Abstract
Objectives
To examine the characteristics of incident reporting systems in neonatal intensive care units (NICUs) in relation to type, aetiology, outcome and preventability of incidents.
Methods
Systematic review. Search strategy: Medline, Embase, Cochrane Library. Included: relevant systematic reviews, randomised controlled trials, observational studies and qualitative research. Excluded: non‐systematic reviews, expert opinions, case reports and letters. Participants: hospital units supplying neonatal intensive care. Intervention: none. Outcome: characteristics of incident reporting systems; type, aetiology, outcome and preventability of incidents.
Results
No relevant systematic reviews or randomised controlled trials were found. Eight prospective and two retrospective studies were included. Overall, medication incidents were most frequently reported. Available data in the NICU showed that the total error rate was much higher in studies using voluntary reporting than in a study using mandatory reporting. Multi‐institutional reporting identified rare but important errors. A substantial number of incidents were potentially harmful. When a system approach was used, many contributing factors were identified. Information about the impact of system changes on patient safety was scarce.
Conclusions
Multi‐institutional, voluntary, non‐punitive, system based incident reporting is likely to generate valuable information on type, aetiology, outcome and preventability of incidents in the NICU. However, the beneficial effects of incident reporting systems and consecutive system changes on patient safety are difficult to assess from the available evidence and therefore remain to be investigated.
Keywords: intensive care, neonatal, medical errors, incident reporting system, NICU, patient safety
Almost every healthcare professional has at some time made a mistake resulting in injury or possible injury to a patient. In the industrial sector it has been acknowledged that human errors will occur, and therefore systems are designed in such a way that errors are prevented or detected before they develop into a true accident. In clinical practice, however, the magnitude of this problem has long been underestimated, despite several large studies confirming the occurrence of medical error with (possible) patient harm.1 In 1991, the Harvard Medical Practice Study reported that adverse events occurred in 3.7% of acute‐care hospital admissions in New York. Subsequent analysis showed that more than 50% were caused by errors.2,3 Yet, only since the release of report, “To err is human”, by the Institute of Medicine (IOM) in 1999, has the importance of good patient safety management been recognised by healthcare workers all over the world.
The IOM defines safety as “freedom from accidental injury”. Their report mentioned that the problem of accidental injury is serious, and that patient safety must become a national priority. It was emphasised that the cause is not careless people but faulty systems.4 The IOM recommended that all healthcare settings should establish comprehensive patient safety programmes executed by trained personnel within a culture of safety, and emphasised that reporting systems are one of the key strategies for learning from errors and for monitoring progress in the prevention of their recurrence.5
A good internal reporting system makes all responsible healthcare workers aware of the major hazards, and external reporting allows lessons to be shared so that others can avoid making the same mistakes. Also, external reporting systems will yield a larger sample size, increasing the potential to identify patterns of infrequent, yet striking errors.6,7 According to Leape, most of the benefits can be obtained with specialty based or system‐wide reporting programmes.7
Data exist for specialty based reporting systems. The Australian Incident Monitoring System in Anaesthesia used voluntary, anonymous incident reporting, which elicited large volumes of specific information about incidents that could be analysed for root causes and contributing factors.8,9 Through systematic analysis of incident reports, this study, and several others, provided important information about factors associated with adverse events in their specialty.8,10,11,12 Patients who are more severely ill, those who are subjected to multiple interventions, and those who remain in hospital longer seem to be more likely to receive a serious injury as a result of medical mistakes.9,13
Thus, newborns in the neonatal intensive care unit (NICU) are a particularly vulnerable group, owing to their small size, physiological immaturity and limited compensatory abilities.14 In a study on medication errors and adverse drug events (ADEs) in two academic paediatric hospitals, the rate of potential ADEs was considerably higher in neonates than in other age groups. Moreover, neonates in the NICU had significantly higher medication error and potential ADE rates than neonates in other wards.15 In establishing a specialty based incident reporting system for NICUs in The Netherlands, the objective was to examine the characteristics of incident reporting systems in NICUs in relation to type, aetiology, outcome and preventability of incidents.
Methods
Search strategy
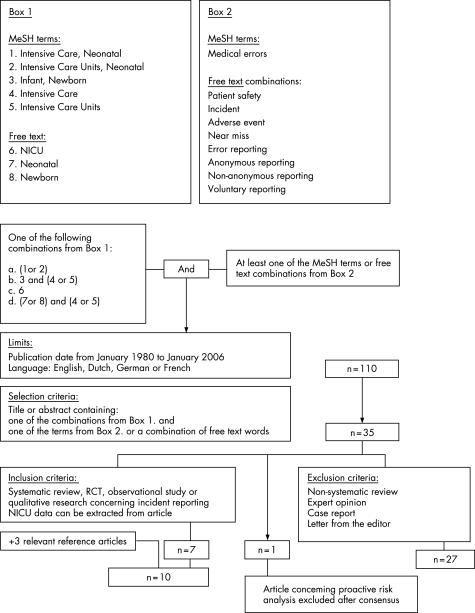
Two authors (CS and RAvL) independently searched Medline on PubMed for English, Dutch, German, or French language articles (January 1980 to January 2006). Figure 1 outlines the search strategy. Subsequently, Embase and the Cochrane library were searched with similar search terms as in Medline. The titles or abstracts, or both, of all identified studies were reviewed and full manuscripts obtained for articles meeting the selection criteria (n = 35, fig 1).14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 The reference lists of the articles included and of the review articles were checked manually to look for other potentially relevant articles.
Figure 1 Search strategy.
Analysis of search results
The same authors (CS and RAvL) independently assessed each article for eligibility according to the inclusion criteria (fig 1), and extracted relevant information on study design and results using a predefined data abstraction form. The definition of an incident or error was recorded according to the original author's definition. If possible, the total number of incident reports was expressed per 100 admissions and per 1000 patient days. Differences between reviewers were discussed within the research group, and agreement was reached by consensus.
Results
Search results
The Medline search yielded no relevant systematic reviews or randomised controlled trials. Eight prospective and two retrospective studies were included (table 1).14,15,23,24,39,41,43,47,50,51
Table 1 Study design.
| Reference | Study design | Study period | Setting | Study population | Aims and objectives |
|---|---|---|---|---|---|
| Folli50 USA 1987 | Prospective | 6 Months (1985) | (1) Paediatric university teaching hospital(2) Paediatric community teaching hospital | (1) 145 Beds (18 PICU, 54 NICU beds)(2) 100 Beds (16 PICU, 33 NICU beds) | To report findings of (potential) severity of errant medication orders To assess the impact of pharmacist intervention to prevent harm |
| Vincer47 Canada 1989 | Prospective | 2 Years (1986–7) | University affiliated teaching hospital | 1200 NICU admissions (exclusively inborn nursery) each year, with 50 000 drug doses or IV infusions administered each year | To document experience, with particular emphasis on the cause of all medication errors and incidents |
| Raju39 USA 1989 | Prospective | 4 Years (1985–8) | University hospital | 2147 Admissions, 1224 (57%) to a 17 bed NICU and 923 (43%) to a 7 bed PICU | To establish a baseline pattern of errors To assess the frequency of drug related iatrogenic complications and to institute some corrective measures |
| Frey23 Switzerland 2000 | Prospective | 1 Year (1998–9) | Non‐university teaching hospital | 467 Admissions in a multidisciplinary 12 bed NICU/PICU (56% neonates) | To examine the occurrence of critical incidents in order to improve quality of care |
| Ross51 UK 2000 | Retrospective | 5 Years (1994–9) | Paediatric teaching hospital | From April 1995 to March 1999:112 536 Admissions (3373 to a 28 bed tertiary referral NICU) | To determine the incidence and type of medication errors in a large UK paediatric hospital over a 5 year period To evaluate the potential impact of prevention strategies |
| Kaushal15 USA 2001 | Prospective | 6 Weeks (1999) | Two urban teaching hospitals | 1120 Admissions to 9 wards (1 NICU), mainly children 183 (16%) Neonates and 36 (3%) adults | To assess the rates of medication errors and (potential) ADEs To compare paediatric rates with adult rates To analyse the major types of errors To evaluate the potential impact of prevention strategies |
| Frey24 Switzerland 2002 | Prospective | 1 Year (2001) | University teaching children's hospital | Multidisciplinary 23 bed NICU/PICU | To analyse drug related critical incidents, with an emphasis on how they contributed to system changes |
| Simpson41 UK 2004 | Prospective | 1 Year (2002) | University maternity hospital | Large tertiary referral NICU | To describe the medication errors occurring in the NICU To assess the impact of a combined risk management/ward based, clinical pharmacist led education programme on these errors |
| Suresh43 USA 2004 | Prospective: phase 1 Prospective: phase 2 | 17 Months (2000–2)10 Months (2002–3) | 54 NICUs participating in the NICQ Collaborative (Vermont Oxford Network) | Patients in NICU, step‐down unit, well‐infant newborn nursery, delivery room, newborn resuscitation room, mother's hospital room, other hospital inpatient unit, operating room, newborn infants during interhospital transport | To implement a voluntary, anonymous, internet based reporting system for medical errors in neonatal intensive care and to evaluate its feasibility To identify errors that affect high risk neonates and their families |
| Kanter14 USA 2004 | Retrospective | 1 Year (1997) | Discharge data from community hospitals in >20 states (from the Healthcare Cost and Utilisation Project database) | All discharges of neonates from 1997: total 815 296 Premature (<2500 g) 66 146 (8%)Full term 749 150 (92%) | To determine the national rate of hospital reported medical errors in premature neonates and describe the patient and organisational characteristics associated with their occurrence |
The authors search results were identical, except for two articles.14,30 The authors agreed to include a retrospective study on reported complications14 and to exclude a study concerning proactive risk analysis of the medication‐use process.30 Three relevant articles were added after reference checking.15,50,51 An article describing errant drug orders collected by pharmacist review and not by incident reporting was also included because of its relevance.50 A search in Embase and the Cochrane library yielded no additional relevant articles.
Characteristics of incident reporting systems
In most studies, all staff were encouraged to fill out a report on awareness of an error or incident. (table 2).
Table 2 Characteristics of reporting systems.
| Reference | What is reported? | Reporting climate | Area of reporting | Reporter | Characteristics of report form | Review/analysis of reports | Systems‐oriented52 | Time until feedback(months) |
|---|---|---|---|---|---|---|---|---|
| Folli50 USA 1987 | Errant medication order: ordering physician and pharmacist agreed on the need to change the order | Not described | Paediatric wards including NICU | Pharmacist reviews all medication orders and reports all errant orders | Type of medication order Patient age Severity Unit in which patient received the order | Member of the paediatric faculty or attending physician Two paediatric clinical pharmacist practitioners | – | >3 |
| Vincer47 Canada 1989 | Medication incidents Incident has reached the patient (except for errors of omission). | Non‐punitive Voluntary | NICU | Either self reported or reported by another person who identified incident | Type of incident Patient information and severity Cause of incident Time | Committee of three:Neonatologist Nursing unit coordinator Clinical neonatal pharmacist | +/− | <1 |
| Raju39 USA 1989 | Medication errors Medication dose must reach the patient, except for errors of omission (criteria by American Society of Hospital Pharmacists, 1988) | Non‐punitive Anonymous Voluntary | NICU/PICU | ICU staff, person who noticed the error | Type Severity Error attribution Time | Manager from the pharmacy department Department of quality assurance | ? | <1 |
| Frey23 Switzerland 2000 | Overall critical incident monitoring Critical incident: any event which might have reduced, or did reduce, the safety margin for the patient | Non‐punitive Anonymous Voluntary | NICU/PICU | ICU staff fills out form immediately on becoming aware of a critical incident | Narrative, including contributing factors Patient information and severity Incident attributionTime and location | Critical incident group:Two nurses One consultant | + | 1–3 |
| Ross51 UK 2000 | Medication error: wrong medicine, wrong dose, wrong route, wrong preparation, wrong time, unauthorised drug or omission, wrong dispensing, or to someone known to be allergic | Gradual change from punitive to non‐punitive Mandatory | Paediatric wards, including NICU | Hospital‐wide reporting, all staff | Standardised form in all departments Not further specified | Head of department | + | 1–3 |
| Kaushal15 USA 2001 | Medication errors and (potential) (intercepted) ADEs. An ADE is an injury that results from a drug. A preventable ADE is an ADE associated with a medication error | Non‐punitive Non‐anonymous Voluntary | Paediatric wards including NICU | House officers, nurses and pharmacists report verbally to trained data collectors | Type of error Name, dose, route and category of drug Point in system where error occurred | Two physicians(severity, preventability and attribution were recorded) | + | <1 |
| Frey24 Switzerland 2002 | Drug related critical incidents A critical incident is a harmful and potentially harmful event | Non‐punitive Anonymous Voluntary | NICU/PICU | ICU staff fill out form immediately on becoming aware of a critical incident | Narrative, including contributing factors Patient information and severity Time and location Was patient harm prevented by check?Proposals for prevention Were patient/parents informed? | Quality assurance group:One consultant Three senior nurses One teaching nurse One junior nurse One person responsible for ICU equipment | + | 1–3 |
| Simpson41 UK 2004 | Medication errors identified through critical incident reports | Non‐punitive | NICU | Nursing or medical staff involved in the error, or the clinical pharmacist | Not specified | Risk management group:Clinical pharmacist Consultant neonatologist Neonatal specialist registrar Senior nurse(severity was recorded) | + | <1 |
| Suresh43 USA 2004 | Errors that resulted in harm to the patient as well as near misses | Non‐punitive Anonymous Voluntary | NICU | 739 Healthcare providers (physicians, nurses, pharmacists and others) from a total of 54 NICUs were authorised to report to hypertext mark‐up language forms | External, internet based Phase 1: 4 free text boxes (title, description, key words, references)Phase 2: structured scroll‐down form:severity, time and location, type, contributing and mitigating factors, changes to prevent recurrence | Members of Center for Patient Safety in neonatal intensive care | + | <1 |
| Kanter14 USA 2004 | Medical error: International Classification of Diseases (ICD)‐9 diagnosis codes 996–999 (complications of medical or surgical care) | Not specified | Hospital discharge data of neonates | Not specified | Discharge records with diagnostic, utilisation and patient information | Healthcare Cost and Utilisation Project of the Agency for Healthcare Research and Quality | – | Not specified |
Most of these studies mentioned the benefits of a voluntary, non‐punitive approach to error.15,23,24,39,41,43,47 One study reported a fourfold to sixfold annual increase in the reporting of medication incidents after efforts to reduce the punitive aspect of reporting incidents and to use the educational value of these reports through teaching sessions for nurses.47 Many studies used anonymous reporting to ensure confidentiality. However, because of the anonymity, people could not be contacted for details of reported events.43 In the study by Suresh and colleagues, multi‐institutional reporting identified rare but important errors, and error patterns that were unique to the specialty—such as infants being fed breast milk from the wrong mother. This study also mentioned meetings, conference calls and email discussion lists for collaborative learning and systems thinking.43
Type and aetiology
Seven studies reported only medication related incidents or errors.15,24,39,41,47,50,51 Two studies concerned different types of incidents and errors.23,43 Overall, however, medication errors were reported most frequently. Although definitions of an incident or error varied across studies (table 2), data on medication errors in the NICU showed that the total medication error rate was much higher in studies using voluntary reporting than in studies using mandatory reporting (13–14.7 per 1000 NICU patient days, compared with 0.97 per 1000 NICU patient days; and 13–91 per 100 NICU admissions, compared with 0.83 per 100 NICU admissions) (table 3).15,41,47,51
Table 3 Study results.
| Reference | Total number of incidents included in study period | Incidents by type No (%) | Aetiology No (%) | Degree of harm No (%) | Preventability No (%) |
|---|---|---|---|---|---|
| Folli50 USA 1987 | 281 (hospital 1) and 198 (hospital 2) errant medication orders (4.9/1000 and 4.5/1000 medication orders, respectively)Total error rate: 15.2/1000 patient days (PICU 32.6/1000, NICU 8.2/1000, ward 19.4/1000) | Overdose 264 (55.1)Underdose 129 (26.9)Wrong drug 27 (5.6)IV incompatibility 13 (2.7)Wrong route 9 (1.9)Drug interaction 9 (1.9)Drug allergy 2 (0.4)Other 26 (5.4) | Frequency of errant orders declined as physicians' training status increased (p<0.001) | All areas: no actual harm NICU:No actual harm Potentially lethal 0.04/100 patient days Serious 0.23/100 patient days Significant 0.55/100 patient days | Paediatric pharmacists were able to detect errant medication orders and prevent medical errors |
| Vincer47 Canada 1989 | 313 Medication incidents on 23 307 patient days (13.4/1000 patient days, approximately 13/100 admissions) | Human error 274 (87.5)Mechanical failures 8 (2.6)Other events 24 (7.7)Unknown 7 (2.2) | Administration 84 (27):Neglecting to give a drug on scheduled time 52 (17)Failure to follow procedures 56 (18):Intravenous infusion not properly regulated 32 (10)Physician's orders incorrect 51 (16)Faulty drug preparation 26 (8)Transcription of physician's order 26 (8)Interstitial intravenous line 18 (6)Other 52 (17)Relative risk of medication incidents increased with increasing level of care (p<0.01)Three serious errors were caused by verbal orders that differed from the subsequently written order | Errors in physician's orders resulted in more serious incidents (incidents with (potential for) patient morbidity), 20% compared with 6% of all other causes (p<0.001) | Not described |
| Raju39 USA 1989 | 315 Medication related errors among 2147 admissions (14.7/100 admissions, 8.8/1000 patient days) | Wrong time 68 (21.6)Wrong rate 43 (13.7)Wrong dose 43 (13.7)Unauthorised drug 42 (13.3)Wrong technique 41 (13.0)Omission 39 (12.4)Wrong preparation 26 (8.3)Wrong route 13 (4.1) | Improper placement of the decimal point was the commonest error in calculation | Substantial injury (long term injury, toxic effects or death) 1 (0.3)Mild injury (no substantial treatment or intervention) 32 (10.2)No apparent injury 250 (79.4)Potentially serious (drug serum level in toxic range, or insufficient dose of a life‐saving drug) 33 (10.5) | Not described |
| Frey23 Switzerland 2000 | 211 (45/100 neonatal admissions, 40/100 paediatric admissions) | Management/environment 62 (29)Drugs 62 (29):Wrong dose 37 Wrong drug 11 Procedures 37 (18)Respiration 29 (14)Equipment dysfunction 15 (7)Nosocomial infections 6 (3) | Human error (63)Communication (14)Organisational problems (10)Equipment dysfunction (7)Milieu (3)No contributing factor identified (3) | Major: death (0), need for therapeutic intervention specific to the ICU (30)Moderate (requiring routine treatment available outside ICU) (25)Minor (no intervention required) (45)Most severe: incidents relating to respiration | Not described |
| Ross51 UK 2000 | Total hospital: 195 medication errors (0.15/100 admissions, 0.51/1000 patient days)NICU: 33 medication errors (0.83/100 admissions, 0.97/1000 patient days)PICU: 20 medication errors (0.61/100 admissions, 1.6/1000 patient days) | Parenteral medicines 109 (56):Antibiotics 48 Oral medicines 66 (34)Other route 20 (10)Incorrect IV infusion rate 32 (15.8)Incorrect dose given 30 (14.8)Extra dose given 28 (13.8)Dose omitted 25 (12.3)Incorrect drug given 25 (12.3)Incorrect IV concentration 21 (10.3)Labelling error 20 (9.9)Incorrect route 9 (4.4)Incorrect patient 8 (3.9)Incorrect strength 1 (0.5)Other 4 (2) | Double check did not occur 58 (30)Unknown whether checking occurred 7 (3)Intravenous pump errors 23: many different types of syringe pump and volumetric pump in use Tenfold dosing errors 15 (8):5 Miscalculations of dose despite clear prescribing 4 Incorrect or unclear prescribing 1 Inaccurate verbal communication | Long term morbidity or mortality 0 Serious (potential severe harm) 2 (1)Medium severity (clinical symptoms aggravated by error) 3 (2)Minor (no actual harm resulted) (96) Errors requiring active patient intervention 18 (9.2) | Errors involving morphine sulphate occurred when 10 mg, 15 mg and 30 mg ampoules were available. In one case ampoules had been confused |
| Kaushal15 USA 2001 | 616 Medication errors (5.7/100 orders, 55/100 admissions, 157/1000 patient days)115 Potential ADEs 26 ADEs Neonates in the NICU:Medication errors 91/100 admissions Potential ADEs 46/100 admissions Neonates in other wards:Medication errors 50/100 admissions Potential ADEs 9/100 admissions | Dose 175 (28)Frequency 58 (9.4)Route 109 (18)Administration 85 (14)Wrong drug 8 (1.3)Wrong patient 1 (0.16)Known allergy 8 (1.3)Illegible order 14 (2.3)Missing or wrong weight 74 (12)No or wrong date 74 (12)Other 61 (9.9) | Prescription 454 (74)Transcription 62 (10)Administration 78 (13)Patient monitoring 4 (0.6)Missing 12 (1.9) | ADEs:Fatal or life‐threatening 2 (7.7)Serious 9 (34.6)Significant 15 (57.7)Potential ADEs:Fatal or life threatening 18 (15.7)Serious 52 (45.2)Significant 45 (39.1) | Preventable ADEs: 5 (0.52/100 admissions)Non‐preventable ADEs: 21 (1.9/100 admissions) |
| Frey24 Switzerland 2002 | 284 Drug related incidents (including IV fluids and enteral and parenteral nutrition) | Catecholamines 31 (11)Anticoagulants 30 (11)Electrolytes 30 (11)Crystalloids 22 (8)Opiates 24 (9)Antibiotics 17 (6)Other 95 (34) | Prescription 102 (37)Preparation 162 (59)Administration 200 (73) | Major: death (0), need for therapeutic intervention specific to the ICU (5)Moderate (requiring routine treatment available outside ICU) (19)Minor (no intervention required) (76)Potentially life‐threatening 24 (8)Most severe: sedative drugs, crystalloids and enteral nutrition | 75 (27) Incidents were caught before administration |
| Simpson41 UK 2004 | 105 Medication errors (14.7/1000 patient days):24.1/1000 Patient days before intervention 5.1/1000 Patient days after intervention (pharmacist‐led education programme)12.2/1000 Patient days after start of new junior medical staff | Parenteral medicines 63 (60):Antibiotics 40 Morphine 6 Oral medicines 41 (39)Topical medicines 1 (1) | Prescription 75 (71):37 Incorrect doses 19 Incorrect dose intervals 14 Incomplete prescriptions 5 Incorrect units Administration 30 (29):16 Poor documentation or communication | Most severe: two 10‐fold dose miscalculations Serious (actual harm or very high risk of harm to the infant) 4 (4)Potentially serious (potential harm to the infant) 45 (43)Minor 56 (53) | A change over of junior medical staff was associated with an increase in medication errors |
| Suresh43 USA 2004 | 1230 Reports: 522 from phase 1 (free text reports) and 708 from phase 2 (structured reports) | Errors of diagnosis 137 (11.2)Errors of treatment 949 (77.2)Errors of prevention 0 Other errors 144 (11.7)Of all reported events, 581 (47%) were related to medication, nutritional agents or blood products:Administration (31)Dispensing (25)Ordering (16)Transcribing (12)Monitoring (1.4)Wrong drug (8.4)Uncertain (6) | In 584 (82.5) phase 2 reports at least one contributing factor was reported. In 52 (8.9) reports, 5– 8 factors were selected for each report Most frequent contributing factors in these 584 reports:Failure to follow policy/protocol 273 (47)Inattention 157 (27)Communication problem 131 (22)Charting or documentation error 78 (13)Distraction 69 (12)Inexperience 59 (10)Labelling error 56 (10)Poor teamwork 50 (9) | Outcome reported in 673 phase 2 reports:Actual harm 181 (27):Death 1 (0.2)Serious (threat to life, impaired outcome) 13 (1.9)Minor (increased monitoring, intervention) 167 (25)Potential harm, reached patient, no harm (34)Potential harm, did not reach patient (25)No potential for harm (14) | Not described |
| Kanter14 USA 2004 | 824 (1.2/100) Premature neonates experienced a medical error | Procedural complications (60), including mechanical complications of device implants and grafts Medical care complications (25) | Significant inverse linear association between birth weight and medical error rates (birth weight 2000–2499 g, 0.6%, versus birth weight 500–749 g, 5.2%, p<0.001)More errors in urban teaching centres than in rural or urban non‐teaching centres (OR = 1.69; CI 1.18 to 2.43) | Not described | Not described |
Despite these differences in reporting climate and error rates, almost all authors reported a considerable number of dosing errors due to wrong prescription or wrong administration. When a system approach was used,52 many contributing factors were identified. In one study, up to eight factors were selected for each report.43 Important aetiological factors were failure to follow procedures, inattention and poor documentation or communication (table 3).
Outcome
Although there were many differences in degree and definition of harm across the studies, several conclusions can be drawn. In only one study was no actual harm reported during the study period.50 Potentially harmful incidents, on the other hand, were reported in almost every study. In studies on medication errors, fatal or life‐threatening incidents were not often reported. However, in a study describing critical incidents, the need for therapeutic intervention specific to the NICU was 30% (table 3). In this study, incidents relating to ventilation were the most severe and incidents involving drugs the least severe events.23
Preventability
Two studies described the implementation of system changes.24,39 One study found that 63% of the incidents which triggered system changes were classified as minor. System changes involved a standardised prescription form and a computerised system for ordering infant formula bottles.24 In the other study, variations in both the error rate and distribution of error type led to the identification of the cause of wrong‐time errors, and subsequently, to the reinstitution of satellite pharmacies near the NICU and PICU.39
Two other studies described the potential impact of prevention strategies.15,51 Kaushal and colleagues reported five preventable ADEs (0.52 per 100 admissions).15 Errors associated with these five incidents included two overdoses, one missing dose, one drug administration error, and giving medication to a patient with a known allergy to this drug. Physician reviewers judged that 93% of the potential ADEs were preventable by physician computer order entry with clinical decision support, and 94% by ward based clinical pharmacists.15 Comparable medication prescription or preparation errors were reported by several other studies.24,39,47,50 Ross and colleagues implemented preconstituted syringes and the double checking of content and administration, after the occurrence of errors caused by confusion of ampoules of different strengths.51
However, only two studies evaluated the impact of preventive strategies.41,50 Results of the first study indicated that paediatric pharmacists could detect errant medication orders and prevent medical errors.50 The second study reported similar results: after the introduction of a pharmacist‐led review of medication orders and the introduction of system changes based on incident analysis, medication errors fell significantly from 24.1 (1.7) per 1000 neonatal activity days in the first 4 months and to 5.1 (3.6) per 1000 in the next 3 months.41 However, as these authors pointed out, within the context of their overall risk management approach it is difficult to quantify the proportion of errors reduced by any one change in practice.
Discussion
This review shows that there are few studies of incident reporting systems in neonatal intensive care. Moreover, these studies have limited generalisability, and it is difficult to compare categories used across their systems. However, several conclusions can be drawn from the available data.
Most incident reporting systems in neonatal intensive care use a voluntary, non‐punitive approach to incidents. The available data on errors in the NICU suggest that these reporting systems elicit many more incidents in the NICU than a mandatory system, yielding more information in a shorter period of time.
Medication incidents are most often reported in the NICU. This is in concurrence with the literature on incidents in other specialties.13 Fatal or life‐threatening harm due to medication incidents was not often reported.23 However, most studies reported that the potential for patient harm as a result of an incident was a significant problem. Moreover, when a system approach was used, many contributing factors were identified. These data suggest that with the use of these incident reporting systems, repeated occurrence of incidents and contributing factors can be identified, thus facilitating their clarification and preventing their recurrence.
What is already known on this topic
The importance of acknowledging error in medicine and the need to improve patient safety is gaining more and more attention across all healthcare facilities.
Several studies suggest that incident reporting systems might improve safety.
However, there are also several limitations to incident reporting systems.
First, owing to anonymity of reports, people cannot be contacted for details of reported events.43 On the other hand, the success of non‐anonymous reporting of incidents strongly depends on the creation of a non‐punitive climate which allows staff to report incidents without disciplinary sanctions.53
Second, when using a voluntary incident reporting system, only a fraction of incidents may be detected. In an NICU, more complex errors, such as prioritisation of clinical tasks or failure to perform diagnostic assessments, might also result in adverse outcomes. These errors are difficult to measure and, therefore, are not often reported.42 Collaborative learning from incident reports through a neonatal network is likely to offer a solution to this problem.43 However, evaluation of the incidence of actual errors by additional detection methods may be a more accurate marker.15,54 These detection methods can be used for incidents that are likely to be under‐reported in voluntary reporting systems.
What this study adds
A systematic review of the characteristics and benefits of incident reporting systems in neonatal intensive care.
Multi‐institutional, voluntary, non‐punitive, system based incident reporting is likely to generate valuable information on type, aetiology, outcome and preventability of incidents in the NICU.
In addition, this review provides the insight that the beneficial effects of incident reporting systems and consecutive system changes on patient safety in neonatal intensive care are difficult to assess from the available evidence.
Finally, assessing the impact of preventive strategies on patient safety remains a challenge. Although voluntary reporting systems increase the number of reports, under‐reporting is still likely. Also, a system change can increase the number of incident reports. Therefore, the impact of incident reporting systems and consecutive system changes on patient safety remains a subject for our future investigations.
Conclusions
Multi‐institutional, voluntary, non‐punitive, system based incident reporting is likely to generate valuable information on type, aetiology, outcome and preventability of incidents in the NICU. However, the beneficial effects of incident reporting systems and consecutive system changes on patient safety are difficult to assess from the available evidence and therefore remain to be investigated.
Acknowledgements
We thank the hospital librarians for their help with searching and obtaining papers.
Abbreviations
ADEs - adverse drug events
IOM - Institute of Medicine
NICU - neonatal intensive care unit
Footnotes
Funding: CS was funded by the Dutch Association of Medical Specialists.
Competing interests: None.
References
- 1.Van der Schaaf T W. Medical applications of industrial safety science. Qual Saf Health Care 200211205–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan T A, Leape L L, Laird N M.et al Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med 1991324370–376. [DOI] [PubMed] [Google Scholar]
- 3.Leape L L, Brennan T A, Laird N.et al The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med 1991324377–384. [DOI] [PubMed] [Google Scholar]
- 4.Kohn L T, Corrigan J M, Donaldson M S.To err is human. Building a safer health system. Washington DC: National Academy Press, 2000 [PubMed]
- 5.IOM report Patient safety—achieving a new standard for care. Acad Emerg Med 2005121011–1012. [DOI] [PubMed] [Google Scholar]
- 6.Leape L L. Why should we report adverse incidents? J Eval Clin Pract 199951–4. [DOI] [PubMed] [Google Scholar]
- 7.Leape L L. Reporting of adverse events. N Engl J Med 20023471633–1638. [DOI] [PubMed] [Google Scholar]
- 8.Runciman W B, Sellen A, Webb R K.et al The Australian Incident Monitoring Study. Errors, incidents and accidents in anaesthetic practice. Anaesth Intensive Care 199321506–519. [DOI] [PubMed] [Google Scholar]
- 9.Wu A W, Pronovost P, Morlock L. ICU incident reporting systems. J Crit Care 20021786–94. [DOI] [PubMed] [Google Scholar]
- 10.Beckmann U, West L F, Groombridge G J.et al The Australian Incident Monitoring Study in Intensive Care: AIMS‐ICU. The development and evaluation of an incident reporting system in intensive care. Anaesth Intensive Care 199624314–319. [DOI] [PubMed] [Google Scholar]
- 11.Beckmann U, Baldwin I, Hart G K.et al The Australian Incident Monitoring Study in Intensive Care: AIMS‐ICU. An analysis of the first year of reporting. Anaesth Intensive Care 199624320–329. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan H S, Battles J B, Van der Schaaf T W.et al Identification and classification of the causes of events in transfusion medicine. Transfusion 1998381071–1081. [DOI] [PubMed] [Google Scholar]
- 13.Weingart S N, Wilson R M, Gibberd R W.et al Epidemiology of medical error. BMJ 2000320774–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanter D E, Turenne W, Slonim A D. Hospital‐reported medical errors in premature neonates. Pediatr Crit Care Med 20045119–123. [DOI] [PubMed] [Google Scholar]
- 15.Kaushal R, Bates D W, Landrigan C.et al Medication errors and adverse drug events in pediatric inpatients. JAMA 20012852114–2120. [DOI] [PubMed] [Google Scholar]
- 16.Basinski A. Variations in mortality rates among Canadian NICUs—and anonymous reporting. CMAJ 2002167120–121. [PMC free article] [PubMed] [Google Scholar]
- 17.Carroll A E, Tarczy‐Hornoch P, O'Reilly E.et al Resident documentation discrepancies in a neonatal intensive care unit. Pediatrics 2003111(Pt 1)976–980. [DOI] [PubMed] [Google Scholar]
- 18.Chappell K, Newman C. Potential tenfold drug overdoses on a neonatal unit. Arch Dis Child Fetal Neonatal Ed 200489F483–F484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark P A. What residents are not learning: observations in an NICU. Acad Med 200176419–424. [DOI] [PubMed] [Google Scholar]
- 20.Cordero L, Kuehn L, Kumar R R.et al Impact of computerized physician order entry on clinical practice in a newborn intensive care unit. J Perinatol 20042488–93. [DOI] [PubMed] [Google Scholar]
- 21.Edwards W H. Patient safety in the neonatal intensive care unit. Clin Perinatol 20053297–106, vi. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher M A, Brown D R, Landers S.et al Umbilical arterial catheter use: report of an audit conducted by the Study Group for Complications of Perinatal Care. Am J Perinatol 19941194–99. [DOI] [PubMed] [Google Scholar]
- 23.Frey B, Kehrer B, Losa M.et al Comprehensive critical incident monitoring in a neonatal‐pediatric intensive care unit: experience with the system approach. Intensive Care Med 20002669–74. [DOI] [PubMed] [Google Scholar]
- 24.Frey B, Buettiker V, Hug M I.et al Does critical incident reporting contribute to medication error prevention? Eur J Pediatr 2002161594–599. [DOI] [PubMed] [Google Scholar]
- 25.Gray J E, Goldmann D A. Medication errors in the neonatal intensive care unit: special patients, unique issues. Arch Dis Child Fetal Neonatal Ed 200489F472–F473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray J E, Suresh G, Ursprung R.et al Patient misidentification in the neonatal intensive care unit: quantification of risk. Pediatrics 2006117e43–e47. [DOI] [PubMed] [Google Scholar]
- 27.Horns K M, Loper D L. Medication errors: analysis not blame. J Obstet Gynecol Neonatal Nurs 200231347–354. [DOI] [PubMed] [Google Scholar]
- 28.Kelly K J, Neu J, Rice T B.et al Efficacy of a programmed calculator for constant‐infusion medication calculations. Pediatrics 19847368–70. [PubMed] [Google Scholar]
- 29.Kostopoulou O, Shepherd A. Fragmentation of treatment and the potential for human error in neonatal intensive care. Top Health Inf Manage 20002078–92. [PubMed] [Google Scholar]
- 30.Kunac D L, Reith D M. Identification of priorities for medication safety in neonatal intensive care. Drug Saf 200528251–261. [DOI] [PubMed] [Google Scholar]
- 31.Larsen G Y, Parker H B, Cash J.et al Standard drug concentrations and smart‐pump technology reduce continuous‐medication‐infusion errors in pediatric patients. Pediatrics 2005116e21–e25. [DOI] [PubMed] [Google Scholar]
- 32.Lefrak L. Moving toward safer practice: reducing medication errors in neonatal care. J Perinat Neonatal Nurs 20021673–84. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann C U, Conner K G, Cox J M. Preventing provider errors: online total parenteral nutrition calculator. Pediatrics 2004113748–753. [DOI] [PubMed] [Google Scholar]
- 34.Lillis K. Automated dosing. Computerized physician order entry reduces risk of medication and dosing errors in neonatal ICU. Health Manag Technol 20032436–37. [PubMed] [Google Scholar]
- 35.Lucas A J. Improving medication safety in a neonatal intensive care unit. Am J Health Syst Pharm 20046133–37. [DOI] [PubMed] [Google Scholar]
- 36.McGrath J M. Patient safety: examples in the NICU. J Perinat Neonatal Nurs 2005197–8. [DOI] [PubMed] [Google Scholar]
- 37.Needham D M, Sinopoli D J, Thompson D A.et al A system factors analysis of “line, tube, and drain” incidents in the intensive care unit. Crit Care Med 2005331701–1707. [DOI] [PubMed] [Google Scholar]
- 38.Proctor M L, Pastore J, Gerstle J T.et al Incidence of medical error and adverse outcomes on a pediatric general surgery service. J Pediatr Surg 2003381361–1365. [DOI] [PubMed] [Google Scholar]
- 39.Raju T N, Kecskes S, Thornton J P.et al Medication errors in neonatal and paediatric intensive‐care units. Lancet 19892374–376. [DOI] [PubMed] [Google Scholar]
- 40.Ryan C A, Mohammad I, Murphy B. Normal neurologic and developmental outcome after an accidental intravenous infusion of expressed breast milk in a neonate. Pediatrics 2006117236–238. [DOI] [PubMed] [Google Scholar]
- 41.Simpson J H, Lynch R, Grant J.et al Reducing medication errors in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed 200489F480–F482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stokowski L A. Working conditions and patient safety. Adv Neonatal Care 20033257. [PubMed] [Google Scholar]
- 43.Suresh G, Horbar J D, Plsek P.et al Voluntary anonymous reporting of medical errors for neonatal intensive care. Pediatrics 20041131609–1618. [DOI] [PubMed] [Google Scholar]
- 44.Tisdale J E. Justifying a pediatric critical‐care satellite pharmacy by medication‐error reporting. Am J Hosp Pharm 198643368–371. [PubMed] [Google Scholar]
- 45.van den Anker J N. Managing drugs safely. Semin Fetal Neonatal Med 20051073–81. [DOI] [PubMed] [Google Scholar]
- 46.Verklan M T. Malpractice and the neonatal intensive‐care nurse. J Obstet Gynecol Neonatal Nurs 200433116–123. [DOI] [PubMed] [Google Scholar]
- 47.Vincer M J, Murray J M, Yuill A.et al Drug errors and incidents in a neonatal intensive care unit. A quality assurance activity. Am J Dis Child 1989143737–740. [DOI] [PubMed] [Google Scholar]
- 48.Walsh‐Sukys M, Reitenbach A, Hudson‐Barr D.et al Reducing light and sound in the neonatal intensive care unit: an evaluation of patient safety, staff satisfaction and costs. J Perinatol 200121230–235. [DOI] [PubMed] [Google Scholar]
- 49.White J R, Veltri M A, Fackler J C. Preventing adverse events in the pediatric intensive care unit: prospectively targeting factors that lead to intravenous potassium chloride order errors. Pediatr Crit Care Med 2005625–32. [DOI] [PubMed] [Google Scholar]
- 50.Folli H L, Poole R L, Benitz W E.et al Medication error prevention by clinical pharmacists in two children's hospitals. Pediatrics 198779718–722. [PubMed] [Google Scholar]
- 51.Ross L M, Wallace J, Paton J Y. Medication errors in a paediatric teaching hospital in the UK: five years operational experience. Arch Dis Child 200083492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reason J. Human error: models and management. BMJ 2000320768–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen L L.Act on Patient Safety in the Danish Health Care System. http://www.patientsikkerhed.dk/admin/media/pdf/133907d0940e4d5f751852ec8f6b1795.pd (accessed 28 January 2007)
- 54.Beckmann U, Bohringer C, Carless R.et al Evaluation of two methods for quality improvement in intensive care: facilitated incident monitoring and retrospective medical chart review. Crit Care Med 2003311006–1011. [DOI] [PubMed] [Google Scholar]