Abstract
Objective
With changes in the predominant pathogenic factors in the new form of bronchopulmonary dysplasia (BPD), a different pattern of CT findings may be expected. This study aimed to (1) describe CT findings in infants with BPD and (2) correlate the CT findings with lung function abnormalities.
Study design and method
Retrospective review of 41 very low birthweight infants with BPD, who were referred for pulmonary investigations at between 10 and 20 months after birth because of persistent respiratory symptoms, and underwent CT and lung function tests.
Results
None of the infants had normal CT findings. The most frequent abnormalities were hyperlucent areas (n = 36; 88%), linear opacities (n = 39; 95%), and triangular subpleural opacities (n = 26; 63%). Bronchiectasis was not seen. None of the CT abnormalities correlated with the maximum expiratory flow at functional residual capacity (VmaxFRC). In contrast, increased number of subpleural opacities and limited linear opacities were associated with low FRC and longer duration of neonatal oxygen exposure. The numbers of triangular subpleural opacities also correlated with duration of mechanical ventilation.
Conclusions
Despite advances in neonatal care, many CT findings in infants with BPD are similar to those observed in the pre‐surfactant era, and are still associated with duration of supplemental oxygen and mechanical ventilation. The absence of bronchial involvement in the present study was the most striking difference from previous studies.
Despite considerable obstetric and neonatal advances in the care of very low birthweight infants, bronchopulmonary dysplasia (BPD) continues to occur in 25–30% of surviving infants.1 BPD was initially described in premature infants who were supported with only supplemental oxygen and mechanical ventilation. As new treatment options became available, the characteristics of BPD changed. Before the surfactant treatment era, airway injury, inflammation and parenchymal fibrosis were the prominent findings in BPD.2,3 The “new” BPD seems to be a result of arrested lung development, characterised by abnormal alveolar septation and microvascular maturation. Husain and colleagues characterised this “new” BPD on the basis of autopsy lung specimens from surfactant‐treated infants with BPD.4 The consistent findings were negligible epithelial changes in the airways and a simplified distal lung due to impaired alveolarisation.4,5 The reduced risk of severe airway injury and the primary importance of arrested lung growth are associated with modifications in long‐term alterations in lung function tests. Small lung volumes are now a characteristic feature of infants with BPD whereas severe chronic airway obstruction is no longer seen.6,7 Similar to the changes seen in lung function tests, a different pattern of CT findings may be expected with changes in the main pathogenic factors of BPD. Previous CT findings included reticular opacities, multifocal areas of reduced density and marked bronchial wall thickening.8,9,10
No recent study has reported both the results of lung function tests and CT evaluation in infants with BPD. Here we report on CT lesions in 1–2‐year‐old infants with BPD who were born before 32 weeks' gestation and underwent investigations for persistent respiratory symptoms. We also correlated the CT findings with lung function abnormalities and examined the relative contributions of birth weight, gestational age and neonatal respiratory illness to the CT and lung function test findings.
Patients and methods
Study design
We retrospectively studied infants with BPD who were referred to our centre because of persistent respiratory symptoms and who underwent CT and lung function tests.
Patients
The study population consists of 41 infants, born between January 1999 and March 2001, who needed oxygen supplementation for at least 28 days and were referred to our centre because of uncontrolled respiratory symptoms. According to the definition proposed by Jobe and Bancalari, the infants were classified as having mild, moderate or severe BPD according to the need for supplemental oxygen at 36 weeks' postmenstrual age (PMA).3 Twenty‐two infants were breathing room air at 36 weeks' PMA and had mild BPD, whereas 19 infants still needed supplemental oxygen at 36 weeks' PMA and had moderate or severe BPD. All the infants had experienced several exacerbations during the previous 6 months, and underwent CT and lung function tests. Respiratory exacerbation was defined as the need for oral corticosteroids, emergency visits or hospitalisation. Infants with one or more respiratory exacerbation a month during the previous 6 months (n = 21) were considered to have frequent symptoms. At the time of this evaluation, the mean (SEM) chronologic age was 16.0 (3.2) months (range 10.6–20.2 months). Mean gestational age and birth weight were 27.2 (0.2) weeks (range 23.5–30.8 weeks) and 914 (37) g (range 540–1490 g), respectively. Six infants were small for gestational age, as defined by a birth weight below the fifth percentile; 10 infants had proved maternofetal infection; and 17 had a postnatal infection, defined as either positive blood culture or empirical antibiotic treatment indicated for tracheal microorganisms, clinical deterioration, changes in biological parameters or evidence of pneumonitis in the chest x ray. Other features of the study population are summarised in table 1.
Table 1 Characteristics of the 41 neonates participating in the present study.
| All infants (n = 41) | Infants with frequent symptoms (n = 21) | Infants with infrequent symptoms (n = 20) | |
|---|---|---|---|
| Birth weight (g), mean (SEM) | 914 (37) | 915 (51) | 913 (55) |
| Gestational age (weeks), mean (SEM) | 27.2 (0.2) | 27.3 (0.4) | 27.0 (0.3) |
| Hospital admissions in previous 6 months, n (%) | 14 (34) | 11 (52) | 3 (15)* |
| Inhaled glucocorticoids for ⩾6 months, n (%) | 22 (54) | 10 (48) | 12 (60) |
| Surfactant‐treated newborns, n (%) | 28 (68) | 13 (62) | 15 (75) |
| Patent ductus arteriosus, n (%) | 12 (29) | 7 (33) | 5 (25) |
| Intrauterine growth retardation, n (%) | 6 (5) | 2 (10) | 4 (20) |
| Maternofetal infection, n (%) | 11 (27) | 6 (29) | 5 (25) |
| Postnatal infection, n (%) | 17 (41) | 7 (33) | 10 (50) |
| Duration of oxygen supplementation (days), mean (SEM) | 68 (6) | 65 (7) | 73 (8) |
| PMA at end of oxygen supplementation (weeks), mean (SEM) | 37.0 (0.7) | 36.5 (0.9) | 37.5 (1.1) |
| PMA at end of mechanical ventilation (days), mean (SEM) | 29.5 (0.4) | 29.1 (0.4) | 29.8 (0.7) |
PMA, postmenstrual age.
The study population was divided into two subgroups according to the intensity of clinical manifestations at the time of the study. Infants with frequent symptoms were those with one or more respiratory exacerbation a month.
*p<0.05 between the two subgroups.
Lung function tests
The maximum expiratory flow at functional residual capacity was assessed by the squeeze technique and has been previously described.11 Briefly, the infants were sedated with chloral hydrate (75 mg/kg). Maximal partial expiratory flow volume (PEFV) was determined with the squeeze technique by rapidly inflating a thoracoabdominal jacket at different pressure levels at the beginning of expiration (Medical Engineering Department, Royal Postgraduate Medical School, Hammersmith Hospital, London). The jacket was wrapped around the infant's chest and abdomen, and the neck was extended to minimise airway and glottic obstruction. All measurements and calculations were done using a paediatric mobile measurement module (SensorMedics Corporation 2600, Yorba Linda, California, USA). Flow was measured at the infant's mouth, using a facemask attached to a 0–30 LPM triple‐screen pneumotachograph with a flow resolution of 0.06 ml/s, a volume resolution of 0.12 ml, and a volume range of 0–255 ml. Forced expiration was measured as the maximum expiratory flow at functional residual capacity (VmaxFRC). The FRC level was defined as the end‐expiratory level obtained from the respiratory cycle preceding the forced expiratory test. Three partial expiratory flow volume (PEFV) curves were produced for each pressure level, and the mean baseline value was determined from the highest three of six technically acceptable values. Criteria for an acceptable PEFV curve included a rapid rise in forced expiratory flow so that peak flow occurred before 50% of the tidal volume was expired. Data were expressed as absolute values and as z scores after adjustment for height.12
The FRC was measured by helium dilution. The measurements were made with the infants in supine position and breathing into the closed helium dilution circuit via a facemask. Helium equilibration was assumed to be complete when the concentration remained constant. FRC was expressed as the absolute value, the absolute value per kg of body weight and the z score after adjustment for height.13
Computed tomography
High‐resolution CT scans were obtained on sedated infants (pentobarbital 5 mg/kg intrarectally) with a Prospeed Advantage scanner (General Electric Medical System, Milwaukee, Wisconsin, USA) and the following parameters: 1 mm sections at 6 mm intervals, bone E‐3 enhanced algorithm, 512×512 matrix, 120 kpV, 100 mAs, and a scan time of 1.0 s, from the apices of the lungs to their bases. Lung windows were photographed at a window width of 1600 Hounsfield units (HU) and a window level of 600 HU. Two experienced reviewers analysed the CT findings qualitatively by describing the most consistently found lesions and their frequencies: hyperlucent areas and bullae, linear opacities and triangular subpleural opacities, bronchiectasis and bronchiolectasis. The reviewers were unaware of the infants' neonatal history.
Hyperlucent areas were defined as areas of decreased density in which parenchymal structures (vessels and bronchi) could be identified. They were recorded as large if their size exceed 2 cm. Bullae were defined as rings free of parenchymal structures. Linear opacities were defined as a reticular pattern of condensed parenchymal structures and were recorded as extensive or limited. Extensive linear opacities were observed on three adjacent slices (vertical extension) or exceeded two‐thirds of the lung cross‐sectional area (horizontal extension). Triangular subpleural opacities were recorded if a linear opacity with an external base and an internal apex was noted adjacent to the pleura. Bronchiectasis and bronchiolectasis were recorded if the internal diameter of an airway was larger than that of an adjacent pulmonary artery branch. We evaluated inter‐reviewer agreement using the correlation z test. The agreement ranged from 82.7% (95% CI 65.7% to 92.7%; p<0.0001) for small hyperlucent areas to 94.7% (88.7% to 97.5%; p<0.0001) for large hyperlucent areas. When all the findings were pooled, the correlation between two reviewers was 89.7% (85.3% to 92.8%; p<0.0001).
Statistical analysis
Quantitative data are expressed as mean (SEM). Univariate analysis of all potential risk factors for CT or lung function abnormalities was based on the χ2 test for qualitative variables and analysis of variance for quantitative variables. We tested the following factors: birth weight, gestational age, intrauterine growth retardation, maternofetal infection, postnatal infection, duration of oxygen exposure, PMA at the end of oxygen supplementation, and PMA at the end of mechanical ventilation.
Results
We obtained CT scans for all 41 infants, but lung function data could not be obtained for three infants (parents refusal (one infant) and unreliable data because of glottis closure (two infants)). Mean (SEM) age, weight and height at the time of the investigations was 16 (3.2) months, 8.6 (1.6) kg and 73 (4.5) cm, respectively.
Lung function test results
Most of the infants had low VmaxFRC values suggesting an obstructive pattern; 63% had a VmaxFRC z score below −1 (median −1.2). Mean VmaxFRC was 148 (11) ml/s (range 33–350 ml/s).
We were able to measure FRC in only 31 infants, because of early waking after sedation. Mean FRC was 207 (10) ml (range 100–309 ml). The median z score was −0.5, showing that most of our study population had low FRC values, suggesting a restrictive pattern. Although VmaxFRC values tended to be lower in infants with lower FRC values, there was no correlation between FRC and VmaxFRC z scores. Strong correlations were observed between FRC measurements and oxygen exposure. FRC z scores correlated negatively with the duration of oxygen exposure (r = −0.596; p = 0.0004), and with PMA at the end of oxygen supplementation (r = −0.593; p = 0.0003). None of the neonatal risk factors were significantly associated with VmaxFRC. Lung function variables did not differ significantly between the infants with frequent and infrequent symptoms (table 2).
Table 2 CT findings and lung function test results of the 41 neonates participating in the study.
| All infants (n = 41) | Infants with frequent symptoms (n = 21) | Infants with infrequent symptoms (n = 20) | |
|---|---|---|---|
| Subpleural opacities, n (%) | 26 (63) | 12 (57) | 14 (70) |
| Subpleural opacities per infant, mean (SEM) | 1.1 (0.2) | 1.1 (0.3) | 1.1 (0.2) |
| Extended linear opacities, n (%) | 24 (59) | 11 (52) | 13 (65) |
| Limited linear opacities, n (%) | 39 (95) | 19 (90) | 20 (100) |
| Limited linear opacities per infant, mean (SEM) | 5.0 (0.5) | 4.3 (0.6) | 5.8 (0.6) |
| Large hyperlucent area(s), n (%) | 14 (34) | 7 (33) | 7 (35) |
| Presence of bullae, n (%) | 21 (51) | 9 (43) | 12 (60) |
| FRC (ml), mean (SEM) | 207 (10) | 207 (14) | 207 (14) |
| FRC (ml/kg), mean (SEM) | 23.3 (1.0) | 23.0 (1.4) | 23.6 (1.5) |
| FRC (z score), mean (SEM) | 0.1 (0.4) | 0.2 (0.7) | −0.04 (0.5) |
| VmaxFRC (ml/s), mean (SEM) | 148 (11) | 135 (14) | 160 (18) |
| VmaxFRC (z score), mean (SEM) | −1.3 (0.2) | −1.4 (0.2) | −1.2 (0.2) |
The study population is divided into two subgroups according to the intensity of clinical manifestations at the time of the study. Infants with frequent symptoms had one or more respiratory exacerbations a month. None of the results differed significantly between the two subgroups.
CT findings and correlation with lung function test results
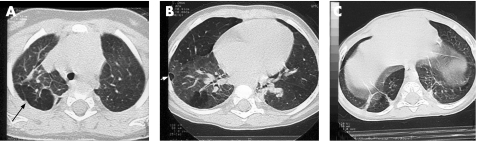
None of the infants had normal CT findings. The most frequent abnormalities were hyperlucent areas (88%), linear opacities (95%), triangular subpleural opacities (63%) and bullae (51%) (table 3). None of the infants had bronchiectasis. The largest diameter of hyperlucent areas was generally <2 cm. Large hyperlucent areas (>2 cm) were observed in 14 (34%) infants. Limited linear opacities were present in all but 2 of the infants (median value 5 per infant), 5 infants had up to 10 limited linear opacities and 20 infants had between 1 and 4 extensive linear opacities. Eight infants had bullae >1 cm in diameter. Triangular subpleural opacities were few in number (median 1 per infant), but six infants each had three such features. Typical lesions are shown fig 1.
Table 3 CT findings and lung function test results.
| Mean z score (SEM), n | |||
|---|---|---|---|
| FRC | VmaxFRC | ||
| Subpleural | None | 0.78 (0.67), 12 | −0.95 (0.31), 14 |
| opacities | 1 | 0.07 (0.92), 7 | −1.66 (0.27), 11 |
| 2 | −0.35 (0.50), 7 | −1.47 (0.27), 7 | |
| 3 | −1.97 (0.60*), 5 | −1.28 (0.52), 6 | |
| Extended linear | No | 1.03 (0.73) (12) | −1.22 (0.32), 15 |
| opacities | Yes | −0.78 (0.36*), 19 | −1.35 (0.19), 23 |
| Limited linear | No | 1.76 (2.64), 2 | −2.01 (1.11), 2 |
| opacities | Yes | 0.11 (0.40), 29 | −1.26 (0.17), 36 |
| Limited linear | 0–2 | 2.10 (1.08), 7 | −0.76 (0.45), 9 |
| opacities | 3–6 | −0.18 (0.38*), 12 | −1.54 (0.22), 16 |
| ⩾7 | −0.50 (0.62*), 12 | −1.39 (0.25), 13 | |
| Large hyperlucent | No | 0.39 (0.48), 21 | −1.29 (0.17), 27 |
| area(s) | Yes | −0.98 (0.66), 10 | −1.32 (0.43), 11 |
| Presence of | No | 187 (20) | 140 (28) |
| bullae | Yes | 216 (11) | 151 (12) |
*p<0.05 compared with the reference subgroup (ie, first listed subgroup).
Figure 1 Various aspects of CT in infants with bronchopulmonary dysplasia. (A) Characteristic aspects of the right lung with hyperlucent areas adjacent to condensed parenchymal structures with a reticular pattern (linear opacities). One triangular subpleural opacity is also present (arrow). (B) Large hyperlucent area of the right lung, with one bleb (white arrow head). (C) Triangular subpleural opacities and large linear opacities.
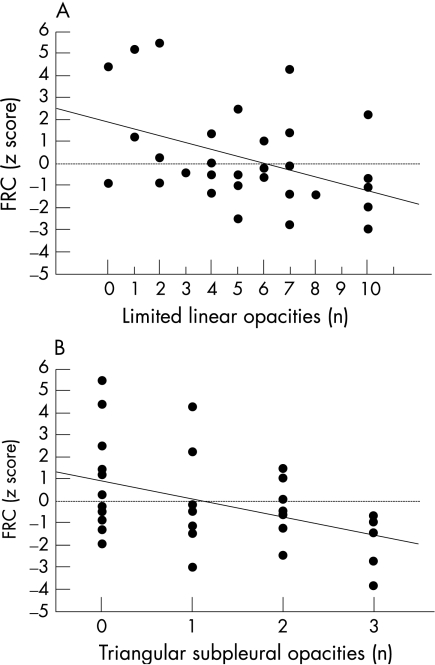
CT abnormalities were found to correlate inversely with FRC measurements but not with VmaxFRC values (table 2). The number of triangular subpleural opacities and the number of limited linear opacities correlated negatively with the FRC z score (r = −0.426, p<0.02 and r = −0.421, p<0.02, respectively). Infants with minor CT lesions had obviously higher FRC values (fig 2). Similarly, infants with extensive linear opacities had significantly lower FRC than those without these opacities (p = 0.02).
Figure 2 Relationship between functional residual capacity (FRC) z score and (A) number of triangular subpleural opacities and (B) limited linear opacities on CT.
Correlation between CT findings and neonatal events
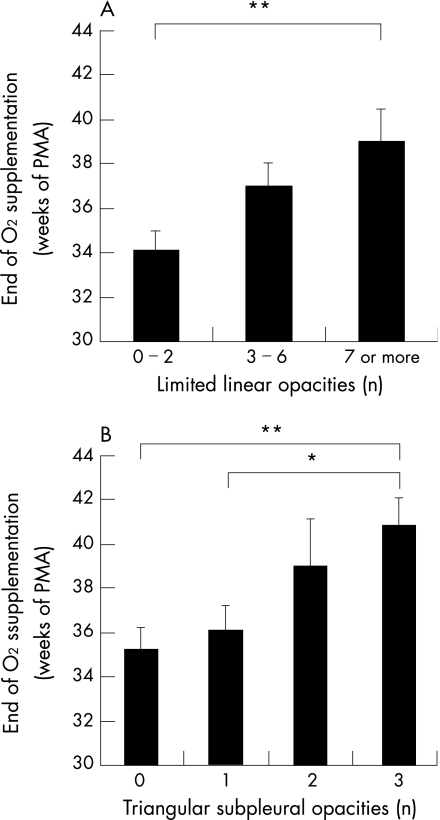
Hyperlucent areas (regardless of size), bullae and extensive linear opacities did not correlate with any of the neonatal risk factors. In contrast, triangular subpleural opacities and limited linear opacities correlated with the occurrence of one or more neonatal event. Oxygen exposure was the factor most strongly associated with these CT abnormalities. There was no relationship with gestational age or birth weight. Linear regression analysis of continuous variables showed that the number of triangular subpleural opacities and the number of limited linear opacities correlated with the duration of oxygen exposure (r = 0.379, p<0.02 and r = 0.339 p = 0.03, respectively) and with PMA at the end of oxygen supplementation (r = 0.431, p<0.005 and r = 0.372, p<0.02, respectively) (fig 3). The numbers of triangular subpleural opacities also correlated with PMA at the end of mechanical ventilation (r = 0.366 p<0.02). Finally, the number of triangular subpleural opacities was the sole CT parameter that significantly differentiated infants with moderate or severe BPD from those with mild BPD: 1.5 (0.3) versus 0.8 (0.2), respectively (p<0.03). In contrast, none of the CT parameters correlated with the severity of current symptoms (table 2).
Figure 3 Relationship between the number of (A) limited linear opacities and (B) of triangular subpleural opacities and the postmenstrual age at end of oxygen supplementation. *p<0.05 and **p<0.02 between groups.
What is already known on this topic
Before the surfactant treatment era, airway injury, inflammation and parenchymal fibrosis were the prominent findings in BPD; however the “new” BPD appears to be a result of arrested lung development, with abnormalities of alveolar septation and microvascular maturation.
CT findings in children with “old” BPD included reticular opacities, multifocal areas of reduced density and marked bronchial wall thickening.
BPD is associated with long‐term alterations in lung function tests.
What this study adds
Bronchiectasis is not observed in infants with “new” BPD.
Hyperlucent areas are common but do not correlate with airway obstruction.
Linear opacities and subpleural opacities are associated with low FRC, suggesting persistent fibrotic pulmonary lesions despite advances in neonatal care.
Discussion
The pattern of chronic lung disease in infants born prematurely has changed considerably in the past decades, reflecting both better perinatal management and increasing lung immaturity with the survival of increasingly premature infants.14 However, the pathologic description of this new BPD is mostly based on autopsy specimens from the most severely ill infants.3 In infants with less severe BPD, little is known about the correlation between CT and lung function test findings and the neonatal history. We describe, for the first time, CT and lung function test findings before 2 years of life in infants with symptomatic BPD who benefited from optimised neonatal care and a high rate of administration of surfactant. Although our results are based on a selected population with persistent respiratory symptoms, and therefore do not constitute a global description of the respiratory outcome in very low birthweight infants, many findings of the present study are of interest. First, we have shown that multifocal hyperlucent areas, linear opacities and subpleural opacities are the prominent CT features. In contrast, no bronchial involvement was observed. Second, linear opacities and subpleural opacities, but not hyperlucent areas, correlated with FRC and neonatal management, suggesting different mechanisms for these CT findings.
All the infants had abnormal CT findings, although some CT scans showed only minor lesions. The cardinal CT features included multifocal hyperlucent areas, linear opacities and subpleural opacities. Many of our CT findings have been previously observed in children with “old” BPD.8,9,10 This is in agreement with many of the histopathological features of BPD described over the past 20 years, which are similar whether or not the infants benefited from optimised management.5 Thus, our CT findings strongly suggest that infants with BPD who have been treated with exogenous surfactant still show variable association between impaired alveolarisation, abnormal vascularisation and interstitial fibroproliferation. In previous CT studies, reticular opacities and multifocal areas of reduced density were routinely observed, and areas of architectural distortion,8 air trapping,8 triangular subpleural opacities10 and a reduced bronchus‐to‐pulmonary artery diameter ratio9 were less frequent. These lesions may be induced by several mechanisms. Architectural distortions and reticular opacities are probably related mainly to fibrosis, which is the endpoint of abnormal lung repair. In our study, such lesions were still seen and we described them as linear opacities and triangular subpleural opacities. Areas of reduced density may be related to air trapping associated with bronchiolar obstruction. The airways are noticeably involved in classic BPD, and both bronchial wall thickening and decreased bronchial diameter have been observed in adulthood.9 We observed no such lesions in our population with new BPD, and the relative absence of bronchial involvement seems to confirm that reported in recent autopsy studies of infants having died of BPD.4 Furthermore, we found no relationship between the presence of areas of decreased density on CT and airway obstruction. These hyperlucent areas may therefore correspond mainly to abnormal alveolar development and reduced distal vascularisation, which constitute the key findings in new BPD.4,15 These features were present in nearly 90% of our infants and did not correlate with neonatal history, or with immaturity itself (reflected by low gestational age or birth weight), suggesting that prematurity per se interferes with alveolar development, as has been demonstrated in animal models.16
In contrast with hyperlucent areas, linear opacities and subpleural opacities correlated with neonatal insults such as oxygen supplementation and mechanical ventilation. Oxygen supplementation was the most consistent neonatal factor associated with these CT abnormalities. The number of triangular subpleural opacities had the strongest association with disease severity, as it was the unique finding that significantly differed between infants with mild BPD and those with moderate or severe disease. Aquino et al similarly found in classic BPD a correlation with architectural distortion but not with areas of decreased density.8 However, the correlations we found between CT findings and oxygen exposure or mechanical ventilation do not imply a causal relationship. Oxygen supplementation may be the indicator of severe lung disease rather than the inducer of the lesions. Nevertheless, our results show that less aggressive care of very low birthweight neonates can still induce tissue injury and interfere with alveolar and pulmonary vascular development. Oxygen supplementation,17 mechanical ventilation18 and inflammation19 have been all shown to inhibit alveolarisation.
Our lung function test findings are consistent with the above mentioned observations. The alterations in lung volume and airway flow were similar to those previously reported.7 However, only reduced FRC correlated with neonatal events. Similarly, Baraldi et al did not find any relationship between VmaxFRC and the duration of supplemental oxygen exposure in premature infants evaluated at 16 months of age.6 The decreased values observed in our population of infants with symptoms may therefore reflect hyper‐reactive airway disease rather than oxygen‐induced airway sequelae. The absence of bronchial lesions on CT reinforces this hypothesis. However, in absence of measurement of hyper‐reactivity, we cannot exclude that a fixed, reduced airway calibre may have contributed to the low VmaxFRC values. In the absence of appreciable airway disease, low FRC may therefore be related to fibrotic pulmonary lesions and/or to reduced absolute lung volume due to arrested septation. This is consistent with the significant association that we found between FRC and the number of triangular subpleural opacities or linear opacities on CT.
Finally, CT and lung function abnormalities did not correlate with the severity of respiratory symptoms following discharge. This suggests that these lesions were mainly influenced by the neonatal insults in our population of very low birthweight infants, and are poor predictors of the severity of persistent symptoms after discharge. These results do not argue for considering CT or lung function tests as helpful in routine practice in the care of infants with symptomatic BPD.
In conclusion, CT lesions are illustrative of the pathologic mechanisms responsible for BPD: alveolar and vascular growth disorders, as well as abnormal tissue repair and fibrosis. Despite advances in neonatal care, many CT findings are similar to those observed in the pre‐surfactant era, and are still associated with duration of oxygen supplementation and mechanical ventilation. The absence of bronchial involvement was the most striking difference with previous studies, in agreement with autopsy data.
Abbreviations
BPD - bronchopulmonary dysplasia
CT - computed tomography
PMA - postmenstrual age
VmaxFRC - maximum expiratory flow at functional residual capacity
Footnotes
Competing interests: None.
References
- 1.Fanaroff A A, Hack M, Walsh M C. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol 200327281–287. [DOI] [PubMed] [Google Scholar]
- 2.Chess P R, D'Angio C T, Pryhuber G S.et al Pathogenesis of bronchopulmonary dysplasia. Semin Perinatol 200630171–178. [DOI] [PubMed] [Google Scholar]
- 3.Jobe A H, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 20011631723–1729. [DOI] [PubMed] [Google Scholar]
- 4.Husain A N, Siddiqui N H, Stocker J T. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 199829710–717. [DOI] [PubMed] [Google Scholar]
- 5.Coalson J J. Pathology of bronchopulmonary dysplasia. Semin Perinatol 200630179–184. [DOI] [PubMed] [Google Scholar]
- 6.Baraldi E, Filippone M, Trevisanuto D.et al Pulmonary function until two years of life in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med 1997155149–155. [DOI] [PubMed] [Google Scholar]
- 7.Hofhuis W, Huysman M W, Van Der Wiel E C.et al Worsening of V′maxFRC in infants with chronic lung disease in the first year of life: a more favorable outcome after high‐frequency oscillation ventilation. Am J Respir Crit Care Med 20021661539–1543. [DOI] [PubMed] [Google Scholar]
- 8.Aquino S L, Schechter M S, Chiles C.et al High‐resolution inspiratory and expiratory CT in older children and adults with bronchopulmonary dysplasia. AJR Am J Roentgenol 1999173963–967. [DOI] [PubMed] [Google Scholar]
- 9.Howling S J, Northway W H, Jr, Hansell D M.et al Pulmonary sequelae of bronchopulmonary dysplasia survivors: high‐resolution CT findings. AJR Am J Roentgenol 20001741323–1326. [DOI] [PubMed] [Google Scholar]
- 10.Oppenheim C, Mamou‐Mani T, Sayegh N.et al Bronchopulmonary dysplasia: value of CT in identifying pulmonary sequelae. AJR Am J Roentgenol 1994163169–172. [DOI] [PubMed] [Google Scholar]
- 11.Delacourt C, Benoist M R, Waernessyckle S.et al Relationship between bronchial responsiveness and clinical evolution in infants who wheeze: a four‐year prospective study. Am J Respir Crit Care Med 20011641382–1386. [DOI] [PubMed] [Google Scholar]
- 12.Hoo A F, Dezateux C, Hanrahan J P.et al Sex‐specific prediction equations for Vmax(FRC) in infancy: a multicenter collaborative study. Am J Respir Crit Care Med 20021651084–1092. [DOI] [PubMed] [Google Scholar]
- 13.Merth I T, de Winter J P, Borsboom G J.et al Pulmonary function during the first year of life in healthy infants born prematurely. Eur Respir J 199581141–1147. [DOI] [PubMed] [Google Scholar]
- 14.Eber E, Zach M S. Long term sequelae of bronchopulmonary dysplasia (chronic lung disease of infancy). Thorax 200156317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt A J, Pryhuber G S, Huyck H.et al Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt‐1, and TIE‐2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 20011641971–1980. [DOI] [PubMed] [Google Scholar]
- 16.Coalson J J, Winter V T, Siler‐Khodr T.et al Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 19991601333–1346. [DOI] [PubMed] [Google Scholar]
- 17.Warner B B, Stuart L A, Papes R A.et al Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 1998275L110–L117. [DOI] [PubMed] [Google Scholar]
- 18.Albertine K H, Jones G P, Starcher B C.et al Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med 1999159945–958. [DOI] [PubMed] [Google Scholar]
- 19.Franco M L, Waszak P, Banalec G.et al LPS‐induced lung injury in neonatal rats: changes in gelatinase activities and consequences on lung growth. Am J Physiol Lung Cell Mol Physiol 2002282L491–L500. [DOI] [PubMed] [Google Scholar]