Abstract
Objective
To determine the effects of maternal diabetes on fetal iron status using serum transferrin receptors (STfR) and their ratio to ferritin (TfR‐F index) in cord blood.
Methods
Iron, ferritin, erythropoietin, STfR and haemoglobin concentration were measured and TfR‐F index calculated in 97 maternal/cord blood pairs. Forty‐nine women had type 1 diabetes (diagnosed before pregnancy) and these were compared with forty‐eight non‐ diabetic controls. The women with type 1 diabetes were recruited consecutively from attendance at the joint antenatal endocrine clinic while the control group of women was recruited from consecutive attendance at the remaining antenatal clinics.
Results
The infants of the diabetic women had significantly lower levels of ferritin (47 vs 169 μg/l; p<0.01) and higher STfR (17.4 vs 12.9 mg/l; p<0.01) and TfR‐F index (10.4 vs 5.8; p<0.01) than controls. They were also significantly more acidotic at birth (7.25 vs 7.30; p<0.01), were born at an earlier gestation (36.7 vs 39.7 weeks; p<0.01) and had higher z Scores for weight (0.53 vs 0.02; p = 0.016).
Conclusions
Maternal diabetes causes depletion of fetal iron stores and is associated with higher fetal iron demands as indicated by higher STfR level and TfR‐F index in cord blood.
Iron is essential to many cellular processes and transport across the cell membrane is facilitated by the transferrin receptor (TfR) on the cell surface.1 The number of surface transferrin receptors is inversely proportional to intracellular iron.1 Cellular iron homeostasis is regulated through the action of iron regulatory proteins (IRPs). The IRPs bind to the mRNA of both ferritin and the transferrin receptor and as intracellular iron concentration falls the IRPs upregulate the expression of transferrin receptors while simultaneously reducing serum ferritin.2,3
Serum transferrin receptor (STfR) is a soluble truncated form of the transferrin receptor, which has been demonstrated in several studies to have a constant relationship with tissue transferrin receptor expression.4,5 STfR levels are thought to reflect red cell precursor TfR turnover which is determined by cellular iron demand and cellular proliferation rate. In healthy individuals serum ferritin levels are a reliable marker of tissue iron stores. The ratio of the STfR to the log of the ferritin (TfR‐F index) has been proposed to give an even more accurate reflection of tissue iron status.1
Many factors can influence the iron status of the fetus at birth. Iron stores are mainly deposited in the third trimester and are therefore reduced in preterm infants.6 Maternal smoking increases fetal iron requirements for erythropoiesis.7 Maternal diabetes mellitus is associated with depleted fetal iron stores and this is proportionate to the degree of maternal control and presence or absence of complications of diabetes and not maternal iron status.8 Infants of diabetic women tend to be polycythaemic and this may reflect a shift of iron from serum and storage pools to support increased erythropoiesis.
The aims of this study were to examine the effects of maternal diabetes on fetal iron status using STfR, ferritin levels and their ratio in samples of cord blood. We hypothesised that STfR levels and the TfR‐F index would be higher in infants of diabetic women, reflecting a shift of iron out of ferritin stores during increased erythropoiesis and would be proportionate to the degree of polycythaemia. As erythropoiesis occurs in response to hypoxaemia, we also hypothesised that the TfR‐F index would be an indirect measure of fetal hypoxaemia as reflected by erythropoietin levels and cord blood pH.9
Methods
Subjects
The study was approved by the Research Ethics Committee of Queen's University Belfast. Sixty patients with type‐1 diabetes mellitus and 50 non‐diabetic control subjects who attended the Royal Maternity Hospital were recruited consecutively in the first trimester of pregnancy for a study on fetal and maternal leptin levels in diabetic pregnancy which has been previously published.10 The diabetic subjects were confirmed as type‐1 before pregnancy. The controls were excluded if they had glucose intolerance on routine screening, previous gestational diabetes or a first degree relative with diabetes mellitus. Women who reported smoking cigarettes during pregnancy were taken to be smokers. Maternal body mass index was calculated using the formula: weight (kg)/ height (m) squared using booking values for weight and height. The neonatal birth weight z Score was calculated using standard British growth charts for sex and gestation.11
Samples and assays
After birth 20 ml of venous blood was taken from the cord immediately after it was clamped and the baby had been removed. A small aliquot was used for blood gas analysis to record the fetal pH at birth and haemoglobin concentration. The remainder was left to clot at room temperature and after centrifugation the serum was separated and stored at −70°C for subsequent analysis. Erythropoietin was determined by radioimmunoassay.12 Ferritin was measured by immunoradiometric assay using mouse antibody (ICN Pharmaceuticals, Costa Mesa, CA, USA). STfR was measured using an immunoenzymometric assay (Orion Diagnostica Oy, Finland). Blood glucose at one hour of age was measured using Precision Q‐I‐D™ monitor (Abbott Laboratories, Maidenhead, England).
Statistical analysis
Analysis of the data was performed using SPSS v13. The Student's t test was used for continuous variables, with log transformation of non‐normally distributed data such as STfR before analysis. Analysis of variance was performed to determine the effect of potential confounding factors including gestation, mode of delivery and maternal smoking.
Results
Table 1 shows the clinical obstetric and neonatal descriptive variables. Of the 110 subjects recruited to the study a total of 97 cord samples were obtained, 49 from infants of women with diabetes mellitus. Although all samples were suitable for serum ferritin, STfR and erythropoietin analysis, only 29 of the samples were not clotted and therefore suitable for analysis of haemoglobin concentration, providing insufficient data for comparison of haemoglobin concentration between the two cohorts.
Table1 Maternal and neonatal characteristics of the study groups.
| Variable | Diabetic group (n = 49) | Control group (n = 48) | Significance (p) |
|---|---|---|---|
| Maternal BMI | 27.6 (5.6) | 25.5 (5.4) | 0.06 |
| Maternal smoking, n (%) | 6 (10%) | 19 (38%) | <0.01 |
| Maternal Hb at 30 week (g/l) | 115 (14) | 120 (11) | 0.06 |
| Delivery by Caesarean section | 30 (61%) | 12 (25%) | <0.01 |
| Gestation (weeks) | 36.7 (2.3) | 39.7 (1.4) | <0.01 |
| Birth weight (g) | 3299 (746) | 3455 (519) | 0.24 |
| z Score for birth weight | 0.529 (1.04) | 0.022 (0.996) | 0.016 |
| Admission to neonatal unit n (%) | 20 (41%) | 0 | <0.001 |
Mean (SD) except where shown.
Hb, haemoglobin; BMI, body mass index
Infants of women with diabetes mellitus were more likely to be born earlier by caesarean section and to be admitted to the neonatal unit. This was due to predominantly more elective sections being performed in the diabetic group. Although there was no significant difference in birth weight between the two groups of infants, the calculated z Scores for weight were significantly higher in the infants of diabetic women.
Table 2 shows the distribution of the measured variables between the two groups. Cord blood pH, blood glucose at one hour of age and serum ferritin were significantly lower whereas STfR (17.4 vs 12.9 mg/l; p<0.01) and TfR‐F index (10.4 vs 5.8; p<0.01) were significantly higher in infants of diabetic women.
Table 2 Comparison of outcome measurements between the study groups.
| Infants of diabetic (n = 49) | Infants control group (n = 48) | Significance (p) | |
|---|---|---|---|
| Cord pH | 7.30 (0.08) (n = 35) | 7.302 (0.07) (n = 32) | <0.01 |
| Erythropoietin (IU/l) | 45.7 (80.8) | 41.9 (62.7) | 0.058 |
| STfR (mg/l) | 17.4 (11.7–23.2) | 12.9 (8.3–18.7) | <0.01 |
| Ferritin (μg/l) | 47 (24–117) | 169 (136–202) | <0.01 |
| TfR‐F index | 10.4 (6.0–19.4) | 5.8 (3.7–8.5) | <0.01 |
| Blood glucose at 1 h (mmol/l) | 2.5 (1.4) | 3.5 (0.9) | <0.01 |
STfR, serum transferrin receptors; TfR‐F index, transferrin receptor to ferritin index.
Data shown as mean (SD) or as median (interquartile range)
What is already known on this subject
TfR‐F index is proposed to be a more accurate reflection of tissue iron status than ferritin.
Many factors influence fetal iron status including gestation, smoking and maternal diabetes mellitus.
What this paper adds
Infants of mothers with type 1 diabetes have higher fetal iron demands confirmed by increase in cord blood TfR‐F index.
This is at least partly explained by increased erythropoiesis secondary to intra‐uterine hypoxia.
Analysis of variance performed on the data demonstrated that mode of delivery and gestation were not significant confounding variables for either cord pH or TfR‐F index. Maternal smoking masked the effect of maternal diabetes on the TfR‐F index but did not reach statistical significance.
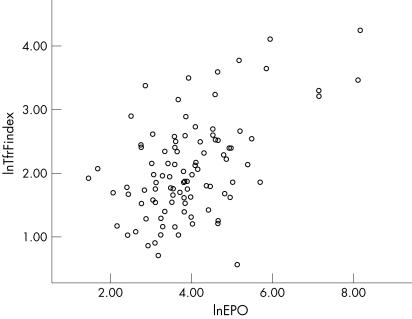
There is a positive correlation between erythropoietin levels and TfR‐F index in the study population, Spearman's rho correlation coefficient being 0.4, p<0.01, although the difference in erythropoietin levels between the two groups failed to reach statistical significance (fig 1).
Figure 1 Correlation between TfR‐F index and erythropoietin levels (r = 0.4; p<0.01)
Discussion
This is the first study on fetal iron status in diabetic pregnancy using the TfR‐F index as a measure of cellular iron status. Infants of diabetic women are often polycythaemic at birth and this may be a reflection of fetal response to chronic intrauterine hypoxia which leads to increased erythropoiesis. STfR levels are increased during periods of increased erythropoiesis,13 reflecting increases in red cell precursor production and expression of TfR. Increased erythropoiesis gives rise to increased fetal iron demand which may exceed placental iron transport capacity. The transferrin binding capacity of the transferrin receptor in the placentae of women with insulin dependent diabetes mellitus (IDDM) is reduced14 and this contributes to decreased placental transfer capacity, thereby reducing fetal iron stores.
This study shows that infants of diabetic women have significantly lower iron stores and significantly higher STfR levels than infants born to women who do not have diabetes, suggesting a state of increased erythropoiesis. It has previously been shown that infants of mothers with type 1 diabetes mellitus who have high levels of erythropoietin in amniotic fluid have an increased rate of neonatal morbidity.15 There was a positive correlation between erythropoietin levels and TfR‐F index in this study, and the infants of the mothers with type 1 diabetes were more likely to be admitted to the neonatal unit, reflecting higher rates of neonatal morbidity within this group.
A number of factors may account for decreased iron stores and increased erythropoiesis. The infants of the women with IDDM were significantly more acidotic at birth, which would suggest exposure to a degree of chronic intra‐uterine hypoxia,9 perhaps related to placental vascular disease. Chronic hypoxia is known to increase erythropoeisis and hence iron demand. A further contributory factor may be the gestation at delivery as the infants of women with IDDM were more likely to be born at an earlier gestation, for reasons such as macrosomia. It has previously been shown that fetal iron stores are directly related to gestational age where earlier gestation is associated with lower iron stores,6 and the lowest stores are found in extremely preterm infants. This was not found to be a significant contributory factor in this study. Maternal smoking is also associated with reduced fetal iron stores7 and the higher incidence of maternal smoking in the control group tended to mask the effect of maternal diabetes on fetal iron stores and so was not a significant confounding variable.
Maternal diabetes is known to have many effects on the fetus and interaction of a number of hormones, such as insulin‐like growth factor may have significant influence. The insulin‐like growth factor system is essential for growth and development and there is a direct positive correlation between levels of IGF‐1 and birth weight, cord haemoglobin and ferritin levels in healthy term infants.16,17,18 The higher z Scores for birth weight of infants born to women with diabetes mellitus would suggest that these infants had higher growth rates in‐utero. The effect of maternal diabetes on these infants is also reflected in the significantly lower blood glucose levels at one hour of age. Increased growth and more rapid cell turnover further increases cellular iron demands. It may be that the combination of increased growth, causing more rapid cell proliferation, with intrauterine hypoxia leads to excessive demands on the placental transfer capacity of iron, which is reduced in diabetic pregnancy,14 contributing to depleted fetal iron stores which are used to meet the cellular requirements.
In conclusion, this study confirms that iron stores are lower at birth in infants of women with diabetes mellitus. This appears to be due to the effects of increased erythropoiesis secondary to chronic intrauterine hypoxia.
Acknowledgements
We would like to thank Geraldine Savage of the Department of Haematology, Royal Victoria Hospital who carried out the laboratory analyses.
Abbreviations
IRPs - iron regulatory proteins
STfR - serum transferrin receptors
TfR - transferrin receptor
TfR‐F index - transferrin receptor ferritin index
Footnotes
Competing interests: None.
References
- 1.Feelders R A, Kuiper‐Kramer E P A, van Eijk H G. Structure, function and clinical significance of transferrin receptors. Clin Chem Lab Med 1999371–10. [DOI] [PubMed] [Google Scholar]
- 2.Klausner R D, Rouault T A, Harford J B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell 19937219–28. [DOI] [PubMed] [Google Scholar]
- 3.Skikne B S, Flowers C H, Cook J D. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 1990751870–1876. [PubMed] [Google Scholar]
- 4.Nunez M T, Garate M A, Arredondo M.et al The cellular mechanism of body iron homeostasis. Biol Res 200033133–142. [DOI] [PubMed] [Google Scholar]
- 5.Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood 1997891052–1057. [PubMed] [Google Scholar]
- 6.Sweet D G, Savage G A, Tubman R.et al Cord blood transferrin receptors to assess fetal iron status. Arch Dis Child Fetal Neonatal Ed 200185F46–F48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweet D G, Savage G A, Tubman T R.et al Study of maternal influences on fetal iron status at term using cord blood transferrin receptors. Arch Dis Child Fetal Neonatal Ed 200184F40–F43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgieff M K, Landon M B, Mills M M.et al Abnormal iron distribution in infants of diabetic women: spectrum and maternal antecedents. J Pediatr 1990117455–461. [DOI] [PubMed] [Google Scholar]
- 9.Rollins M D, Maxwell A P, Afrasiabi M.et al Cord blood erythropoietin, pH, PaO2 and haematocrit following caesarean section before labour. Biol Neonate 199363147–152. [DOI] [PubMed] [Google Scholar]
- 10.Manderson J G, Patterson C C, Hadden D R.et al Leptin concentrations in maternal serum and cord blood in diabetic and nondiabetic pregnancy. Am J Obstet Gynecol 20031881326–1332. [DOI] [PubMed] [Google Scholar]
- 11.Gairdner D, Pearson J. Growth and developmental record; preterm to 2 years. Published by Castlemead Publications, Brickendonbury (1988).
- 12.Lappin T R, Elder G E, Taylor T.et al Comparison of the mouse spleen cell assay and a radioimmunoassay for the measurement of serum erythropoietin. Br J Haematol 198870117–120. [DOI] [PubMed] [Google Scholar]
- 13.Kohgo Y, Niitsu Y, Kondo H.et al Serum transferrin receptor as a new index of erythropoiesis. Blood 1987701955–1958. [PubMed] [Google Scholar]
- 14.Georgieff M K, Petry C D, Mills M M.et al Increased N‐glycosylation and reduced transferrin‐binding capacity of transferrin receptor isolated from placentae of diabetic women. Placenta 199718563–568. [DOI] [PubMed] [Google Scholar]
- 15.Teramo K, Kari M A, Eronen M.et al High amniotic fluid erythropoietin levels are associated with an increased frequency of fetal and neonatal morbidity in type 1 diabetic pregnancies. Diabetologia 2004471695–1703. [DOI] [PubMed] [Google Scholar]
- 16.Skalkidou A, Petridou E, Papathoma E.et al Determinants and consequences of major insulin‐like growth factor components among full‐term healthy neonates. Cancer Epidemiol Biomarkers Prev 200312860–865. [PubMed] [Google Scholar]
- 17.Koc E, Bideci A, Cinaz P.et al Insulin‐like growth factor (IGF)‐I and IGF‐binding protein‐3 in relation to hemoglobin concentration in healthy term infants. J Pediatr Endocrinol Metab 199710609–613. [DOI] [PubMed] [Google Scholar]
- 18.Kurtoglu S, Atabek M E, Gunes T.et al Relationship between cord blood levels of IGF‐I and ferritin in healthy term neonates. J Pediatr Endocrinol Metab 200417737–742. [DOI] [PubMed] [Google Scholar]