Abstract
Objective
To evaluate whether lying in a nest affects the posture and spontaneous movements of healthy preterm infants.
Method
10 healthy preterm infants underwent serial video recording in the supine position, when lying in a nest and outside it, at three ages: 30–33 weeks postmenstrual age (PMA) (early preterm), 34–36 weeks PMA (late preterm) and 37–40 weeks PMA (term). The nest was shell‐shaped, made by putting two rolled blankets in a form of an oval. Posture was assessed both before and after general movements by scoring the predominant postural pattern. Movements towards and across the midline, elegant wrist movements, abrupt hand and/or limb movements, rolling to side, and frozen postures of the arms and legs were assessed during four general movements. All data relating to motor and postural items were normalised into frequencies of events per minute because the general movements varied in duration.
Results
When lying in the nest, the infants more often displayed a flexed posture with shoulder adduction and elbow, and hip and knee flexion, and the head was frequently in the midline. The nest was also associated with an increase in elegant wrist movements and movements towards and across the midline and a reduction in abrupt movements and frozen postures of the limbs. The nest did not affect the occurrence of asymmetrical tonic neck posture.
Conclusions
A nest promotes a flexed posture of the limbs with adduction of shoulders, facilitates elegant wrist movements and movements towards and across the midline and reduces abrupt movements and frozen postures of the arms and legs.
Keywords: general movements, nest, posture, preterm infant, spontaneous movement
Neonatal posture requires a number of active postural control mechanisms—that is, neuromotor functions—which allow a living system to control its body posture at rest, during displacement and during active movements.1 Postural control is intimately linked to motor control: dynamic motor actions cannot be performed without first stabilising body posture.2 This is true for voluntary as well for involuntary movements.3
Systematic observations of fetal posture show that, although the fetus does not have a preferred posture for most of the time, it has a certain repertoire of repeated active postures. The observed postures cannot be considered as random configurations of head and limb position: the fetus and the young infant have an active, but variable, posture that is relatively unrelated to the orientation of the force of gravity.1,4 As pregnancy approaches its end, body size of the fetus increases and room for movement inside the womb decreases; the head of the fetus is predominantly flexed or semi‐flexed, the shoulders and hips are flexed and adducted, and the limbs are close to the trunk.
The neonatal intensive care unit (NICU) exposes the preterm infant to a non‐optimal physiological environment and to invasive procedures and handling. These may induce pain and stress, along with the frequent manipulations by medical and nursing staff that disrupt rest activity cycles and sleep, which may lead to chronic and prolonged stress in the preterm infant.5 Acute stress may induce abrupt movements and startles. In addition, motor behaviour of the preterm infant is affected by the force of gravity, the limited ability to control the position of the head and—if the infant is not placed in a nest‐like environment—a lack of containment and boundaries.6 As a result, startles, rolling to the side, abrupt movements of the limbs, frozen postures of arms and legs, either spontaneous or induced by handling, are commonly observed behaviours in preterm infants, in particular when the clinical condition is not yet stable.7 In turn, the abrupt movements and frozen postures of the limbs may add stress to stress.
The nest, which has been adopted by most European NICUs, aims to stabilise body posture, positioning the head towards the midline, and facilitating a flexed or semi‐flexed posture of the head. It also seems to prevent abrupt and distressing movements.8,9,10,11 To our knowledge, no studies have evaluated whether the use of a nest in preterm infants results in the above mentioned effects.
The present explorative study aimed to evaluate the effect of a nest on motor behaviour of preterm infants. We first hypothesised that—notwithstanding the endogenous origin of the basic pattern of general movement12—some aspects of spontaneous motor behaviour of preterm infants may improve when placed in a nest. We expected that the nest, by means of its passive constraints, would favour a midline position of the head and flexion/adduction postures of the limbs. We also expected that placement in a nest would result in fewer large and abrupt arm movements. These may trigger Moro‐like movements and startles, as at this early age, the proprioceptors in neck, and the vestibulum, are very sensitive to stimulation.6,13 The Moro‐like movements and startles in turn may trigger abrupt Moro‐like movements and startles. The abrupt movements may also trigger frozen postures, as motoneurons at this early age are easily triggered into the plateau phase of bistability. This means that a single trigger, for instance, stretch evoked by an abrupt movement, may elicit motoneuronal firing for about 30 s.14,15,16 Thus, our second hypothesis was that putting an infant in a nest would increase the chances of the head being positioned in the midline, flexion/adduction postures of the limbs, limb movements towards and across the midline and elegant wrist movements during general movements, and decrease abrupt movements and frozen postures of the limbs.
To test these hypotheses we evaluated posture and motility in 10 preterm infants at three ages (early preterm, late preterm and term) when placed in a nest and outside it.
Subjects and methods
Subjects
Ten preterm infants (five boys and five girls; gestational age at birth 25–31 weeks (median 30 weeks); birth weight 685–1650 g (median 1292 g)) participated in the study. The parents of the infants gave informed consent and the ethics committee of the University Hospital of Modena approved the study. The infants had been admitted to the NICU at the University Hospital of Modena between 1999 and 2001. We had strict inclusion criteria: prematurity; birth weight >25th and <90th centile according to the American National Center for Health Statistics growth charts17; uneventful pregnancy and delivery according to a detailed list of optimal prenatal and paranatal conditions18; and absence of obvious neurological syndromes, severe sepsis, chromosomal defects or other recognisable malformations of the brain or other organs, or metabolic disorders. Between 1999 and 2001, 17 infants met these inclusion criteria. Parents of two infants declined consent. Five infants were excluded from the study because at the age of 2 years they showed an atypical developmental outcome as determined by Griffith's scales of development19 and Touwen's neurological examination.20
All the 10 infants who participated in the study needed respiratory support. One of them needed intermittent positive pressure ventilation (IPPV) for six days, six infants required IPPV–intermittent mandatory ventilation for one to three days and one needed high‐flow oxygen for five days. All infants were given continuous positive airway pressure (CPAP) for a few days. Ultrasound scans, serially performed from birth until term age, showed normal findings in six infants and transient increased echogenicity lasting less than four days in three infants; one infant had GMH‐IVH (germinal matrix‐intraventricular haemorrhage) grade 1.21
Method
All infants underwent three video recordings, each lasting an hour. The recordings were done at 30–33 weeks postmenstrual age (PMA) (early preterm age), 34–36 weeks PMA (late preterm age) and 37–40 weeks PMA (term age). The first video recording was done when the infant's clinical condition was reasonably stable and respiratory support was no longer necessary. The video recordings were done in a standardised order—that is, half an hour in the nest and half an hour outside the nest, in an incubator or in a cot, in supine position, naked or in a nappy, completely free to move.22 One infant missed one early preterm age video recording.
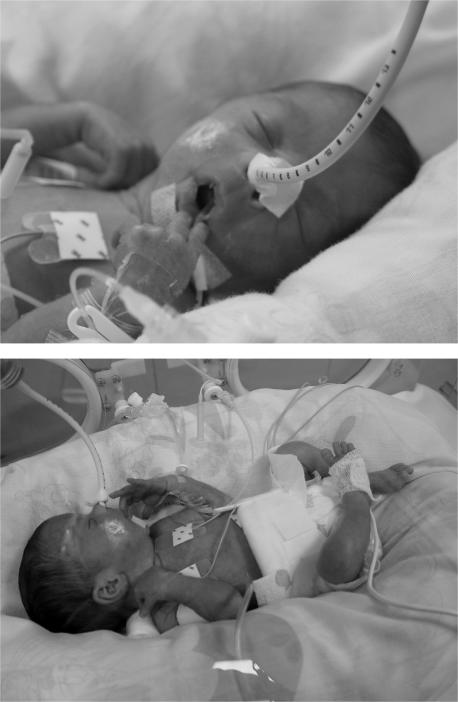
The nest is oval‐shaped and made of cloth (we use two rolled‐up blankets), in which the infant lies when inside the incubator (fig 1). The infant is placed in the nest to facilitate a semiflexed and adducted position of the shoulders and hips and to reduce environmental stimuli. The postural support provided by the nest is in accordance with the strategy of individualised developmental care in the NICU.23 We placed a small roll under the infant's neck to help the proper alignment of the neck and trunk. This was done mainly to provide an optimal position for breathing.
Figure 1 Infant in the nest: lying in the nest facilitates arm and hand movement during which various parts of the body are touched, and promotes flexion of arms and legs, and adduction of shoulders and hips. Parental/guardian informed consent was obtained for publication of this figure.
Our analysis of the videos focused on motor behaviour during activity periods—that is periods with repeated general movements and other non‐sporadic movement patterns.16 General movements are gross movements involving the whole body, lasting from a few seconds to several minutes.22 Four general movements were randomly selected from each video. The movement analysis focused on motor behaviour immediately before, during and immediately after the four general movements. We took care that the posture after one general movement did not overlap with the posture prior to another general movement.
We analysed posture at rest immediately before a general movement started and immediately after this general movement ended. The postural items analysed are given in appendix A and were designed for the present study. A posture was considered to be a posture (and no longer a movement) when it lasted for at least 10 s. When evaluating the position of the head, we used a simple dichotomy: midline position versus rotated to either the right or the left side (20–70°). On the basis of the four postures before and after a general movement, the predominant postural pattern before and after general movements was determined. If two patterns were observed equally often, the more flexed, adducted or midline pattern was scored—that is, for the elbows, hips and knees the flexed posture, for the shoulders the adducted posture, for the head the midline position, and absence rather than presence of the asymmetrical tonic neck posture. We also evaluated the four general movements. In the analysis of spontaneous motility we focused on the items listed in appendix B.24
The video recordings were assessed by three observers. One observer (MC) scored the postural items in eight infants and another observer (CG) scored the items relating to spontaneous movements in the same eight infants. A third observer (QB) evaluated posture and motility data of the remaining two infants. As three observers carried out the scoring, interobserver agreement was calculated. For this, the three observers scored posture and spontaneous movements in another five preterm infants. The interobserver agreement for all items of appendix A and most items of appendix B was between 81% and 100%. The three items for which agreement was somewhat less were items 4, 5, 9 of the section “movements towards and across midline”, varying between 61% and 80%.
We carried out the statistical analyses using SPSS (version 11.0). As the general movements varied in duration, all data relating to spontaneous movements were normalised into frequencies of events per minute per general movement. Next, a median value was calculated per infant, age and the condition. The effect of the condition—that is, in the nest or outside the nest—was evaluated using the non‐parametric Wilcoxon's signed rank test as the data were not normally distributed. A p value <0.05 was considered to be statistically significant (two‐tailed test).
What is already known on this topic
Being placed in a nest reduces the distress in the preterm infant during screening for retinopathy of prematurity.
The nest‐like support provided by pillows in young infants after term age does not affect the occurrence of abnormal general movements.
What this study adds
Being placed in a nest reduces abrupt movements, facilitates elegant wrist movements and movements towards and across the midline.
Nest‐like support promotes a flexed and adducted posture of the limbs.
Results
Lying in a nest had a clear effect on the infant's postural behaviour before and after a general movement (table 1). Before a general movement, an infant's shoulders were more adducted and limbs somewhat more flexed in the nest than outside the nest. After a general movement, lying in a nest was particularly associated with an increase in limb flexion, and at term age, also with an increase in shoulder adduction and head in midline position. Interestingly, the asymmetrical tonic neck posture was never a predominant posture in these relatively healthy preterm infants.
Table 1 Differences in posture during rest between general movements in infants lying in and outside a nest.
| Early preterm age (30–33 weeks) | Late preterm age (34–36 weeks) | Term age (37–40 weeks) |
|---|---|---|
| Posture before general movements | ||
| NEST more adduction | NEST more adduction | NEST more adduction |
| L shoulder* | L shoulder* | L shoulder** |
| R shoulder* | R shoulder* | R shoulder** |
| NEST more flexion | NEST more flexion | NEST more flexion |
| R elbow* | R hip* | L hip* |
| L hip* | R knee* | R hip* |
| R hip* | L knee* | |
| L knee* | R knee* | |
| NEST more often | ||
| Head in the midline* | ||
| Posture after general movements | ||
| NEST more adduction | NEST more adduction | NEST more adduction |
| L shoulder* | ||
| R shoulder** | ||
| NEST more flexion | NEST more flexion | NEST more flexion |
| L elbow* | L elbow* | L hip* |
| L hip* | L hip** | R hip* |
| R hip* | R hip** | L knee* |
| L knee* | L knee** | |
| R knee* | R knee* | |
| NEST more often | ||
| Head in the midline* | ||
L, left, R, right.
*p<0.05, **p<0.01: differences between lying in a nest and without a nest (Wilcoxon's signed rank test).
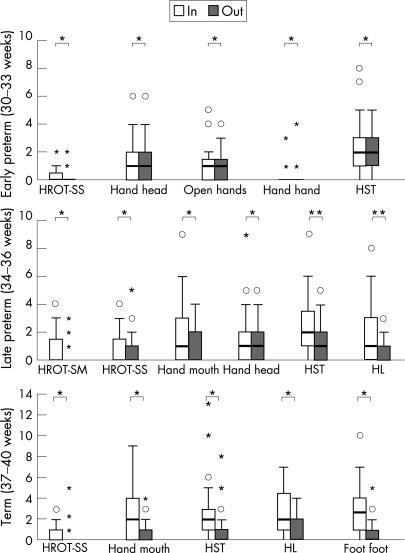
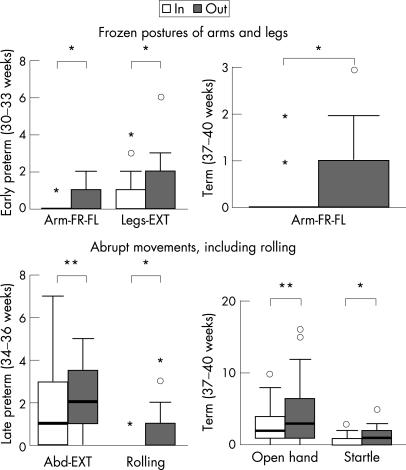
Lying in a nest also had a substantial effect on spontaneous motor behaviour. Throughout the early and late preterm and term periods lying in a nest increased the number of movements towards or crossing the midline (fig 2). In the same period the nest also increased the occurrence of elegant wrist movements (p<0.05; item considered separately, data not shown). The nest was also associated with a reduction in frozen postures and, from late preterm term onwards, abrupt movements (fig 3).
Figure 2 Differences between the various spontaneous movements when in and outside the nest. The short horizontal lines denote median values, the boxes denote the interquartile ranges and the vertical lines denote the ranges. Outliers are denoted by stars and dots. Foot foot, foot–foot contact; Hand hand, hand–hand contact; Hand head, hand–head contact; Hand mouth, hand–mouth contact; HL, hand–leg contact; HROT‐SM, head rotation from side to midline and back; HROT‐SS, head rotation from side to side; HST, hand touching contralateral shoulder and trunk; Open hands, gently striking head with open hands. *p<0.05, **p<0.01 (Wilcoxon's signed rank test). Note that the differences in the early preterm age cannot be seen clearly, but the statistical analysis shows a significant difference.
Figure 3 Upper panel: effect of lying in a nest on occurrence of frozen postures of arms and legs. ARM‐FR‐FL, arms in frozen flexion and fisting; LEGS‐EXT, legs in frozen extension. Lower panel: effect of lying in a nest on the occurrence of abrupt movements. ABD‐EXT, abrupt abduction–extension movements of the arms; Open hand, abrupt opening of hands and fingers; Startle, abrupt abduction–extension of the four limbs; Rolling, abrupt rolling to side. *p<0.05, **p<0.01 (Wilcoxon's signed rank test).
Discussion
The present study has shown that placing an infant in a nest facilitates a flexed and adducted posture, promotes elegant wrist movements and movements towards or crossing the midline, and it reduces abrupt movements and frozen postures. The strength of the present study was the standardised and detailed assessment of the effect of lying in a nest in relatively healthy preterm infants. Each infant acted as its own control.
There were some weaknesses of this explorative study. First, the infants, for the rest of their stay in hospital, were placed in the nest. This meant that when they were taken out of the nest to be studied, they experienced an abrupt interruption of the environment and posture to which they had got used to. However, we could not overcome this limitation because of the strong opinion of the NICU nursing staff regarding the beneficial effect of the nest. A firm belief in a method, despite the absence of research evidence, is a well‐known obstacle in the evaluation of new forms of developmental care.25 Second, the observers, unavoidably, were not blinded to the nesting condition and might have had a bias favouring the nest, and a team of observers evaluated different aspects of motor behaviour. Third, the conditions were not applied in a random order, and there was no “washout” period. However, the data did not reveal changes in motor and postural behaviour over time within a condition—that is, lying in the nest or outside it.
Until now few studies have addressed the effect of nest‐like support in preterm infants in the NICU.26,27,28,29,30 There is no evidence that lying in a nest shortens the duration of hospital stay and increases weight before discharge.28 Slevin et al29 showed that the distress caused by retinopathy of prematurity screening in a cohort of preterm infants was considerably less in infants lying in a nest that in the non‐nested group, for both movement activity and crying. De Graaf‐Peters et al30 studied the immediate effects of the nest‐like support provided by pillows in infants with and without minor neurological dysfunction at 1–5 months post‐term age. They found that support using a pillow did not affect the quality of general movements—that is, the occurrence of abnormal general movements. It did, however, promote the variation in specific movements—movements that can be considered as precursors of goal‐directed movements. The effect was particularly noticeable in the infants with minor neurological dysfunction.
The nest affected posture and motility at all the three ages at which this was studied. The overall effect was similar for the three periods, but some age‐related differences were observed:
during the early preterm age, the nest did not affect head position, but from late preterm onwards it did;
at term age the nest had the strongest effect on shoulder adduction;
at early preterm age, lying in a nest was not associated with a reduction of abrupt movements.
These age‐related effects might be related to the changes that occur in the nervous system around 36–38 weeks PMA.31 After these changes, general movements become slower and have a writhing aspect, which probably counteracts the production of abrupt movements.31
Our study indicates that lying in a nest reduces the abrupt movements that increase the stress in the infant, and it facilitates age‐specific postures and movements. The age‐specific flexed and adducted posture of the limbs promotes movements towards and across the midline. This, in turn, enhances contact of the limbs with other parts of the body, just as in fetal life. Hand–hand contact, hand–head contact, hands touching the contralateral shoulder and trunk, hand–leg contact and foot–foot contact offer rich and continual exteroceptive and proprioceptive feedback to the central nervous system. This type of self‐generated feedback—that is, continuous motor and sensory exploration—is currently considered to be a major driving force of motor development.32,33
The intrauterine environment and postural stability offered by the womb represent the ideal setting for the preterm infant.34 The results of our study underscore the importance of individualised developmental care5,23 to promote age‐adequate postures, thereby reducing the distressing conditions of extrauterine life in preterm infants.
Acknowledgements
We are grateful to Quinta Bergman and Manuela Corradini for their help in data collection.
Abbreviations
NICU - neonatal intensive care unit
PMA - postmenstrual age
Appendix A
Items in the assessment of posture
| Item | Description | |
|---|---|---|
| (1) Left shoulder | 0 = adduction | 0–20° |
| 1 = neutral | 21–90° | |
| 2 = abduction | >90° | |
| (2) Left elbow | 0 = flexion | 0–30° |
| 1 = semiflexion | 31–150° | |
| 2 = extension | >150° | |
| (3) Right shoulder | 0 = adduction | 0–20° |
| 1 = neutral | 21–90° | |
| 2 = abduction | >90° | |
| (4) Right elbow | 0 = flexion | 0–30° |
| 1 = semiflexion | 31–150° | |
| 2 = extension | >150° | |
| (5) Left hip | 0 = flexion | 0–80° |
| 1 = semiflexion | 81–150° | |
| 2 = extension | >150° | |
| (6) Left knee | 0 = flexion | 0–60° |
| 1 = semiflexion | 61–150° | |
| 2 = extension | >150° | |
| (7) Right hip | 0 = flexion | 0–80° |
| 1 = semiflexion | 81–150° | |
| 2 = extension | >150° | |
| (8) Right knee | 0 = flexion | 0–60° |
| 1 = semiflexion | 61–150° | |
| 2 = extension | >150° | |
| (9) Asymmetrical tonic neck posture | = absent | |
| = present | ||
| (10) Head position | 0 = head in the midline | Rotated <20° |
| 1 = head to side | Rotated 20–70° |
Appendix B
Items in the assessment of details of general movements and spontaneous movements
| Movements towards and across the midline | Elegant wrist movements | Abrupt hand and/or limb movements and rolling to side | Frozen postures of arms and legs |
|---|---|---|---|
| (1) Head rotation from side to midline and back | (1) Wrist move‐ ments with superimposed rotations | (1) Abrupt opening of hands and fingers | (1) Arms in frozen extension |
| (2) Head rotation from side to side | (2) Abrupt abduction–extension of the arms | (2) Arms in frozen flexion and fisting | |
| (3) Hand–mouth contact | (3) Abrupt abduction–extension of the four limbs | (3) Legs in frozen extension | |
| (4) Hand–head contact | (4) Abrupt rolling to side | ||
| (5) Gently striking head with open hands | |||
| (6) Hand–hand contact | |||
| (7) Hands touching contralateral shoulder and trunk | |||
| (8) Hand–leg contact | |||
| (9) Foot–foot contact |
Footnotes
Parental/guardian informed consent was obtained for publication of fig 1.
Competing interests: None.
References
- 1.Casaer P.Postural behaviour in newborn infants. Clinics in Developmental Medicine, no. 72. London: Heinemann Medical, 1979
- 2.Kernell D. The final common pathway in postural control—developmental perspective. Neurosci Biobehav Rev 199822479–484. [DOI] [PubMed] [Google Scholar]
- 3.De Groot L. Posture and motility in preterm infants. Dev Med Child Neurol 20004265–68. [DOI] [PubMed] [Google Scholar]
- 4.Ververs I A, Van Gelder‐Hasker M R, De Vries J I.et al Prenatal development of arm posture. Early Hum Dev 19985161–70. [DOI] [PubMed] [Google Scholar]
- 5.Als H, Lawhon G, Duffy H F.et al Individualized developmental care for the very low‐birth‐weight preterm infant: medical and neurofunctional effects. JAMA 1994272853–858. [PubMed] [Google Scholar]
- 6.Dubowitz L M S, Dubowitz V, Mercuri E.The neurological assessment of the preterm and full‐term newborn infant, 2nd edn. Clinics in Developmental Medicine, no. 148. Cambridge: MacKeith Press, 1999
- 7.Ferrari F, Roversi M F. Fattori di rischio pre, peri e neonatali e la “care” del neonato pretermine in Terapia Intensiva Neonatale. Riabilitazione Oggi 199887–14. [Google Scholar]
- 8.Cuttini M, Maraschini A, Greisen G.et al Developmental care for preterm neonates: a survey of practices in European neonatal units. Book of Abstracts, European Society Paediatric Research, October 2006, Barcelona
- 9.Fleisher B E, VandenBerg K, Constantinou J.et al Individualized developmental care for very low‐birth‐weight premature infants. Clin Pediatr 199534523–529. [DOI] [PubMed] [Google Scholar]
- 10.Westrup B, Kleberg A, von Eichwald K.et al A randomized controlled trial to evaluate the effects of the newborn individualized developmental care and assessment program in a Swedish setting. Pediatrics 200010566–72. [DOI] [PubMed] [Google Scholar]
- 11.Young J. Positioning premature babies. Nursing preterm babies in intensive care. Which position is the best? J Neonatal Nurs 1994127–31. [Google Scholar]
- 12.Prechtl H F R. Qualitative changes of spontaneous movements in fetus and preterm infant are a marker of neurological dysfunction. Early Hum Dev 199023151–158. [DOI] [PubMed] [Google Scholar]
- 13.Prechtl H F R.The neurological examination of the full‐term newborn infant, 2nd edn. Clinics in Developmental Medicine, no. 63. London: Heinemann Medical, 1977
- 14.Ferrari F, Cioni G, Prechtl H F R. Qualitative changes of general movements in preterm infants with brain lesions. Early Hum Dev 199023193–231. [DOI] [PubMed] [Google Scholar]
- 15.Eken T, Hultborn H, Kiehn O. Possible functions of transmitter‐controlled plateau potentials in alpha motoneurones. Prog Brain Res 198980257–267. [DOI] [PubMed] [Google Scholar]
- 16.Hadders‐Algra M, Van Eykern L A, Klip‐van den Nieuwendijk A W J.et al Developmental course of general movements in early infancy. II. EMG correlates. Early Hum Dev 199228231–252. [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics NCHS growth charts. Mon Vital Stat Rep 197625(Suppl3)(HRA)76–112. [Google Scholar]
- 18.Prechtl H F R. Neurological findings after pre‐ and paranatal complications. In: Jonxis JHP, Visser HKA, Troelstra JA, eds. Aspects of Praematurity and Dysmaturity. Leiden: Stenfert Kroese, 1968303–321.
- 19.Griffith R.The abilities of young children. A comprehensive system of mental measurement for the first 8 years of life. London: Child Development Research Centre, Young and Son, 1970
- 20.Touwen B C L.Neurological development in infancy. Clinics in Developmental Medicine, no. 58. Philadelphia: Lippincott, 1976
- 21.Trounce J Q, Rutter N, Levene M I. A prospective study of the incidence of periventricular leukomalacia and ventricular haemorrhage in the preterm neonate. Arch Dis Child 1986611196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Einspieler C, Prechtl H F R, Ferrari F.et al The qualitative assessment of general movements in preterm, term and young infants—review of the methodology. Early Hum Dev 19975047–60. [DOI] [PubMed] [Google Scholar]
- 23.Bauer K. Interventions involving positioning and handling in the neonatal intensive care unit: early developmental care and skin‐to‐skin holding. Research on Early Developmental Care for preterm neonates. Paris: John Libbey Eurotext, 200559–65.
- 24.Ferrari F, Bertoncelli N, Roversi M F.et al Motor and postural behavior in low‐risk preterm infants from 30–33 to 46–54 weeks postmenstrual age: an observational study. Prenat Neonat Med 20016166–183. [Google Scholar]
- 25.Westrup B, Bohm B, Lagercrantz H.et al Preschool outcome in children born very preterm and cared according to NIDCAP. Acta Paediatr 200493498–507. [DOI] [PubMed] [Google Scholar]
- 26.Stevens B, Petryshen P, Hawkins J.et al Developmental versus conventional care: a comparison of clinical outcomes for very low birth weight infants. Can J Nurs Res 19962897–113. [PubMed] [Google Scholar]
- 27.Brown L D, Heermann J A. The effect of developmental care on preterm infant outcome. Appl Nurs Res 199710190–197. [DOI] [PubMed] [Google Scholar]
- 28.Symington A, Pinelli J. Developmental care for promoting development and preventing morbidity in preterm infants. Cochrane Database Syst Rev 20062CD001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slevin M, Murphy J F A, Daly L.et al Retinopathy of prematurity screening, stress related responses, the role of nesting. Br J Ophthalmol 199781762–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Graaf‐Peters V B, De Groot‐Hornstra A H, Dirks T.et al Specific postural support promotes variation in motor behaviour of infants with minor neurological dysfunction. Dev Med Child Neurol 200648966–972. [DOI] [PubMed] [Google Scholar]
- 31.Hadders‐Algra M, Klip‐Van den Nieuwendijk A W J, Martijn A.et al Assessment of general movements: towards a better understanding of a sensitive method to evaluate brain function in young infants. Dev Med Child Neurol 19973988–98. [DOI] [PubMed] [Google Scholar]
- 32.Woollacott M, Sveistrup H, Swinnen S P, Massion J, Heuer H et a l. The development of sensorimotor integration underlying posture control in infants during the transition to independence stance. In: eds. Interlimb coordination:neural dynamical and cognitive constraints. San Diego, CA: Academic Presss, 1994371–389.
- 33.Hadders‐Algra M. The Neuronal Group Selection Theory: an attractive framework to explain variation in normal motor development. Dev Med Child Neurol 200042566–572. [DOI] [PubMed] [Google Scholar]
- 34.Blauw‐Hospers C H, Hadders‐Algra M. A systematic review on the effects of early intervention on motor development. Dev Med Child Neurol 200547421–432. [DOI] [PubMed] [Google Scholar]