Abstract
Background
Positive pressure ventilation in premature infants can improve oxygenation but may diminish cerebral blood flow and cardiac output. Low superior vena cava (SVC) flow increases risk of intraventricular haemorrhage, and higher mean airway pressure is associated with low SVC flow. Whether this is a direct effect of positive pressure ventilation or a reflection of severity of lung disease is not known. This study aimed to determine if positive end expiratory pressure (PEEP) in ventilated newborns could be increased without clinically relevant cardiorespiratory changes.
Method
Ventilated newborns were studied before and 10 min after increasing PEEP (5 cm H2O to 8 cmH2O) and again when PEEP returned to baseline. Echocardiographic and respiratory function measurements were collected during the intervention.
Results
In 50 infants, increased PEEP was associated with a non‐significant difference in mean SVC flow of −5 ml/kg/min (95% CI −12 to 3 ml/kg/min) but a significant reduction in right ventricular output of 17 ml/kg/min (95% CI 5 to 28 ml/kg/min). The increase in lung compliance was non‐significant (median difference 0.02 ml/cmH2O/kg) and the decrease in lung resistance (18 cmH2O/l/s; 95% CI 10 to 26 cm H2O/l/s) was significant. Changes (%) in lung compliance and SVC flow, when corrected for Paco2, were positively associated (regression coefficient 0.4%; 95% CI 0.2% to 0.6%).
Conclusion
A short‐term increase in PEEP does not lead to significant changes in systemic blood flow, although 36% of infants in the present study had clinically important changes in flow (±25%). The intervention can improve dynamic lung function, especially airway resistance. Improvements in compliance tend to be associated with improvements in blood flow.
Low systemic blood flow is common in babies born before 30 weeks and is associated with considerable morbidity. Therefore an understanding of the factors that might affect blood flow in this population is essential.1,2 Previous studies have shown that higher mean airway pressure is a determinant of low superior vena cava (SVC) flow.3 Whether this is a direct effect of positive pressure ventilation on reducing systemic venous return, as suggested by animal studies, or a reflection of severity of lung disease, is not known.4,5
Positive end expiratory pressure (PEEP) is used to increase end expiratory lung volume to improve arterial oxygen content, decrease the alveolar to arterial oxygen difference, and decrease shunt fraction by preventing alveolar collapse.6,7 Most neonatal intensive care units use a static level of PEEP, varying between 3 cm H2O and 8 cm H2O. The haemodynamic safety of different PEEP levels in a clinical setting has not been investigated yet. Theoretically there is potential for higher PEEP, as by opening up the lungs, it may reduce pulmonary vascular resistance and increase pulmonary and systemic blood flow. Our goal was to study whether end expiratory pressure in ventilated newborns could be increased from 5 cm H2O to 8 cm H2O without causing clinically relevant cardiorespiratory changes.
Methods
All newborn infants admitted to RPA Newborn Care between April 2005 and March 2006, who required assisted ventilation in the first 3 days, were eligible. We excluded infants if they had a major congenital malformation, severe perinatal asphyxia defined as a Sarnat score greater than 2 at any point, or severe hypoxia with fractional inspired oxygen (Fio2) more than 80%. Infants with peak inspiratory pressure below 12 cm H2O with a Fio2 of 21% were also excluded as increasing PEEP in these infants might compromise tidal volume. We obtained informed consent from the parents of eligible infants, and the study was approved by the local ethics committee.
Infants received surfactant as soon as possible after birth on the basis of a surfactant function test (click test) performed on an early tracheal aspirate.8 Ventilator strategies included adjusting the peak inspiratory pressure to achieve an expiratory tidal volume of 4 ml/kg with the rate adjusted to achieve anarterial carbon dioxide pressure (Paco2) of 40–55 mm Hg, and Fio2 adjusted to maintain preductal oxygen saturation between 88% and 95%. At our institution, PEEP is maintained at 5 cm H2O in all infants. Morphine is used selectively to sedate babies considered to be uncomfortable on the ventilator. Circulatory support interventions were not changed during the period of the study.
Doppler echocardiographic measurements were done by using an Aspen ultrasound machine (Siemens Medical, Germany) with a 7 MHz vector array transducer incorporating colour flow and pulsed wave Doppler. SVC flow, Doppler measurement of right ventricular output (RVO), left pulmonary artery velocity, colour Doppler diameter of ductus arteriosus shunt and size, and Doppler of the middle cerebral arteries (MCAs) were performed according to previously published methods.9,10 Doppler assessment of pulmonary artery pressure was also performed using the time to peak velocity divided by the right ventricular ejection time (TPV/RVET).11 All measurements were performed by one investigator (KW).
Blinding at the time of measurement was achieved by allocating each study a random number drawn from a sealed envelope. This number was the only identifier on the ultrasound screen. The scans were recorded to different magnetic optical disks, and measurements were done as a batch away from the bedside at a later time. The intervention was carried out and the data collected, if possible, between 3 h and 9 h of age. Echocardiographic and other parameters were recorded at the initial setting of 5 cm H2O PEEP, after an increase of PEEP to 8 cm H2O for 10 min and 10 min after PEEP was returned to 5 cm H2O. An arterial blood gas was drawn just before the start of the protocol and before the intervention measurement. If no arterial line was in place, transcutaneous measurements of Paco2 and Pao2 were noted. Respiratory function variables (dynamic lung compliance, mean airway pressure, minute volume, Fio2) were measured using the Babylog 8000 ventilator (Dräger, Germany) and were collected together with physiological variables (heart rate, blood pressure) from the Agilent monitor (Phillips, The Netherlands). Average respiratory function variables were calculated from 30 s interval measurements. Respiratory function measurements were excluded if the tube leak was more than 25%. Exit criteria to stop the intervention were an increase in Fio2 more that 30% from baseline or a drop in mean blood pressure of 20% from baseline.
Statistical analysis
Our hypothesis was that an increase in PEEP would not result in a remarkable change in SVC flow of more than 25% from baseline. The cut‐off of 25% was chosen to allow for measurement error that was documented in previous studies and to allow for clinically unimportant changes in SVC flow. We statistically analysed the data with SPSS (version 12.0) for Windows.
General linear models with repeated measurements were performed for normally distributed outcomes to test the within subjects effects at the three measurement time points. Friedman test was used for non‐parametric outcomes, paired t test was used to estimate mean differences in normally distributed outcomes and Wilcoxon signed rank test was used for non‐parametric outcomes after the increase in PEEP compared with baseline. Linear regression was used to identify risk factors for changes in flow before and after the increase in PEEP and to evaluate the possible interaction or confounding effect of gestational age, postnatal age and Paco2 on the relation between PEEP increase and flow changes. Statistical significance was set at 5%. One‐way analysis of variance was used to identify risk factors for changes in compliance. Groups were divided on the basis of a clinically relevant change in compliance of 10% (no clinically relevant change, difference >10% or ⩽10%). The association between severity of lung disease (mean airway pressure, compliance, resistance, FiO2) and flow changes was evaluated with linear regression analysis.
Results
The study included 50 infants (40 preterm and 10 term) with a median gestational age of 30 weeks and a median weight of 1612 g. Of the preterm infants, 22 were less than 30 weeks' gestation. All preterm infants were ventilated because of respiratory distress syndrome (RDS) and received surfactant. Two of the term infant were ventilated for meconium aspiration, five for non‐specific lung disease with a positive click test for surfactant function, two for pneumonia/sepsis and one infant for mild perinatal asphyxia. Nine infants had their first study after 24 h or 48 h of life because they were transported to our centre or they were not ventilated in the first 24 h of life.
Tables 1 and 2 show the cardiovascular and pulmonary function measurements before, during and after the increase in PEEP. In eight infants, some indices of flow were not measurable due to poor windows, but SVC flow was always measurable. Pathologically low measures of SVC and RVO are defined as less than 40 ml/kg/min and 120 ml/kg/min, respectively.12 No infant met the exit criteria during the study.
Table 1 Cardiovascular response to an increase in positive end expiratory pressure (PEEP) in all 50 infants*.
| Baseline PEEP 5 cmH2O | Intervention PEEP 8 cmH2O | Return to PEEP 5 cmH2O | p Value | |
|---|---|---|---|---|
| Right ventricular output (ml/kg/min) | 234 (103) | 218 (95) | 224 (87) | 0.014 |
| SVC (ml/kg/min) | 92 (36) | 87 (36) | 86 (34) | 0.207 |
| SVC diameter (mm) | 3.7 (1.0) | 3.6 (1.1) | 3.6 (1.0) | 0.040 |
| SVC velocity time integral (m/s) | 0.093 (0.032) | 0.097 (0.035) | 0.090 (0.029) | 0.172 |
| Left pulmonary artery velocity (m/s) | 0.33 (0.11) | 0.32 (0.13) | 0.32 (0.12) | 0.669 |
| Duct diameter (mm) | 2.2 (1.6–3.0) | 2.2 (1.9–28) | 2.1 (1.8–3.1) | 0.628 |
| Ductal shunt (%RL) | 14 (0–25) | 12 (0–27) | 15 (0–28) | 0.760 |
| Middle cerebral artery mean (m/s) | 0.13 (0.09–0.17) | 0.14 (0.10–0.18) | 0.12 (0.09–0.16) | 0.190 |
| Heart rate (beats/min) | 143 (17) | 141 (20) | 143 (19) | 0.198 |
| Systolic blood pressure (mm Hg) | 47 (9) | 48 (9) | 47 (9) | 0.265 |
| Diastolic blood pressure (mm Hg) | 30 (7) | 30 (6) | 30 (6) | 0.602 |
%RL, percentage of cardiac cycle with right to left shunt; SVC, superior vena cava flow; Vti, velocity time integral.
*Values expressed as mean (SD) or median (IQR) where appropriate.
Table 2 Pulmonary response to an increase in positive end expiratory pressure (PEEP) in all 50 infants*.
| Baseline PEEP 5 cm H2O | Intervention PEEP 8 cm H2O | Return to PEEP 5 cm H2O | p Value | |
|---|---|---|---|---|
| Mean airway pressure (cm H2O) | 8.5 (7.3–10.0) | 11.0 (9.6–12.3) | 8.6 (7.2–10.0) | 0.000 |
| Tidal volume† (ml/kg) | 4.1 (3.4–4.9) | 3.3 (2.7–4.3) | 4.1 (3.5–5.3) | 0.000 |
| Compliance† (ml/cmH2O/kg) | 0.43 (0.36–0.69) | 0.53 (0.33–0.81) | 0.45 (0.30–0.67) | 0.409 |
| Resistance† (cmH2O/l/s) | 96 (41) | 78 (33) | 93 (40) | 0.000 |
| Partial pressure of carbon dioxide (mmHg) | 44 (12) | 47 (11) | NA | 0.002 |
| Partial pressure of oxygen (mmHg) | 75 (63–99) | 80 (63–99) | NA | 0.884 |
| Fractional inspired oxygen (%) | 25 (21–34) | 25 (21–31) | 25 (21–30) | 0.043 |
NA, not available.
*Values expressed as mean (SD) or median (IQR) where appropriate.
†Tidal volume, compliance and resistance are reported for 36 infants with less than 25% tube leak.
Effect of higher PEEP on SVC flow and RVO
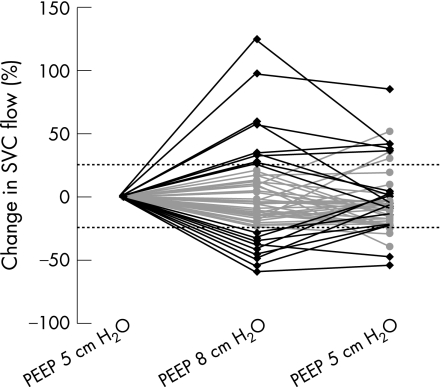
A change in SVC flow of more than 25% from baseline was found in 18 (36%) infants, with 9 infants having increased flows and 9 decreased flows (fig 1). Overall, after the increase in PEEP there was a non‐significant difference in SVC flow of −5 ml/kg/min (95% CI −12 to 3 ml/kg/min) or −0.7% (95% CI −10.7% to 9.2%) due to a small but significant decrease of 1.6 mm (95% CI 0.3 to 2.9 mm) in SVC diameter. The intervention caused a significant reduction in RVO with a mean of 17 ml/kg/min (95% CI 5 to 28 ml/kg/min) or 5% (95% CI 0% to 10%). Gestational age was not found to be an interaction factor or confounding factor in these analyses.
Figure 1 Change in superior vena cava (SVC) flow (%) with the intervention. Black lines show more than 25% change between 8 cm H2O of positive end expiratory pressure (PEEP) and baseline.
Gestational age, birth weight, baseline RVO, mean airway pressure, Fio2, lung compliance or resistance did not predict the direction of the change in SVC flow or RVO. There was a negative association between baseline SVC flow and change in SVC flow after the increase in PEEP (linear regression coefficient −0.3 ml/kg/min; 95%CI −0.5 to −0.1 ml/kg/min). Postnatal age (hours) was positively associated with change in RVO after the increase in PEEP (regression coefficient 0.8 ml/kg/min; 95% CI 0.1 to 0.5 ml/kg/min).
Effect of higher PEEP on other haemodynamic measurements
In 40 infants the ductus arteriosus was patent, with a median ductal diameter of 2.2 mm. The increase in PEEP had no significant effect on the ductal size or the proportion of ductal flow going right to left, a marker of relative pressure between the systemic and pulmonary system. The intervention also did not significantly change the left pulmonary artery mean velocity. As an assessment of pulmonary pressure, we calculated the time to peak velocity divided by the right ventricular ejection time (TPV/RVET). It showed no significant change with the intervention and there was no association between the change in RVO or LPA mean velocity and the change in TPV/RVET. The change in MCA mean velocity after the increase in PEEP was not significant (median difference 0.003 m/s; p = 0.337).
Effect of higher PEEP on respiratory function
Valid analysis of respiratory outcomes was possible in 36 infants who had a tube leak less than 25% (see table 2). Dynamic lung compliance showed a non‐significant increase (median difference 0.02 ml/cm H2O/kg; p = 0.093), but lung resistance was significantly decreased after 10 min of higher PEEP (mean difference at 10 min −18 cm H2O/l/s; 95% CI −26 to −10 cm H2O/l/s). Both compliance and resistance returned to their baseline values within 10 min of the PEEP returning to 5 cm H2O.
Significant associations were found between the following baseline characteristics and change in compliance with the intervention: mean airway pressure (F(2,35) = 3.3, p = 0.049), minute volume (F(2,27) = 6.3, p 0.006) and resistance (F(2,35) = 3.8, p = 0.032). Infants who showed a decrease of more than 10% with the intervention had a higher mean airway pressure, higher minute volume and a lower resistance. This may point to overdistension as the cause for the decrease in compliance with an increase in PEEP.
An increase in PEEP resulted in a significant increase in Paco2 (mean difference 3.0 mm Hg; 95% CI 1.2 to 4.8 mm Hg). Linear regression showed no significant effect of Paco2 changes on changes in RVO, MCA mean velocity or ductal shunting. An increase in Paco2 was, however, associated with a small but significant increase in SVC flow (regression coefficient 1.3 ml/kg/min; 95% CI 0.0 to 2.5 ml/kg/min).
Respiratory function and flow
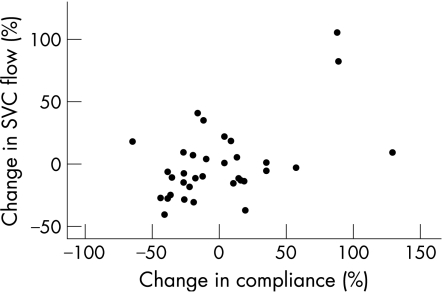
We explored the relationship between changes in respiratory function and changes in flow and found two weak associations. First, an increase of 1% in compliance was associated with a 0.4% (95% CI 0.1% to 0.6%) increase in SVC flow (fig 2). Second, there was a positive association between Paco2 and MCA mean velocity (regression coefficient 0.002 m/s; 95% CI 0.001 to 0.004 m/s), but the association between changes in Paco2 and MCA mean velocity with the intervention was not statistically significant (regression coefficient 0.001; (95% CI −0.001 to 0.003).
Figure 2 Partial regression scatterplot of change in superior vena cava (SVC) flow (%) and change in lung compliance (%) if corrected for Paco2 changes with 8 cm H2O of positive end expiratory pressure (PEEP) compared with baseline.
Discussion
What is already known on this topic
Higher PEEP can optimise lung inflation and improve oxygenation in ventilated newborns.
Higher PEEP can diminish venous return, hence cardiac output and consequently cerebral blood flow.
What this study adds
A short‐term modest increase in PEEP reduces RVO but does not lead to a remarkable change in systemic blood flow in most infants.
Infants with improvements in compliance in response to higher PEEP tend to have improvements in SVC flows.
We have shown that an increase of PEEP from 5 cm H2 to 8 cmH2O for 10 min did not lead to a clinically relevant change in upper body and brain blood flow (SVC flow) in most of the infants in the present study. It was associated with a significant decrease in RVO but there were no clinically relevant changes in any of the other haemodynamic measurements. Improvements in SVC flow were associated with improvements in lung compliance, although this analysis is sensitive to the method of analysis.
In contrast to the literature, our study found relatively little consistent change in the haemodynamic measurements overall.13,14 Potential reasons for this include our heterogeneous study population, or the impact of our intervention. Possibly, an increase of 5 cm H2O to 8 cmH2O in PEEP was not large enough to cause consistent negative effects on blood flow as reported in the literature. Few infants had low systemic blood flows. We expected that infants with low baseline flow would show a larger decrease in flow with the intervention, but they were more likely to have an increase in SVC flow at 8 cmH2O of PEEP. This may be a regression to the mean.
The decrease in RVO of −17 ml/kg/min was significant, but we are not sure if this is of clinical importance considering the normal baseline RVO in this population. In six of the seven babies with low baseline RVO (<120 ml/kg/min) the intervention resulted in no detectable change, but a potential adverse effect of higher PEEP should be evaluated more thoroughly with further research, especially in infants with low flow.
Probably the most important haemodynamic effect of PEEP in preterm infants with respiratory distress syndrome is through the direct effect on alveoli. When PEEP is set too low, blood will be shunted away from collapsed alveoli which produce a regional increase in pulmonary vascular resistance. With optimal lung volume, lung vascular resistance will be at its lowest point, thus maximising RVO and cardiac input. PEEP is capable of optimising lung volume by keeping open (partially) collapsed alveoli. The intervention performed in this study is not considered a lung recruitment manoeuvre, and we can only speculate about lung volumes in the studied infants.15 Lung volume is also related to airway resistance. At low lung volumes due to insufficient PEEP, airway resistance and the work of breathing are high.
In this study compliance increased with the maximum increase after 10 min of PEEP of 8 cmH2O. It can take up to 14 min for the alveoli to stablise after a change in PEEP.16 However, the evidence on the effect of PEEP on compliance is conflicting.17,18,19 Compliance can only be used as a measurement to optimise ventilation without decreasing blood flow, if it is combined with information on lung volume. Two studies investigating lung compliance and blood flow simultaneously in newborn infants showed a progressive decrease in cardiac output with less decrease in the infants with low compliance.13,14 We found a reduction in RVO, but we did not find a significant positive association between an increase in compliance and an increase in RVO as we did for SVC flow. This could be caused by differences in flow measurements. In RVO, we looked at the change in velocity and assumed the diameter did not change, whereas change in SVC depended on change in diameter. This suggests that RVO, although perhaps not such a consistent measure of systemic blood flow as SVC, is less vulnerable to measurement error if only change in velocity is assessed. It may be a better method to detect short‐term changes such as in this study.20
Although our intervention seems to be only a minor increase in mean airway pressure, modest changes in PEEP can result in a clinically important decrease in tidal volume or functional residual capacity.21 This could explain the increase in Paco2 found in our study. Other studies suggest that the increase in Paco2 is responsible for an increase in cerebral blood flow.22,23 The relationship between Paco2 and cerebral blood flow is exponential and a normal Paco2–cerebral blood flow reactivity is about 4% per mm Hg.24 In this study, the increase in Paco2 was associated with an increase in SVC flow, but not with an increase in RVO or MCA mean velocity which makes the Paco2–SVC flow relationship difficult to interpret. The potential negative effect of PEEP on RVO could have been balanced by the potential positive effect of a higher Paco2 on cerebral blood flow and SVC flow.
Conclusion
A short‐term modest increase in PEEP reduces RVO, but does not lead to a clinically important change in systemic blood flow in most infants. In the present study, clinically significant changes (both positive and negative) in systemic blood flow were found in 36% of the infants. Improvements in lung compliance were associated with improvements in SVC flow. The association between ventilation and blood flow is a complex one that needs more study. Future research should focus on more sick infants, especially those with low blood flows, and on the effect of a larger intervention, such as lung recruitment manoeuvres.
Acknowledgement
We thank Dr J H vd Lee, Deparment of Pediatric Clinical Epidemiology Emma Children's Hospital AMC, Amsterdam, for statistical advice.
Abbreviations
Fio2 - fractional inspired oxygen
MCA - middle cerebral artery
Paco2 - arterial carbon dioxide pressure
PEEP - positive end expiratory pressure
RVO - right ventricular outflow
SVC - superior vena cava
TPV/RVET - time to peak velocity divided by the right ventricular ejection time
Footnotes
Funding: Koert A de Waal was partially funded by the Ter Meulen Fund, Royal Netherlands Academy of Arts and Sciences.
Competing interests: None.
References
- 1.Meek J H, Tyszczuk L, Elwell C E.et al Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch Dis Child Fetal Neonatal Ed 199981F15–F18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed 200082F188–F194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborn D A, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics 2003112(1 Pt 1)33–39. [DOI] [PubMed] [Google Scholar]
- 4.Mirro R, Busija D, Green R.et al Relationship between mean airway pressure, cardiac output, and organ blood flow with normal and decreased respiratory compliance. J Pediatr 1987111101–106. [DOI] [PubMed] [Google Scholar]
- 5.Mundie T G, Easa D, Finn K C.et al Effect of baseline lung compliance on the subsequent response to positive end‐expiratory pressure in ventilated piglets with normal lungs. Crit Care Med 1994221631–1638. [PubMed] [Google Scholar]
- 6.Ranieri V M, Eissa N T, Corbeil C.et al Effects of positive end‐expiratory pressure on alveolar recruitment and gas exchange in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 1991144(3 Pt 1)544–551. [DOI] [PubMed] [Google Scholar]
- 7.Michna J, Jobe A H, Ikegami M. Positive end‐expiratory pressure preserves surfactant function in preterm lambs. Am J Respir Crit Care Med 1999160634–639. [DOI] [PubMed] [Google Scholar]
- 8.Osborn D A, Jeffery H E, Bredemeyer S L.et al Targeted early rescue surfactant in ventilated preterm infants using the click test. Pediatrics 2000106E30. [DOI] [PubMed] [Google Scholar]
- 9.Evans N, Kluckow M, Simmons M.et al Which to measure, systemic or organ blood flow? Middle cerebral artery and superior vena cava flow in very preterm infants. Arch Dis Child Fetal Neonatal Ed 200287F181–F184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Hajjar M, Vaksmann G, Rakza T.et al Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed 200590F419–F422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su B H, Watanabe T, Shimizu M.et al Doppler assessment of pulmonary artery pressure in neonates at risk of chronic lung disease. Arch Dis Child Fetal Neonatal Ed 199777F23–F27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans N. Which inotrope for which baby? Arch Dis Child Fetal Neonatal Ed 200691F213–F220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hausdorf G, Hellwege H H. Influence of positive end‐expiratory pressure on cardiac performance in premature infants: a Doppler‐echocardiographic study. Crit Care Med 198715661–664. [DOI] [PubMed] [Google Scholar]
- 14.Trang T T, Tibballs J, Mercier J C.et al Optimization of oxygen transport in mechanically ventilated newborns using oximetry and pulsed Doppler‐derived cardiac output. Crit Care Med 1988161094–1097. [DOI] [PubMed] [Google Scholar]
- 15.De Jaegere A, van Veenendaal M B, Michiels A.et al Lung recruitment using oxygenation during open lung high‐frequency ventilation in preterm infants. Am J Respir Crit Care Med 2006174639–645. [DOI] [PubMed] [Google Scholar]
- 16.Thome U, Topfer A, Schaller P.et al The effect of positive endexpiratory pressure, peak inspiratory pressure, and inspiratory time on functional residual capacity in mechanically ventilated preterm infants. Eur J Pediatr 1998157831–837. [DOI] [PubMed] [Google Scholar]
- 17.Philips J B, Beale E F, Howard J E.et al Effect of positive end‐expiratory pressure on dynamic respiratory compliance in neonates. Biol Neonate 198038270–275. [DOI] [PubMed] [Google Scholar]
- 18.Dinger J, Topfer A, Schaller P.et al Effect of positive end expiratory pressure on functional residual capacity and compliance in surfactant‐treated preterm infants. J Perinat Med 200129137–143. [DOI] [PubMed] [Google Scholar]
- 19.Dimitriou G, Greenough A, Laubscher B. Appropriate positive end expiratory pressure level in surfactant‐treated preterm infants. Eur J Pediatr 1999158888–891. [DOI] [PubMed] [Google Scholar]
- 20.Tsai‐Goodman B, Martin R P, Marlow N.et al The repeatability of echocardiographic determination of right ventricular output in the newborn. Cardiol Young 200111188–194. [DOI] [PubMed] [Google Scholar]
- 21.Bartholomew K M, Brownlee K G, Snowden S.et al To PEEP or not to PEEP? Arch Dis Child Fetal Neonatal Ed 199470F209–F212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shortland D B, Field D, Archer L N.et al Cerebral haemodynamic effects of changes in positive end expiratory pressure in preterm infants. Arch Dis Child 198964465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullaart R A, Hopman J C, Rotteveel J J.et al Influence of end expiratory pressure on cerebral blood flow in preterm infants. Early Hum Dev 199540157–165. [DOI] [PubMed] [Google Scholar]
- 24.Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev 200581423–428. [DOI] [PubMed] [Google Scholar]