Short abstract
Understanding of the specific pathophysiology of acquired brain injury in infants with CHD will help optimise treatment and brain protection strategies
Congenital heart disease (CHD) is a common cause of childhood morbidity, occurring in 6–8/1000 live births, with up to 50% of these children requiring open‐heart surgery to correct their defect.1,2 Most forms of CHD can now be definitively repaired with neonatal surgery resulting in good cardiac function. However, neurological deficits are common, particularly in infants. Given the burden of neurodevelopmental impairment following neonatal cardiac surgery, this article will discuss:
the timing of brain injury in newborns with CHD;
how the pattern of brain abnormalities on imaging studies, such as stroke or white matter injury, informs etiology;
the surprising predominance of white matter injury in term newborns with CHD.
Neurodevelopmental abnormalities are common in infants with CHD
CHD refers to a variety of malformations of the heart present at birth, and includes both cyanotic and acyanotic types. A seminal study of two forms of cardiopulmonary bypass for the correction of transposition of the great arteries (TGA), a relatively homogeneous type of cyanotic CHD, noted neurological abnormalities in more than a third of enrolled patients.3,4 The identified deficits persisted throughout childhood with considerable detriment to school performance.3,4 Others have noted that compared with population norms newborns with TGA are more likely to have abnormal neurological examinations, learning disabilities and behavioural disorders.5,6,7 Motor and global developmental delay is seen in children with multiple types of CHD, in addition to TGA.8 In newborns with hypoplastic left heart syndrome, a type of single ventricle physiology, the incidence of major disabilities in survivors exceeds 60%.9,10 The neurological basis for the high incidence of these global deficits in children with CHD is beginning to be understood with insight from neuroimaging.
The timing of brain injury in newborns with congenital heart disease
The etiology of neurodevelopmental deficits in children with CHD is multifactorial with regard to both timing and mechanisms. Hypothesised mechanisms include disturbance in brain metabolic function, brain injury and abnormal brain development, with some contribution from associated genetic conditions.11 Initial studies of acquired brain injury focused on the operative period and cardiopulmonary bypass technique. Early attempts at correcting complex heart lesions during the neonatal period required a bloodless field and total circulatory arrest. Prolonged circulatory arrest time is identified as a major risk factor for subsequent neurodevelopmental impairments.3,7 However, long‐term neurodevelopmental deficits in newborns with TGA are seen despite attempts to normalise cerebral blood flow during surgical correction of the heart lesion.12 Cardiopulmonary bypass itself may result in brain injury due to embolism, inflammation and ischaemia resulting in impaired delivery of energy substrates (oxygen and glucose).13 Moreover, newborns have a pronounced decrease in mitochondrial oxygenation during induction of hypothermia and a delay in the recovery of mitochondrial oxygenation following circulatory arrest.14,15
Only recently has it been recognised that more than half of newborns with CHD have clinical evidence of neurological abnormalities on examination prior to surgery and that these abnormalities are a major risk factor for later neurodevelopmental impairment.8,16 In recent studies of newborns with CHD with MRI up to 40% have preoperative brain injuries.17,18 By the postoperative MRI, an additional third of those studied acquired new injuries, such that more than half of those studied had cumulatively acquired brain lesions.18,19,20
The pattern of brain abnormalities on MRI informs etiology
More than a third of newborns with CHD have brain injuries noted on MRI prior to cardiac surgery, with an additional third of newborns acquiring brain injuries during or shortly after cardiac surgery. The spectrum of brain injuries and their associated risk factors differ in the preoperative and postoperative periods. In addition, recent data suggest that separate potentially modifiable risk factors exist for each of the major patterns of brain injury: stroke and white matter injury.
Preoperatively, stroke predominates as the brain lesion detected, particularly in newborns with TGA.17,19 Preoperative stroke is specifically and strongly associated with the need for a balloon atrial septostomy, a therapeutic catheterisation procedure needed by many newborns with TGA.17,19 Preoperative white matter injury is also observed with some frequency.18,19 Risk factors for the preoperative brain injuries include lower Apgar score and lower arterial oxygen saturation.17,19 Elevated brain lactate on proton MR spectroscopy, indicating impaired cerebral metabolism, is detected in more than half of newborns preoperatively and is associated with brain injury on MRI.18,21
Brain injury that was not evident before surgery is recognised postoperatively in a third to half of newborns with CHD.18,19 The most common pattern of brain injury on postoperative MRI is white matter injury, particularly in neonates with single ventricle physiology and aortic arch obstruction.18,19
Physiological mechanisms of brain injury
Acquired brain injury detected postoperatively is associated with cardiopulmonary bypass with regional cerebral perfusion and with lower cerebral haemoglobin oxygen saturation during the myocardial ischaemic period of bypass.19 These findings detected with near‐infrared spectroscopy (NIRS) were seen regardless of the bypass method.19 Just as with preoperative injuries, the risk factors for postoperative stroke need to be distinguished from those for white matter injury. In a recent study, all five postoperative strokes occurred following regional cerebral perfusion in infants with a single ventricle who were undergoing the Norwood procedure and had imaging characteristics suggesting embolism as a possible mechanism.19 In contrast, new postoperative white matter injury is specifically associated with low blood pressure during the first postoperative day, and to low postoperative cerebral saturation measured by NIRS (relative cerebral desaturation).19,20,22 Following cardiopulmonary bypass, a pattern of selective cerebral desaturation is often noted, especially with the bypass method of regional cerebral perfusion.23,24 During the first postoperative day in newborns with hypoplastic left heart syndrome, cerebral oxygen saturation below 45% for longer than 3 h22 and low diastolic blood pressure20 are associated with brain injury. In a recent series of newborns with hypoplastic left heart syndrome, those with adverse neurodevelopmental outcome had decreased systemic oxygen delivery postoperatively.25 Postoperative seizures, a marker of brain injury, are variably associated with adverse neurodevelopmental outcome.26,27,28 These observations suggest that intraoperative factors interact with postoperative risk factors such that events during cardiopulmonary bypass may predispose the brain to injury from postoperative low cardiac output.
White matter injury and abnormal brain development in newborns with CHD
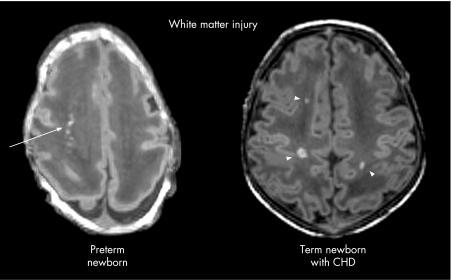
White matter injury is the characteristic pattern of brain injury in premature newborns on MRI and is strongly associated with the risk of adverse neurodevelopmental outcome.29,30 With advances in MRI, a spectrum of white matter injury can now be shown, with cystic periventricular leukomalacia as its most severe manifestation.29,30 New data reveal a strikingly high incidence of white matter injury in term infants with CHD, with imaging characteristics similar to those seen in preterm newborns (fig 1).19,20,31
Figure 1 White matter injury in a premature newborn born at 28 weeks' gestational age and in a term newborn with congenital heart disease, both scanned at 2 weeks of life. The axial images from the spoiled gradient echo volumetric scans show several foci of T1 hyperintensity in the periventricular white matter of the preterm newborn (arrow) and of the term newborn with heart disease (arrowheads).
The pathogenesis of white matter injury in premature newborns is traditionally related to an ischaemic vulnerability secondary to the periventricular vascular anatomy, although more recently the importance of inflammatory states, oxidative stress and the vulnerability of specific cell populations is recognised.32,33 Late oligodendrocyte progenitors and subplate neurons are two cell types that are vulnerable to hypoxia‐ischaemia34,35 and whose development peaks in the white matter throughout the high‐risk period for white matter injury in the premature newborn.36,37 Furthermore, the distribution of susceptible oligodendrocyte progenitor cells, in an ovine model, underlies the spatial anatomy of white matter injury, rather than cerebral blood flow.38
The high frequency of white matter injury in preterm newborns and term newborns with CHD suggests that the white matter in these newborns share a selective vulnerability. Similar to premature newborns, those with CHD are at risk of impaired delivery of energy substrates due to hypoxia‐ischaemia, oxidative stress, and proinflammatory states, particularly with cardiopulmonary bypass. In addition, low preoperative cerebral blood flow is a risk for white matter injury in newborns with CHD.39 However, predominant injury to the deep grey nuclei or intervascular boundary zones would be the expected response to these insults in the term newborn.40 Recently, the characteristic lesions of white matter injury in premature newborns have been produced experimentally in rats by prolonged in utero hypoxia.41 There is considerable evidence that newborns with CHD have impaired in utero brain growth, possibly related to impaired fetal cerebral oxygen delivery as shown in animal models and in human fetuses.42,43,44 Newborns with CHD are more likely to be microcephalic and have an immature cortical mantle on neuropathological examination.45,46 More recently, an immature cortical mantle, reflected in incomplete closure of the operculum, has been identified in approximately 15% of newborns with CHD on preoperative MRI.18,39
There is a complex relationship between brain injuries and abnormal brain development. In premature newborns, white matter injuries are associated with subsequent widespread abnormalities of white matter and cortical development.47,48 Similarly in newborns with CHD, early brain injuries are associated with impaired corticospinal tract development, even when this white matter pathway is normal on conventional MRI.49
Conclusions
The spectrum of neurological abnormalities and their associated cause differ in the periods before, during and after cardiac surgery in newborns with CHD. The pattern of these brain abnormalities on imaging studies, such as stroke or white matter injury, separate specific, and potentially modifiable risk factors. The opportunity to prevent modifiable risk factors for acquired brain injury, as with antithrombotic agents for emboli, maintenance of brain oxygen delivery during cardiopulmonary bypass, and the avoidance of hypotension associated with low cardiac output states postoperatively, necessitates careful clinical trials. Recent observations suggest that prenatal developmental events, cardiac lesion‐specific physiology, intraoperative care and postoperative cardiac output all interact mechanistically to produce the spectrum of injuries observed on MRI. As many of the brain abnormalities detected in recent imaging studies have been clinically silent, the next imperative is to determine the long‐term neurodevelopmental consequence of these lesions. The school‐age developmental outcomes, below population norms, following surgical correction of TGA using low‐flow cardiopulmonary bypass or circulatory arrest suggests a residual burden of injury not attributable to the method of cardiopulmonary bypass. Only with an understanding of the specific pathophysiology of acquired, and potentially preventable, brain injury in infants with CHD will the goal of optimising current treatments and implementing specific brain protection strategies be achieved.
Acknowledgement
We thank Dr Donna M Ferriero for critical review of this manuscript.
Abbreviations
CHD - congenital heart disease
TGA - transposition of the great arteries
Footnotes
Competing interests: None declared.
References
- 1.Hoffman J I, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002391890–1900. [DOI] [PubMed] [Google Scholar]
- 2.Samanek M. Congenital heart malformations: prevalence, severity, survival, and quality of life. Cardiol Young 200010179–185. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger D C, Jonas R A, Rappaport L A.et al Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low‐flow cardiopulmonary bypass. N Engl J Med 1995332549–555. [DOI] [PubMed] [Google Scholar]
- 4.Bellinger D C, Wypij D, Duplessis A J.et al Neurodevelopmental status at eight years in children with dextro‐transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg 20031261385–1396. [DOI] [PubMed] [Google Scholar]
- 5.Ellerbeck K A, Smith M L, Holden E W.et al Neurodevelopmental outcomes in children surviving d‐transposition of the great arteries. J Dev Behav Pediatr 199819335–341. [DOI] [PubMed] [Google Scholar]
- 6.Hovels‐Gurich H H, Konrad K, Wiesner M.et al Long term behavioural outcome after neonatal arterial switch operation for transposition of the great arteries. Arch Dis Child 200287506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hovels‐Gurich H H, Seghaye M C, Schnitker R.et al Long‐term neurodevelopmental outcomes in school‐aged children after neonatal arterial switch operation. J Thorac Cardiovasc Surg 2002124448–458. [DOI] [PubMed] [Google Scholar]
- 8.Limperopoulos C, Majnemer A, Shevell M I.et al Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr 200214151–58. [DOI] [PubMed] [Google Scholar]
- 9.Miller G, Tesman J R, Ramer J C.et al Outcome after open‐heart surgery in infants and children. J Child Neurol 19961149–53. [DOI] [PubMed] [Google Scholar]
- 10.Rogers B T, Msall M E, Buck G M.et al Neurodevelopmental outcome of infants with hypoplastic left heart syndrome. J Pediatr 1995126496–498. [DOI] [PubMed] [Google Scholar]
- 11.Ransom J, Srivastava D. The genetics of cardiac birth defects. Semin Cell Dev Biol 200718132–139. [DOI] [PubMed] [Google Scholar]
- 12.Karl T R, Hall S, Ford G.et al Arterial switch with full‐flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg 2004127213–222. [DOI] [PubMed] [Google Scholar]
- 13.du Plessis A J. Mechanisms of brain injury during infant cardiac surgery. Semin Pediatr Neurol 1999632–47. [DOI] [PubMed] [Google Scholar]
- 14.Kurth C D, Steven J M, Nicolson S C. Cerebral oxygenation during pediatric cardiac surgery using deep hypothermic circulatory arrest. Anesthesiology 19958274–82. [DOI] [PubMed] [Google Scholar]
- 15.du Plessis A J, Newburger J, Jonas R A.et al Cerebral oxygen supply and utilization during infant cardiac surgery. Ann Neurol 199537488–497. [DOI] [PubMed] [Google Scholar]
- 16.Limperopoulos C, Majnemer A, Shevell M I.et al Neurologic status of newborns with congenital heart defects before open heart surgery. Pediatrics 1999103402–408. [DOI] [PubMed] [Google Scholar]
- 17.McQuillen P S, Hamrick S E, Perez M J.et al Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation 2006113280–285. [DOI] [PubMed] [Google Scholar]
- 18.Mahle W T, Tavani F, Zimmerman R A.et al An MRI study of neurological injury before and after congenital heart surgery. Circulation 2002106I109–I114. [PubMed] [Google Scholar]
- 19.McQuillen P S, Barkovich A J, Hamrick S E.et al Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke 200738736–741. [DOI] [PubMed] [Google Scholar]
- 20.Galli K K, Zimmerman R A, Jarvik G P.et al Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2004127692–704. [DOI] [PubMed] [Google Scholar]
- 21.Miller S P, McQuillen P S, Vigneron D B.et al Preoperative brain injury in newborns with transposition of the great arteries. Ann Thorac Surg 2004771698–1706. [DOI] [PubMed] [Google Scholar]
- 22.Dent C L, Spaeth J P, Jones B V.et al Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 2006131190–197. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman G M, Stuth E A, Jaquiss R D.et al Changes in cerebral and somatic oxygenation during stage 1 palliation of hypoplastic left heart syndrome using continuous regional cerebral perfusion. J Thorac Cardiovasc Surg 2004127223–233. [DOI] [PubMed] [Google Scholar]
- 24.McQuillen P S, Nishimoto M S, Bottrell C L.et al Regional and central venous oxygen saturation monitoring following pediatric cardiac surgery: concordance and association with clinical variables. Pediatr Crit Care Med 20078154–160. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman G M, Mussatto K A, Brosig C L.et al Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg 20051301094–1100. [DOI] [PubMed] [Google Scholar]
- 26.Rappaport L A, Wypij D, Bellinger D C.et al Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation 199897773–779. [DOI] [PubMed] [Google Scholar]
- 27.Gaynor J W, Jarvik G P, Bernbaum J.et al The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg 2006131181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clancy R R, McGaurn S A, Wernovsky G.et al Risk of seizures in survivors of newborn heart surgery using deep hypothermic circulatory arrest. Pediatrics 2003111592–601. [DOI] [PubMed] [Google Scholar]
- 29.Miller S P, Ferriero D M, Leonard C.et al Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 2005147609–616. [DOI] [PubMed] [Google Scholar]
- 30.Woodward L J, Anderson P J, Austin N C.et al Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006355685–694. [DOI] [PubMed] [Google Scholar]
- 31.Miller G, Mamourian A C, Tesman J R.et al Long‐term MRI changes in brain after pediatric open heart surgery. J Child Neurol 19949390–397. [DOI] [PubMed] [Google Scholar]
- 32.Duggan P J, Maalouf E F, Watts T L.et al Intrauterine T‐cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet 20013581699–1700. [DOI] [PubMed] [Google Scholar]
- 33.Inder T, Mocatta T, Darlow B.et al Markers of oxidative injury in the cerebrospinal fluid of a premature infant with meningitis and periventricular leukomalacia. J Pediatr 2002140617–621. [DOI] [PubMed] [Google Scholar]
- 34.Back S A, Han B H, Luo N L.et al Selective vulnerability of late oligodendrocyte progenitors to hypoxia‐ischemia. J Neurosci 200222455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McQuillen P S, Sheldon R A, Shatz C J.et al Selective vulnerability of subplate neurons after early neonatal hypoxia‐ischemia. J Neurosci 2003233308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Back S A, Luo N L, Borenstein N S.et al Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 2001211302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kostovic I, Judas M, Rados M.et al Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb Cortex 200212536–544. [DOI] [PubMed] [Google Scholar]
- 38.Riddle A, Luo N L, Manese M.et al Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J Neurosci 2006263045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Licht D J, Wang J, Silvestre D W.et al Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J Thorac Cardiovasc Surg 2004128841–849. [DOI] [PubMed] [Google Scholar]
- 40.Miller S P, Ramaswamy V, Michelson D.et al Patterns of brain injury in term neonatal encephalopathy. J Pediatr 2005146453–460. [DOI] [PubMed] [Google Scholar]
- 41.Baud O, Daire J L, Dalmaz Y.et al Gestational hypoxia induces white matter damage in neonatal rats: a new model of periventricular leukomalacia. Brain Pathol 2004141–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jouannic J M, Benachi A, Bonnet D.et al Middle cerebral artery Doppler in fetuses with transposition of the great arteries. Ultrasound Obstet Gynecol 200220122–124. [DOI] [PubMed] [Google Scholar]
- 43.Donofrio M T, Bremer Y A, Schieken R M.et al Autoregulation of cerebral blood flow in fetuses with congenital heart disease: the brain sparing effect. Pediatr Cardiol 200324436–443. [DOI] [PubMed] [Google Scholar]
- 44.Rudolph A.Congenital diseases of the heart: clinical‐physiological considerations. 2nd edn. Armonk, NY: Futura Publishing Company, 2001
- 45.Rosenthal G L. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol 1996143505–513. [DOI] [PubMed] [Google Scholar]
- 46.Glauser T A, Rorke L B, Weinberg P M.et al Congenital brain anomalies associated with the hypoplastic left heart syndrome. Pediatrics 199085984–990. [PubMed] [Google Scholar]
- 47.Miller S P, Vigneron D B, Henry R G.et al Serial quantitative diffusion tensor MRI of the premature brain: Development in newborns with and without injury. J Magn Reson Imaging 200216621–632. [DOI] [PubMed] [Google Scholar]
- 48.Inder T E, Huppi P S, Warfield S.et al Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol 199946755–760. [DOI] [PubMed] [Google Scholar]
- 49.Partridge S C, Vigneron D B, Charlton N N.et al Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol 200659640–651. [DOI] [PubMed] [Google Scholar]