foot and pressure applied to trigger the micrometer. Initially, the sliding arm was depressed several times to allow for soft tissue compression. Then five readings were taken and the result was expressed as the mean of these five readings. In vitro precision has been calculated by making 30 measurements on a 100 mm Perspex rod, which gave a mean (SD) length of 99.99 (0.06) mm. The in vivo precision in our department was 0.27%, calculated on two sequential measurements on 12 stable infants (24–29 weeks' gestation), median age 33 weeks (range 28–34 weeks) when the precision measurement was done.
Statistical analysis
We analysed the data using STATA version 9.1. Pearson correlations were used to analyse the relationships between variables characterising individual babies, such as gestation at birth and initial tSOS. We used generalised estimating equations (GEE) to analyse the weekly repeated measures (range 3–16 weeks) of tSOS and lower limb length in relation to gestation, BwSDS and the daily intake of calcium, phosphate and vitamin D. The model estimated the effect of BwSDS, gestation and nutrient intake on the overall tSOS and lower limb length measures and on the rate of postnatal change in these two variables. For each of the outcome variables only the factor of interest and its interaction with postnatal age were included as predictors in the GEE model.
Results
Of 111 parents approached, 106 (95%) consented to their infant participating in the study. Of the 106 infants recruited, 21 were not on the neonatal unit for sufficient time to have three weekly measurements (5 died and 16 were transferred back to their referring hospitals) and, as previously stated, 1 was excluded. Thus, 84 preterm infants underwent three or more weekly measurements of tSOS and lower limb length; 50 (60%) infants were receiving full ventilatory support and 25 (30%) were receiving continuous positive airway pressure when initial tSOS and lower limb length were measured at a median age of 5 days (range 2–9 days). The median duration of stay on the unit was 4 weeks (3–16 weeks). Details of infant demographics are presented in table 1.
Cross‐sectional data
The first measure of tSOS and lower limb length increased as gestation progressed (r = 0.42, p<0.001; r = 0.76, p<0.001, respectively). There was also a significant correlation between birth weight and tSOS (r = 0.23, p = 0.038) and lower limb length (r = 0.93, p<0.001). In contrast, BwSDS showed a significant inverse relationship to initial tSOS (r = −0.38, p = 0.001) and gestation at birth (r = −0.46, p<0.001) but not to initial lower limb length (r = 0.09).
Longitudinal data
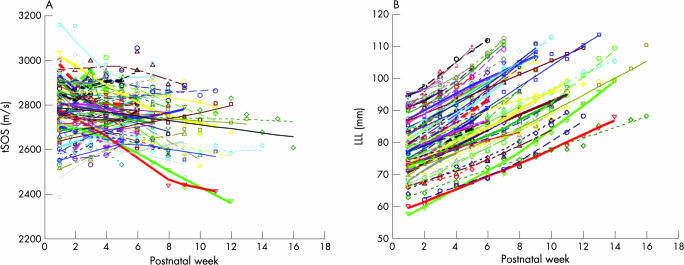
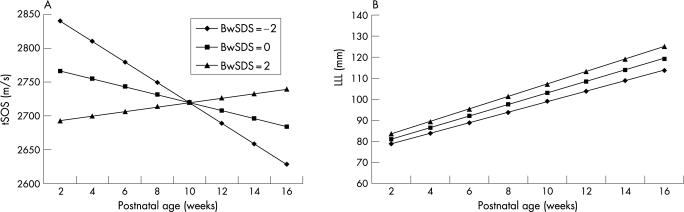
The change in tSOS for individual babies with postnatal age showed a heterogeneous pattern (fig 1A). The overall trend in tSOS showed a decrease (within‐subject correlation, r = −0.15, p = 0.011) with postnatal age (weeks). In contrast, lower limb length for individual babies showed an increase with postnatal age (within‐subject correlation, r = 0.96, p<0.001) (fig 1B). On fitting the GEE model to the tSOS data, BwSDS and its interaction with postnatal age had a significant effect on the tSOS values (p<0.001 and p = 0.012, respectively). The overall effect of BwSDS was negative in the GEE model suggesting that small for gestational age (SGA) babies had a predicted higher initial tSOS value compared with appropriately grown or larger babies. In addition, a positive interaction term indicated that the postnatal decline in tSOS was moderated by the BwSDS. We found no statistically significant effect of BwSDS (p = 0.32) on the overall values of lower limb length or on the postnatal change in lower limb length. Predicted values of tSOS (fig 2A) and lower limb length (fig 2B) were plotted against postnatal age for three BwSDS values, −2, 0 and +2. tSOS decreased in infants with average (0) and lower (−2) BwSDS values, with the decline being more rapid in the latter group. There was a gradual increase in tSOS in infants with higher (+2) BwSDS values (fig 2A). In contrast, lower limb length showed a consistent increase with postnatal age, irrespective of BwSDS (fig 2B).
Figure 1 Longitudinal change in (A) tibial speed of sound (tSOS) and (B) lower limb length (LLL) for individual infants (n = 84) measured at weekly intervals.
Figure 2 Predicted effect of birthweight standard deviation score (BwSDS) of −2, 0 and 2 on postnatal changes in (A) tibial speed of sound (tSOS) using the generalised estimating equations model and (B) lower limb length (LLL) derived from the generalised estimating equations model. ♦, small for gestational age infants (BwSDS = −2); ▪, appropriate for gestational age infants (BwSDS = 0); ▴, larger infants (BwSDS = 2).
There was no interaction between the intake of nutrients and the rate of change in postnatal tSOS, thus changes in tSOS were unaffected by the presence or absence of these nutrients. There was a positive interaction between intake of calcium (p = 0.030), phosphorus (p = 0.012) and vitamin D (p = 0.030) and the rate of change of lower limb length indicating a beneficial effect of these nutrients.
Discussion
What is already known on this topic
Preterm infants are at risk of developing metabolic bone disease of prematurity.
Tibial speed of sound is lower in preterm infants compared with their term counterparts.
What this study adds
In very low birthweight preterm infants, there is postnatal increase in lower limb growth and decline in tibial speed sound.
Nutrient intake seems to relate more to limb growth than tibial speed of sound.
We have shown that tSOS and lower limb length can be measured successfully in critically ill, very low birthweight preterm infants, within a few days of birth and longitudinally thereafter. In the present study, the initial tSOS and lower limb length showed a significant increase with gestational age of infants at birth (p<0.001). The increase in tSOS is in keeping with our16 and other investigators published data.18,21 SOS is known to be influenced by the diaphyseal cortical thickness and bone mineral density,23,24 and therefore the observed increase in tSOS with gestation in part reflects increased tibial cortical thickness in utero. The negative relationship between BwSDS and gestation suggests that the more preterm infants were better nourished in the intrauterine environment than the less preterm infants. As we recruited infants who, at birth, were <1500 g, the infants born at later gestations were probably more growth retarded. Consistent with recent published data20,21 we found that the initial tSOS value was higher in SGA infants. This might simply reflect the increased maturity of these infants—as previously mentioned, the initial tSOS increased with gestation. Lower limb length has previously been shown to be associated with gestation25,26 and reflects the growth of the tibia both in length and volume in utero.
Turning to the longitudinal data, we found that lower limb length increased with postnatal age, indicating a continuing increase in tibial length after birth. In contrast, tSOS showed a heterogeneous pattern of change, which may somewhat account for the small r value. A possible explanation for this overall decline with postnatal age is the thinning of the tibial cortical bone due to endocortical bone resorption27,28 in the postnatal period. The predicted postnatal trend in tSOS showing a more rapid decline in SGA infants compared with appropriately grown infants and an upward trend for larger infants is intriguing. To the best of our knowledge, these findings have not been reported before.
The finding of the overall postnatal decline in tSOS needs to be considered in the context of Frost's mechanostat model29 as applied to intrauterine skeletal development.5,30 Frost stated that bones adapt their strength to the mechanical forces that they are subjected to, which primarily arise from muscles. Such forces result in changes in the dimensions of the bone or “strains”, which are sensed by a regulatory feedback system in the bone called the “mechanostat”. Bones respond to increased loading by adding net bone to the site that is mechanically challenged, thereby keeping bone strains within physiological limits. Fetal activity in the first trimester of pregnancy increases, and during the second trimester the movements become more organised and complex31 against an increasingly limited intrauterine environment. The loading of the fetal skeleton arises from movement against the resistance of the uterine muscle,30 such as the fetus kicking against the uterine wall. This skeletal loading leads to a progressive increase in the thickness of the diaphyseal cortex of the long bones—a plausible explanation for the observed increase in tSOS. Decreased movement due to neuromuscular disorders with intrauterine onset, results in low bone mass at birth.32 Furthermore, the fetus is exposed to high placental oestrogen levels during intrauterine life.33 Oestrogen lowers the mechanostat strain set point on endosteal bone surfaces, and this causes cortical thickening through increased endocortical bone accrual.34,35 Following birth, there is unloading of the skeleton because of cessation of resistance movements, which along with loss of the placental oestrogen leads to endocortical bone resorption and hence thinning of the cortex. Therefore, our observation of postnatal decline in tSOS is probably because of cortical thinning following endocortical bone resorption, which has previously been reported by Beyers and colleagues.28 The importance of physical activity for bone mass density and tSOS of very low birthweight infants comes from trials of postnatal physical activity interventions. Moyer‐Mileur and colleagues showed that a programme of passive postnatal physical activity of upper and lower extremities resulted in increased area bone mineral density measured by DXA in very low birthweight infants compared with a non‐exercising control group.13 Litmanovitz and colleagues13 used a protocol similar to that of Moyer‐Mileur and colleagues and found that physical activity prevented the postnatal decline of tSOS in infants in the intervention group.36
We found that the bone nutrients had a positive effect on postnatal changes in lower limb length but not tSOS. A meta‐analysis of calcium and activity trials in adults found that a response to physical activity was achieved only when calcium intake was sufficient and likewise a response to increased calcium was only observed when physical activity was increased.37 In other words, calcium or other bone nutrients will probably not cause skeletal mineralisation in the absence of physical activity; this concept is supported by two randomised controlled trials of calcium supplementation in older children.38,39 It is therefore plausible that under conditions of relative immobilisation after birth and associated loss of excess bone accrued in the intrauterine environment,5,6 bone nutrients are used for bone growth rather than for mineralisation of the skeletal matrix.13 Or the bone nutrients we studied may well be surrogate markers of better intake of other nutrients, such as daily total calorie, carbohydrate, fat and protein intake, which we did not study.
In summary, the postnatal increase in lower limb length and decline in tSOS provides new insight into the development of long bones and possibly MBDP in very low birthweight preterm infants receiving contemporary neonatal care.
Acknowledgements
We thank the infants and their families who took part in the study. We are grateful to Sunlight Medical Ltd, Israel, for the loan of the Sunlight Omnisense Premier 7000P quantitative ultrasound device used in this study.
Abbreviations
BwSDS - birthweight standard deviation score
DXA - dual energy x ray absorptiometry
GEE - generalised estimating equations
MBDP - metabolic bone disease of prematurity
SGA - small for gestational age
SOS - speed of sound
tSOS - tibial speed of sound
Footnotes
The study was funded by St Mary's Regional Neonatal Medical Unit Research Endowment Fund.
Competing interests: None.
References
- 1.Koo W, Steichen J. Osteopenia and rickets of prematurity. In: Polin R, Fox W, eds. Fetal and neonatal physiology. Philadelphia: WB Saunders, 19982335–2349.
- 2.Care A. Unique aspects of calcium and vitamin D metabolism in the placenta and fetus. In: Gluckman P, Heymann M, eds. Bone and cartilage. Pediatrics and perinatology: the scientific basis. London: Arnold, 1996540–542.
- 3.Koo W, Tsang R. Calcium, magnesium, phosphorus and vitamin D. In: Tsang R, Lucas A, Uauy R, Zlotkin S, eds. Nutritional needs of the preterm infant. Baltimore: Williams & Wilkins, 1993135–155.
- 4.Weiler H, Paes B, Shah Jea Longitudinal assessment of growth and bone mineral accretion in prematurely born infants treated for chronic lung disease with dexamethasone. Early Hum Dev 19977271–286. [DOI] [PubMed] [Google Scholar]
- 5.Miller M E. The bone disease of preterm birth: a biomechanical perspective. Pediatr Res 20035310–15. [DOI] [PubMed] [Google Scholar]
- 6.Rauch F, Schöenau E. Skeletal development in premature infants: a review of bone physiology beyond nutritional aspects. Arch Dis Child Fetal Neonatal Ed 200286F82–F85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan S. Nutritional aspects of metabolic bone disease in the newborn. Arch Dis Child 199674F145–F148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dominguez R. Musculoskeletal abnormalities of the high risk newborn. In: Dominguez R, ed. Diagnostic imaging of the premature infant. New York: Churchill Livingstone, 1992243–263.
- 9.Koo W, Gupta J, Nayanar V.et al Skeletal changes in preterm infants. Arch Dis Child 198257447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazess R, Peppler W, Chesney R.et al Does bone measurement of the radius indicate skeletal status? J Nucl Med 198425281–288. [PubMed] [Google Scholar]
- 11.Avila‐Diaz M, Flores‐Huerta S, Martinez‐Muniz I.et al Increments in whole body mineral content associated with weight and length in pre‐term and full‐term infants during the first 6 months of life. Arch Med Res 200132288–292. [DOI] [PubMed] [Google Scholar]
- 12.Koo W, Hockman E. Physiologic predictors of lumbar spine bone mass in neonates. Pediatr Res 200048485–489. [DOI] [PubMed] [Google Scholar]
- 13.Moyer‐Mileur L J, Brunstetter V, McNaught T P.et al Daily physical activity program increases bone mineralization and growth in preterm very low birth weight infants. Pediatrics 20001061088–1092. [DOI] [PubMed] [Google Scholar]
- 14.Langton C, Palmer S, Porter R. The measurement of broadband ultrasonic attenuation in cancellous bone. Eng Med 19841389–91. [DOI] [PubMed] [Google Scholar]
- 15.Hans D, Dargent‐Molina P, Schott A. Ultrasonic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet 1996348511–514. [DOI] [PubMed] [Google Scholar]
- 16.Yiallourides M, Savoia M, May J.et al Tibial speed of sound in term and preterm infants. Biol Neonate 200485225–228. [DOI] [PubMed] [Google Scholar]
- 17.Eliakim A, Nemet D, Freidland O.et al Spontaneous activity in premature infants affects bone strength. J Perinatol 200222650–652. [DOI] [PubMed] [Google Scholar]
- 18.Nemet D, Dolfin T, Wolach B.et al Quantitative ultrasound measurements of bone speed of sound in premature infants. Eur J Pediatr 2001160736–740. [DOI] [PubMed] [Google Scholar]
- 19.Cole T J, Freeman J V, Preece M A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 199817407–429. [PubMed] [Google Scholar]
- 20.McDevitt H, Tomlinson C, White M.et al Quantitative ultrasound assessment of bone in preterm and term neonates. Arch Dis Child Fetal Neonatal Ed 200590F341–F342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Littner Y, Mandel D, Mimouni F.et al Bone ultrasound velocity of infants born small for gestational age. J Pediatr Endocrinol Metab 200518793–797. [DOI] [PubMed] [Google Scholar]
- 22.Michaelsen K, Skov L, Badsberg J.et al Short‐term measurement of linear growth in preterm infants: validation of a hand‐held knemometer. Pediatr Res 199130464–468. [DOI] [PubMed] [Google Scholar]
- 23.Njeh C F, Hans D, Wu C.et al An in vitro investigation of the dependence on sample thickness of the speed of sound along the specimen. Med Eng Phys 199921651–659. [DOI] [PubMed] [Google Scholar]
- 24.Prevrhal S, Fuerst T, Fan B.et al Quantitative ultrasound of the tibia depends on both cortical density and thickness. Osteoporos Int 20011228–34. [DOI] [PubMed] [Google Scholar]
- 25.Chitty L, Altman D. Charts of fetal size: limb bones. BJOG 2002109919–929. [DOI] [PubMed] [Google Scholar]
- 26.Gibson A, Pearse R, Wales J. Knemometry and the assessment of growth in premature babies. Arch Dis Child 199369498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palacios J, Rodriguez S, Rodriguez J I. Intra‐uterine long bone growth in small‐for‐gestational‐age infants. Eur J Pediatr 1992151304–307. [DOI] [PubMed] [Google Scholar]
- 28.Beyers N, Alheit B, Taljaard J F.et al High turnover osteopenia in preterm babies. Bone 1994155–13. [DOI] [PubMed] [Google Scholar]
- 29.Frost H M. Perspectives: a proposed general model of the “mechanostat” (suggestions from a new skeletal‐biologic paradigm). Anat Rec 1996244139–147. [DOI] [PubMed] [Google Scholar]
- 30.Rauch F, Schöenau E. The developing bone: slave or master of its cells and molecules? Pediatr Res 200150309–314. [DOI] [PubMed] [Google Scholar]
- 31.Kurjak A, Carrera J, Medic M.et al The antenatal development of fetal behavioral patterns assessed by four‐dimensional sonography. J Matern Fetal Neonatal Med 200517401–416. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez J, Garcia‐Alix A, Palacios J.et al Effects of immobilization on fetal bone development: a morphometric study in newborns with congenital neuromuscular diseases with intrauterine onset. Calcif Tiss Int 198843335–339. [DOI] [PubMed] [Google Scholar]
- 33.Kaijser M, Granath F, Jacobsen G.et al Maternal pregnancy estriol levels in relation to anamnestic and fetal anthropometric data. Epidemiology 200011315–319. [DOI] [PubMed] [Google Scholar]
- 34.Lanyon L. Using functional loading to influence bone mass and architecture: objectives, mechanism, and relationship with estrogen of the mechanically adaptive process in bone. Bone 199618S37–S43. [DOI] [PubMed] [Google Scholar]
- 35.Rodan G. Mechanical loading, estrogen deficiency, and the coupling of bone formation to bone resorption. J Bone Miner Res 19916527–530. [DOI] [PubMed] [Google Scholar]
- 36.Litmanovitz I, Dolfin T, Friedland O.et al Early physical activity intervention prevents decrease of bone strength in very low birth weight infants. Pediatrics 2003112(1 Pt 1)15–19. [DOI] [PubMed] [Google Scholar]
- 37.Specker B L. Evidence for an interaction between calcium intake and physical activity on changes in bone mineral density. J Bone Miner Res 1996111539–1544. [DOI] [PubMed] [Google Scholar]
- 38.Iuliano‐Burns S, Saxon L, Naughton G.et al Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. J Bone Miner Res 200318156–162. [DOI] [PubMed] [Google Scholar]
- 39.Specker B, Binkley T. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3‐ to 5‐year‐old children. J Bone Miner Res 200318885–892. [DOI] [PubMed] [Google Scholar]