Abstract
Background
Smoking is a major risk factor for cot death. Many infants smoke passively as a result of parental smoking. This paper reports on infants exposed to a smoking environment and how they accumulate metabolites of cigarette smoke, such as cotinine, which may be physiologically harmful.
Aim
To assess cotinine levels in infants of smoking parents.
Method
Cotinine excretion in urine was assessed in 104 infants, of whom 71 had smoking parents and 33 had non‐smoking parents. All cotinine levels were measured at approximately 12 weeks of age. The subjects were selected from a database of infants in developmental physiological studies which assessed the impact of various factors on early postnatal development.
Results
On average babies with at least one parent who was a current cigarette smoker excreted 5.58 (95% CI 3.4 to 9.5) times as much cotinine in the urine as did the babies of non‐smoking parents. Maternal smoking was the largest contributing factor. Co‐sleeping (p = 0.037) and the minimum room temperature (p = 0.028) were significant contributory factors.
Conclusion
Infants from smoking households accumulate cotinine, a metabolite of nicotine, which may have a detrimental effect on the cardiorespiratory system.
Keywords: passive smoking, infant, cotinine, nicotine, SIDS
The impact of cigarette smoke on health is seen in all age groups. It causes heart and lung disease in adults1 and respiratory illness in children,2,3,4,5 and more recently, it has been linked with smallness at birth6,7,8 and sudden death in infancy.9,10 The active or passive inhalation of cigarette smoke, with its numerous chemical components, is probably the mode of contamination, but the mechanism by which pathophysiological changes occur is unknown. Specifically, antenatal smoking may affect up to 25% of pregnancies,11 causing morphological placental changes that lead to chronic fetal hypoxic stress and abnormal lung and brain development.5 Fetal and infant physiology are disrupted,12,13,14 lung function is reduced15 and arousal mechanisms are impaired,16 with a tendency to central apnoea and reduced ventilatory response to hypoxia. Law et al17 showed the effects of neonatal nicotine withdrawal on infant neurobehaviour. Gergen et al18 have shown that nearly 40% of under‐fives are exposed to environmental tobacco smoke in the home, and that as many as 6000 deaths in young children may be a direct result of this.19
In the present study, we investigated the extent to which the nicotine metabolite, cotinine, is transferred to newborn infants as a consequence of parental smoking.
Methods
During studies of postnatal developmental physiology of human infants, in which deep body temperature was monitored over night, we collected information on parental smoking habits and urine samples from the babies for cotinine estimation. Details of parental reported smoking were recorded and validated by direct observation by a trained researcher.
The studies were carried out over a period of five years up to and including 1998,20,21,22 and for this study, infants were recruited sequentially from the database according to whether the parents smoked (in the original studies, infants who met the inclusion criteria were selected at random from the Birth Notification Register). Infants with insufficient physiological data were excluded. For the purpose of this study, a smoking household was defined as one in which either parent (or main carer) smoked. Co‐sleeping was defined as an infant who routinely bed shared with the carer(s) for the main night‐time sleep over the duration of the study.
The database contained 493 infants. We sequentially selected the first 104 infants who provided a cotinine urine sample and for whom full physiological data were available. The infants were split into those from smoking and non‐smoking households for analysis. All infants were monitored during the same period. Their weight, record of feeding and care practices, and evidence of illness or immunisation were obtained from the database. In the original study, at bedtime, a paediatric urine collector (Hollister U bag), which was modified to reduce the risk of detachment, was attached to the infant to collect the evening urine sample. Samples were frozen within four hours of collection. Care was taken to avoid contamination of the specimen containers with any suspected source of nicotine.
The infants were approximately 10–12 weeks of age at the time of the study. The local ethics committee approved the study, and informed consent was obtained from all parents.
We report on the infants' cotinine levels, in relation to the reported smoking habits of parents, social circumstances and the care practices applied to the infant.
Laboratory techniques
We used the cotinine:creatinine ratio as the measure of exposure to smoke. It was estimated by the enzyme‐linked immunosorbent assay (ELISA) method (Cosazt Bioscience Ltd, UK), a competitive enzyme immunoassay. The logarithm transformation was used to attain normal distribution. All analyses on the ELISA kit were carried out in duplicate and we used cotinine standards from 0 μg/l to 880 μg/l for calibration. All samples with cotinine levels greater than 880 μg/l were reanalysed after diluting in deionised water. Creatinine levels were determined by the Jaffe reaction on a Cobas Fara analyser (Roche Diagnostics, UK). The results were expressed as μg/l of cotinine per mmol/l creatinine. All analyses were performed blind (standards and reagents, Sigma Chemical Co, UK).
Statistical methods
We used Student's t test to compare birth weight, gestational age and other approximately normally distributed variables, according to the smoking status of parents. Cotinine and creatinine levels were found to be largely positively skewed; they were therefore logarithm transformed for analysis. The geometric means are presented here. Homogeneity of variance was formally tested. The residual plots were examined visually and found to be satisfactory. The χ2 test was used to examine the association between parental smoking status and sex, feeding methods, social class of the family and other characteristics.
We used general linear models to examine the association between combinations of characteristics, primarily smoking status and the cotinine:creatinine ratio (on a logarithmic scale). Effects of potential confounders were examined by building models using forwards and backwards selection procedures. The pool of variables used for this consisted of: feeding method; father's employment status; social class; whether the baby co‐slept; inadequate heating in the home; the time of maturation of the adult core body temperature pattern and whether this was delayed; the minimum room temperature where baby slept; and the season.
All analyses were undertaken using SPSS for Windows (version 14.0). The level for statistically significant results was set at 5% (p<0.05), and 95% confidence intervals are presented for the main results.
What is already known on this topic
Smoking is related to cot death.
Babies are able to metabolise nicotine secondary to exposure to environmental tobacco smoke.
What this study adds
If parents smoke, the baby smokes.
Mother smoking is the most important contributing factor.
Co‐sleeping and the temperature of the room the baby sleeps in are also contributory factors.
Results
Characteristics of the study population
We identified 104 infants, 33 (32%) from non‐smoking and 71 (68%) from smoking homes. Both parents of 44 (62%) infants smoked, of 13 (18%) infants only the mother smoked and of 14 (20%) infants only the father smoked. On average each parent smoked 16 cigarettes a day (mean (SD) number of cigarettes smoked: maternal 16 (10.28); paternal 16.6 (10.48)).
Table 1 shows the demographic data on these infants. The most striking feature was a difference of 400 g in birth weight for the same gestational age, with passively smoking babies being lighter. These infants were also from poorer families and were more likely to be bottle fed. The percentage of stillbirths or miscarriage/termination of pregnancy was similar in both groups (20% in non‐smoking group and 37% in smoking group; p = 0.188). Also one mother who smoked had a history of premature labour.
Table 1 Demographic data on infants from non‐smoking and smoking households.
| Non‐smoking (n = 33) | Smoking (n = 71) | p Value | 95% CI* | |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 14 (42) | 43 (61) | ||
| Female | 19 (58) | 28 (39) | 0.080 | −0.02 to 0.37 |
| Birth weight (g), mean (SD) | 3607 (600) | 3205 (544) | 0.002 | 152 to 652 |
| Gestation (weeks), mean (SD) | 39.9 (1.47) | 39.6 (1.66) | 0.424 | −0.42 to 0.98 |
| Feeding, n (%) | ||||
| Breast fed | 24 (73) | 17 (24) | ||
| Bottle fed | 9 (27) | 54 (76) | <0.001 | −0.64 to −0.29 |
| Social class, n (%)† | ||||
| I or II | 15 (50) | 6 (11) | ||
| III or IV | 5 (17) | 10 (18) | −0.61 to −0.05 | |
| V or VI | 7 (23) | 17 (30) | −0.63 to −0.13 | |
| VII or VIII | 3 (10) | 24 (42) | 0.002 | −0.78 to −0.35 |
| Not known | 3 | 14 | ||
| Birth order, mean (SD) | 1.79 (0.86) | 2.66 (1.50) | 0.003 | −1.43 to −0.31 |
| Age (weeks), mean (SD)‡ | 10 (2.97) | 12 (3.59) | 0.090 | −2.90 to 0.25 |
| Weight (g), mean (SD)‡ | 5796 (1053) | 5444 (1206) | 0.278 | −290 to 995 |
*95% CI for differences in mean values (for continuous variables) or differences in % (categorical variables)
†Social class was based the reported occupation of the highest earner in the household.
‡Measurement taken at time of cotinine estimation.
The sex distribution was similar in the two groups. Infants from smoking homes were more likely to be bottle fed and were of higher birth order (range in non‐smoking families was 1–4 children and in smoking families was 1–7 children; p = 0.003). Families who smoked were of lower social class: 72% of smoking families were in social class V or lower compared with 33% of the non‐smoking families (p = 0.001). A third of fathers were unemployed in smoking households compared with only 8% in the non‐smoking group (p = 0.032). There was no difference in the age or weight of the infants between the two groups, at the time of cotinine estimation.
Smoking mothers were on average three years younger than their non‐smoking counterparts (mean maternal age 27.6 years and 30.2 years, respectively; p = 0.011, mean difference 95% CI 0.63 to 4.8 years), as were the smoking fathers (30 years and 32 years, respectively, p = 0.065). Family history of respiratory illness and exposure to pets were similar in the two groups, and the babies experienced similar types and episodes of minor illness.
Infants' sleeping arrangements
The proportion of infants who slept in the same room as their parents was not different between the groups (smoking group, n = 43 (86%) and non‐smoking group, n = 15 (67%), p = 0.067, χ2 = 5.4); similarly the number of babies who slept in the bed with their parents (co‐sleeping) was similar in both groups (n = 3 (13%) of babies from non‐smoking house holds and n = 8 (16%) from smoking households were co‐sleepers; p>0.05, χ2 = 0.13).
Most babies slept supine or in the lateral position. There was 1 (3%) prone sleeper in the non‐smoking household group and 2 (2% of total) prone sleepers in the smoking household group (p>0.05, χ2 = 0.004).
Infants' thermal environment
Most families had central heating and/or gas fires although a large percentage of the central heating in the deprived estates was centrally controlled. The individual tenants could not adjust the temperature in their own flats/homes. All non‐smoking families had full gas central heating. Twenty per cent of smoking households had no or inadequate heating arrangements (p = 0.017, χ2 = 5.65).
The minimum and maximum temperatures in the room in which the baby slept, were on average, higher in the households where a parent smoked (mean minimum room temperature 18.7°C and 17.2°C for smoking and non‐smoking households respectively; p = 0.014; mean maximum room temperature 22.1°C and 21.0°C respectively; p>0.05). There was no difference in how well the babies were wrapped (mean tog value 8.6 v 9.28 for babies from smoking and non‐smoking households, respectively; p>0.05).
Cotinine data
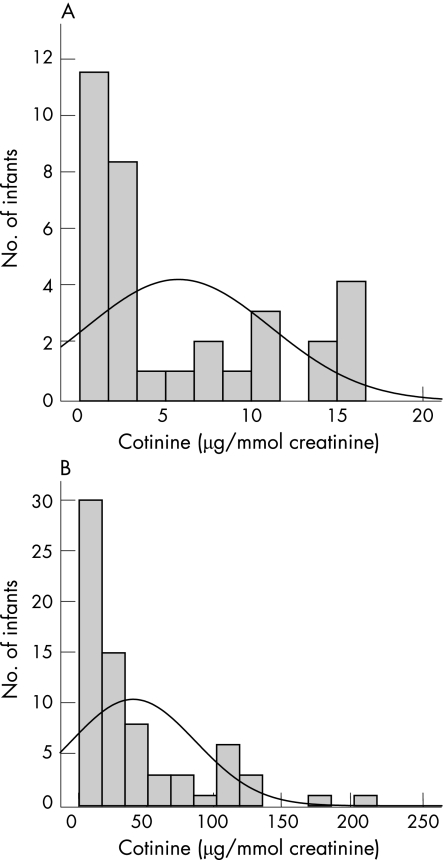
All cotinine data were logarithm transformed to obtain an approximately normal distribution. For comparison with previously published work, we show the “raw” mean cotinine levels calculated without log transformation. The raw data (fig 1) showed that mean cotinine level in infants from the smoking households was markedly higher than that of infants from non‐smoking households (mean (SD) cotinine level 39 (45) μg/mmol creatinine, range 0.35–211.67 μg/mmol creatinine; and 5 (5.39) μg/mmol creatinine, range 0.24–16.66 μg/mmol creatinine, respectively).
Figure 1 Distribution of urinary cotinine levels in infants from (A) non‐smoking households and (B) smoking households. The arithmetic mean level for infants from non‐smoking households was 5 μg/mmol and for those from smoking households was 39 μg/mmol.
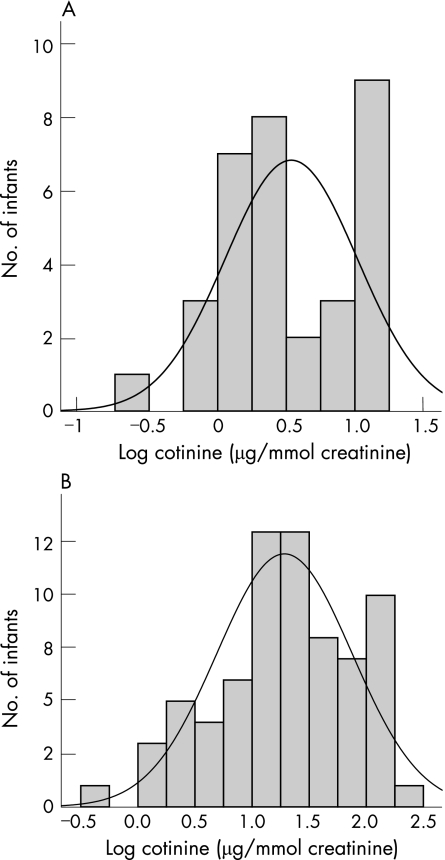
When the data were log transformed (fig 2) the infants from the smoking households still excreted more cotinine than infants from non‐smoking households (1.28 (0.59) μg/mmol creatinine v 0.54 (0.48) μg/mmol creatinine, respectively). The geometric mean value of cotinine levels in infants from households in which at least one parent smoked was 5.58 times higher than that from infants from households in which the parents were non‐smokers (p<0.001).
Figure 2 Distribution of logged cotinine data of infants from (A) non‐smoking households and (B) smoking households. The mean for infants from non‐smoking households was 0.5 μg/mmol and for those from smoking households was 1.28 μg/mmol.
The smoking status of mothers and fathers was treated as separate predictor variables in a multivariable linear model in which each coefficient was adjusted for all other variables. Maternal smoking was the single largest contributing factor, increasing cotinine levels by a factor of four, followed by paternal smoking (table 2).
Table 2 Multivariable linear model comparing smoking status of mothers and fathers.
| Covariate | Ratio of geometric means | 95% CI | p Value |
|---|---|---|---|
| Maternal smoking in household | 3.97 | 2.28 to 6.92 | <0.001 |
| Paternal smoking in household | 1.83 | 1.05 to 3.18 | 0.034 |
Dependent variable: log cotinine:creatinine ratio.
We found no significant interaction between maternal and paternal smoking and infants' cotinine level (p = 0.331). Overall, where the mother smoked, the geometric mean value for cotinine increased by a factor of four, and where the father smoked, it increased by a factor of nearly two. The results indicate that maternal smoking was the single greatest influence on the cotinine levels of a baby.
Other variables
Using a multivariable linear model, the impact of the following variables was assessed:
smoking status of household;
low social class;
paternal unemployment;
inadequate heating in household;
breast feeding;
minimum room temperature;
delay in age baby achieved mature temperature biorhythm;
co‐sleeping;
season in which measurement was taken.
One smoking parent, co‐sleeping and low minimum room temperature were the only three factors found to have a significant effect on the infant's smoke exposure. Other factors such as social class and feeding method had no effect (table 3). The lower the minimum temperature of the room where the baby slept, the higher was the log cotinine:creatinine ratio. Co‐sleeping seemed to have a deleterious effect—that is, babies who bed shared with their parent/main carer had higher cotinine levels. Social class, father's employment status, heating as a proxy for social class, a delay in the age of maturation of temperature rhythm, the season in which cotinine was measured and feeding method did not have independent effects. Both forwards and backwards selection methods, using the 5% level of significance, resulted in a model containing only co‐sleeping, room temperature and household smoking, with p values and parameter estimates similar to those shown in table 3.
Table 3 Results from the multivariable linear model with co‐sleeping and minimum room temperature as predictor values.
| Covariate | Ratio of geometric means | 95% CI | p Value |
|---|---|---|---|
| Baby co‐slept | 4.13 | 1.090 to 15.621 | 0.037 |
| Minimum room temperature where baby slept (°C) | 0.83 | 0.694 to 0.979 | 0.028 |
| Baby with at least one parent smoker | 7.39 | 2.535 to 21.527 | <0.001 |
| Low social class of household | 0.83 | 0.460 to 3.097 | 0.703 |
| Inadequate heating in family home | 0.74 | 0.219 to 2.513 | 0.624 |
| Paternal unemployment | 1.23 | 0.368 to 4.111 | 0.730 |
| Breast feeding | 1.31 | 0.491 to 3.493 | 0.581 |
| Delay in age baby achieved mature temperature biorhythm | 1.01 | 0.893 to 1.160 | 0.784 |
| Season | 0.58 | 0.31 to 1.07 | 0.080 |
Dependent variable: log cotinine:creatinine ratio.
There was no interaction between household smoking and minimum room temperature (p = 0.197). Nor was there an interaction between the season in which the measurement was done and smoking status of the parent (p = 0.740).
Seasonality
There was no seasonal variation in recruitment of babies (p = 0.093). Log cotinine:creatinine ratios and mean minimum room temperature varied as shown in table 4, with the highest cotinine estimation values in winter.
Table 4 Seasonal variation in log cotinine creatinine ratios and mean minimum room temperature.
| Log cotinine/ creatinine ratio | Minimum room temperature | |
|---|---|---|
| Spring | 0.96 | 18.2°C |
| Summer | 0.67 | 18.9°C |
| Autumn | 1.01 | 17.5°C |
| Winter | 1.22 | 18.0°C |
Discussion
Our findings clearly show that by accumulating cotinine, babies become heavy passive smokers secondary to the active smoking of parents. Nicotine, of which cotinine is a byproduct, has recognisable cardiovascular stimulant effects.23 However, it is merely one of the several thousand constituents of tobacco smoke and may not be the most lethal. How it affects infants is largely unknown.
As expected, maternal smoking is the single largest contributor to cotinine levels in infants, quadrupling the level in non‐passively smoking babies. When the father smokes, the level is doubled. This implies that a complex relationship exists between the biomarker and the type of exposure, and varies as a function of environmental and physiological factors. The proximity of the smoker, ventilation, precise location and length of exposure compound the effect of passive smoking in infants.24
“Smoking” babies tend to come from poorer homes,25,26,27 which may have smaller rooms and inadequate heating. The present study has shown an independent effect of low room temperature. There was also a suggestion of summer–winter seasonality of cotinine levels, as shown in previous studies.28,29 This is in keeping with the strong seasonal patterns in sudden infant death syndrome (SIDS). Higher cotinine levels in colder times of the year may be a reflection of the other key factors which influence exposure to passive smoking, such as poorer ventilation or a greater tendency for parents to smoke indoors in winter.
Further, we found that co‐sleeping increased cotinine levels when other factors were corrected for. One simple possible biological mechanism for this may be the direct inhalation or closeness to clothing or other objects contaminated with smoke particles, which in turn are passed to the baby during periods of close contact, such as during sleep. Babies co‐sleeping with “smoking” parents are at increased risk of cot death,30,31,32,33 but the mechanism by which this occurs may not be straightforward. An infant's chronic exposure to smoking whether before and after birth will have an accumulated biological effect, including delayed postnatal physiological maturation,14,34,35 perhaps the basis of vulnerability. How genetic36,37 and other factors such as infection,38,39 impinge on this vulnerability is critical in explaining the causes and the mode of sudden infant death.
Babies and children are routinely exposed to cigarette smoke by their carers in their homes, without the legislative protection available to adults in public places. There are practical difficulties in preventing smoking in family residences, which relies heavily on the goodwill of the parent/carer and accompanying education about strategies to reduce harm related to passive smoking. The well recognised maternal desire to protect the child is the great hope for the future.
Acknowledgement
We would like to thank the Foundation for the Study of Sudden Infant Death and Babes in Arms for their support.
Footnotes
Competing interests: None.
References
- 1.Ockene J K, Kuller L H, Svendsen K H.et al The relationship of smoking cessation to coronary heart disease and lung cancer in the Multiple Risk Factor Intervention Trial (MRFIT). Am J Public Health 199080954–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strachan D P, Cook D G. Parental smoking and childhood asthma: longitudinal and case‐control studies. Thorax 199853204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannino D M, Homa D M, Redd S C. Involuntary smoking and asthma severity in children: data from the Third National Health and Nutrition Examination Survey. Chest 2002122409–415. [DOI] [PubMed] [Google Scholar]
- 4.Cook D G, Strachan D P. Health effects of passive smoking. 3. Parental smoking and prevalence of respiratory symptoms and asthma in school age children. Thorax 1997521081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofhuis W, de Jongste J C, Merkus P J F M. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child 2003881086–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson J S, Moore V M, Owens J A.et al Origins of fetal growth restriction. Eur J Obstet Gynecol Reprod Biol 20009213–19. [DOI] [PubMed] [Google Scholar]
- 7.Yanney M, Marlow N. Paediatric consequences of fetal growth restriction. Sem Neonatol 20049411–418. [DOI] [PubMed] [Google Scholar]
- 8.Vandenbosche R C, Kirchner D O. Intrauterine growth retardation. Am Fam Physician 1998581384–9013934. [PubMed] [Google Scholar]
- 9.Mitchell E A, Tuohy P G, Brunt J M.et al Risk factors for sudden infant death syndrome following the prevention campaign in New Zealand: a prospective study. Pediatrics 1997100835–840. [DOI] [PubMed] [Google Scholar]
- 10.Blair P S, Fleming P J, Smith I J.et al Babies sleeping with parents: case–control study of factors influencing the risk of the sudden infant death syndrome. Commentary: Cot death the story so far, BMJ 19993191457–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owen L. Trends in smoking during pregnancy in England, 1992–7: quota sampling surveys. BMJ 1998317728–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeskind P S, Gingras J L. Maternal cigarette‐smoking during pregnancy disrupts rhythms in fetal heart rate. J Pediatr Psychol 2006315–14. [DOI] [PubMed] [Google Scholar]
- 13.Beratis N G, Panagoulias D, Varvarigou A. Increased blood pressure in neonates and infants whose mothers smoked during pregnancy. J Pediatr 1996128806–812. [DOI] [PubMed] [Google Scholar]
- 14.Petersen S A, Pratt C, Wailoo M P. Relations between the development of patterns of sleeping heart rate and body temperature in infants. Arch Dis Child Fetal Neonatal Ed 200185F133–F136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dezateux C, Stocks J, Dundas I.et al Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Obstet Gynecol 199 159403–410. [DOI] [PubMed] [Google Scholar]
- 16.Chang A B, Wilson S J, Masters I B.et al Altered arousal response in infants exposed to cigarette smoke. Arch Dis Child 20038830–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Law K L, Stroud L R, LaGasse L L.et al Smoking during pregnancy and newborn neurobehaviour. Pediatrics 20031111318–1323. [DOI] [PubMed] [Google Scholar]
- 18.Gergen P J, Fowler J A, Maurer K R.et al The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics 1998101e8. [DOI] [PubMed] [Google Scholar]
- 19.Aligne C A, Stoddard J J. Tobacco and children. An economic evaluation of the medical effects of parental smoking. Arch Pediatr Adolesc Med 2005151648–653. [DOI] [PubMed] [Google Scholar]
- 20.Jackson J A, Wailoo M P, Thompson, et al Early physiological development of infants with intrauterine growth retardation. Arch Dis Child Fetal Neonatal Ed 20048946–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wailoo M P, Westaway J A, Joseph D.et al Overnight deep body temperature and urinary cortisol excretion in infants from economically deprived areas. Child Care Health Dev 200329473–480. [DOI] [PubMed] [Google Scholar]
- 22.Jackson J A, Wailoo M P, Petersen S P.et al Changes in body temperature and urinary cortisol after routine immunisation in babies with intrauterine growth retardation. Acta Paediatr 2001901186–1189. [DOI] [PubMed] [Google Scholar]
- 23.Zhu B Q, Parmley W W. Hemodynamic and vascular effects of active and passive smoking. Am Heart J 19951301270–1275. [DOI] [PubMed] [Google Scholar]
- 24.California Environmental Protection Agency Environmental tobacco smoke report, 1997. http://www.oehha.org/air/pdf/ets.pdf (accessed 4 January 2006)
- 25.Spencer N. Maternal education, lone parenthood, material hardship, maternal smoking, and longstanding respiratory problems in childhood: testing a hierarchical conceptual framework. J Epidemiol Community Health 200559842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbeau E M, Krieger N, Soobader M ‐ J. Working class matters: socioeconomic disadvantage, race/ethnicity, gender and smoking in NHIS 2000. Am J Public Health 200494269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham H, Francis B, Inskip H M.et al Socioeconomic life course influences on women's smoking status in early adulthood. J Epidemiol Community Health 200660228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis M J, Strachan D P, Feyerabend C. Determinants of passive smoking in children in Edinburgh, Scotland. Am J Public Health 1992821225–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvis M J, McNeill A D, Bryant A.et al Factors determining exposure to passive smoking in young adults living at home: qualitative analysis using saliva cotinine concentrations. Int J Epidemiol 199120126–131. [DOI] [PubMed] [Google Scholar]
- 30.Klonoff‐Cohen H, Edelstein S L. Bedsharing and the sudden infant death syndrome. BMJ 19953111269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James C, Klenka H, Manning D. Sudden infant death syndrome: bed sharing with mothers who smoke. Arch Dis Child 200388112–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Task Force on Infant Sleep position and Sudden Infant Death Changing concepts of sudden infant death syndrome: implications for infant sleep environment and sleep position. Pediatrics 2000105650–656. [DOI] [PubMed] [Google Scholar]
- 33.Lahr M B, Rosenburg K D, Lapidus J A. Bedsharing and maternal smoking in a population based survey of new mothers. Pediatrics 2005116530–542. [DOI] [PubMed] [Google Scholar]
- 34.Tuffnell C S, Petersen S A, Wailoo M P. Factors affecting rectal temperature in infancy. Arch Dis Child 199573443–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodemore M R, Petersen S A, Wailoo M P. Factors affecting rectal temperature in infancy. Arch Dis Child 1992671259–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt C E. Gene–environment interactions: implications for sudden unexpected deaths in infancy. Arch Dis Child 20059048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wesse‐Mayer D E, Berry‐Kravis E M, Zhou L.et al Sudden infant death syndrome: case‐control frequency differences at genes pertinent to early autonomic nervous system embryologic development. Pediatr Res 200456391–395. [DOI] [PubMed] [Google Scholar]
- 38.Blackwell C C, Moscovis S M, Gordon A E.et al Ethnicity, infection and sudden infant death. FEMS Immunol Med Microbiol 20044253–65. [DOI] [PubMed] [Google Scholar]
- 39.Moscovis S M, Gordon A E, Madani O M.et al Interleukin‐10 and sudden infant death syndrome. FEMS Immunol Med Microbiol 200442130–138. [DOI] [PubMed] [Google Scholar]