Abstract
Patent ductus arteriosus (PDA) is a common diagnosis among extremely premature infants, especially in those with lung disease. Treatments are often used to close the PDA. Despite nearly three decades of research, the question of whether the benefits of treatments to prevent ductal patency or promote closure outweigh the risks of these treatments remains unanswered. The authors rarely use treatments designed to close the PDA. This article reviews three considerations in support of this restrained approach: rates of spontaneous closure of the ductus arteriosus; adverse effect of persistent ductal patency; and benefits and risks of treatments for closure.
Keywords: patent ductus arteriosus, indomethacin, ibuprofen, ductal ligation
The ductus arteriosus usually closes after a brief period of right‐to‐left and bidirectional shunting following the birth of a healthy term infant. In contrast, in preterm infants, particularly those with lung disease, there is a tendency for the ductus arteriosus to remain patent. Hence, “patent ductus arteriosus” (PDA) is a common diagnosis in these infants. Approximately 65% of infants born at less than 28 weeks' gestation will have persistent patency of the ductus arteriosus and will be assigned the diagnosis of PDA at some time during the early neonatal period.1 PDA is associated with neonatal morbidities such as chronic lung disease (CLD)2,3 and necrotising enterocolitis (NEC).4 Although a cause and effect relationship between PDA and these morbidities has not been established, many neonatologists administer cyclo‐oxygenase (COX) inhibitors (eg, indomethacin or ibuprofen) to promote closure of the ductus arteriosus under the assumption that early closure decreases the likelihood of these and other morbidities.5 In the USA, COX inhibitors are given to more than 10 000 premature infants annually (R H Clark, personal communication, 2006). A smaller number of infants with a diagnosis of PDA undergo surgical ligation, most commonly when medical treatment fails. However, despite nearly three decades of research, we believe that the question of whether these treatments improve outcome remains unanswered. In our practice, we rarely administer medical treatment for a PDA and virtually never recommend surgical closure. This restrained approach to the treatment of PDA is based on three considerations:
rates of spontaneous closure of the ductus arteriosus;
adverse effect of persistent ductal patency;
benefits and risks of treatments for closure.
We review each of these considerations below.
Rates of spontaneous closure of the ductus arteriosus
Nearly all medical treatments are associated with some risk. Patients with conditions that improve or resolve without treatment should not be exposed to even minor risks associated with treatments. Therefore, treatment to promote closure of the ductus arteriosus should be considered only in infants in whom early spontaneous closure will probably not occur.
Although the ductus arteriosus closes spontaneously in nearly all term infants by 3 days of age,6 the natural history of the ductus arteriosus in preterm infants, particularly extremely premature infants with lung disease, is unknown because it is so often perturbed by medical treatments. The best estimates of rates of spontaneous closure can be derived from observations of control infants in placebo‐controlled trials of timing of treatments to close the ductus arteriosus. From these studies, inferences about the rates of spontaneous closure can be drawn from observations made before the age at which treatment for the PDA is prescribed by the study, or occurs outside the confines of the study. Using this approach, Van Overmeire et al provided an estimate of the rate of spontaneous closure of the ductus arteriosus in moderately premature infants during the first week of life.7 They investigated the efficacy of early (3 days of age) compared with late (7 days of age) treatment for PDA in 380 infants of gestational age 26–31 weeks who required ventilatory support (constant positive airway pressure or mechanical ventilation) and a fraction of inspired oxygen greater than 0.30. At 3 days of age, 67% of infants either had no PDA or had a small haemodynamically unimportant ductal shunt. One half of the remaining infants, who all had moderate to severe shunts, were randomised to receive treatment at 7 days of age if ductal patency persisted. Spontaneous closure occurred in 44% of these infants. Therefore, from these data, a rate of spontaneous closure in excess of 80% by 7 days of age can be predicted among moderately premature infants with lung disease.
Although the rate of spontaneous closure for all premature infants is high, there is a direct relationship between gestational age and closure. During the first three to four days of life, the rate of spontaneous closure is approximately 31% among infants born at 26 and 27 weeks' gestation, but it is approximately 21% at 24 and 25 weeks' gestation.8 This relationship was observed by Koch et al who estimated that for each week of increase in gestational age above 23 weeks, the odds of spontaneous closure increased by a ratio of 1.5.9 In this study, other factors that predicted closure were antenatal steroids and the absence of major lung disease. Nemerofsky et al reported that spontaneous ductal closure at 2 weeks of age occurred in 50% of ventilated compared with 80% of non‐ventilated very low birthweight infants.10 Of interest in both of these studies was the observation that spontaneous closure occurred in a number of infants after the first week of life.
Collectively, these observations regarding the rates of spontaneous closure narrow the population in whom early treatment (ie, in the first week of life) would logically be contemplated. Given the high rate of spontaneous closure among most premature infants, consideration of early treatment should be limited to extremely premature infants, in particular, those on respiratory support.
Adverse effect of persistent ductal patency
Decisions regarding treatment should be made with the aim of preventing an adverse outcome resulting from persistent patency, and the outcome should have sufficient impact on long‐term health to justify treatment. These decisions should be based on reasonable certainty of a cause and effect relationship between ductal patency and a specific adverse outcome, not merely association, and treatments should be reserved for infants at sufficient risk for the outcome. Among the relatively large percentage of extremely premature infants and the smaller percentage of more mature infants with PDA, an important determinant of the decision to treat a PDA is whether persistent patency is associated with serious morbidities. The morbidities that have attracted the most attention are CLD and NEC because they occur with relatively high frequency and may be associated with serious consequences later in childhood.
Pulmonary physiology and CLD
Large left‐to‐right ductal shunts may have an effect on pulmonary function, most notably the mechanical properties of the lung. Some investigators have reported a decrease in dynamic lung compliance in the presence of ductal shunting,11,12 although these changes have not been observed by others.13,14 Left‐to‐right shunting does not impair oxygenation, except perhaps when associated with severe left ventricular failure. However, a decrease in lung compliance as a result of shunting may provoke the need to initiate or increase ventilatory support to maintain adequate ventilation. The possibility of this cascade of events has led to the hypothesis that PDA increases the likelihood of respiratory morbidities, including CLD or bronchopulmonary dysplasia (BPD), by increasing exposure to mechanical ventilation, an established risk factor for CLD.15 This hypothesis is supported by a strong association between the presence of PDA and CLD. For example, in a population‐based study of 1460 infants, PDA emerged as a risk factor for CLD with an odds ratio of 1.9 after adjustment for a variety of risk factors (eg, gestational age, sex, etc.).2 In a more recent study, Oh et al showed a similar relationship, although of smaller magnitude (odds ratio 1.4), between PDA and death or BPD.16
Organ blood flow and NEC
A number of authors have hypothesised a “steal syndrome”, in which a large ductal shunt results in decreased diastolic blood flow to the intestines and tissue ischaemia,17 theoretically increasing the likelihood of NEC. Based on ultrasonic measurements, premature infants with PDA have decreased splanchnic and renal blood flow and blood flow velocity compared with infants without PDA.18 These values increase to levels observed in infants without PDA following ductal closure. Whether the decrements in flow result in pathology is uncertain, but epidemiologic data are suggestive. Dollberg et al explored the relationship between the presence of a PDA and NEC among 6146 infants born at gestational age 24–34 weeks, whose outcomes were entered into the Israeli national database.4 The odds ratio for the risk of NEC after adjustment for other risk factors was 1.85.
Collectively, these studies confirm an association between PDA and serious neonatal morbidities, and suggest a plausible explanation for this relationship. However, even with the knowledge that ductal shunting may perturb organ blood flow in a manner that may potentiate pathology, these associations do not prove a cause and effect relationship. It is possible that PDA is simply one manifestation of a “sick neonate” at greater likelihood of developing a number of outcomes of severe illness.
Consequences of long‐term ductal patency
Little is known about persistent patency of the ductus arteriosus beyond early infancy, but there is some reason for concern. Exposure of the pulmonary vasculature to increased flow from large left‐to‐right shunts is associated with pulmonary hypertension in the fourth decade of life.19 Even modest shunts—for example, those resulting from a patent foramen ovale—increase the risk of pulmonary hypertension if they persist into adulthood.20 It is not clear whether similar pathology results from persistent ductal patency. This is because the small calibre of the PDA may result in a diminishing shunt fraction with advancing age as total cardiac output increases. However, it is possible that exposure of the pulmonary vasculature to large shunts during a critical period of development may result in abnormal growth of the pulmonary vasculature and long‐term consequences. An additional risk may be the increased likelihood of endoarteritis.21
Benefits and risks of treatments for closure
As with all medical therapies, treatment to promote closure of the ductus arteriosus should be made with knowledge of the risks and benefits of the treatment. Clinical trials of treatments whose goal is closure of the ductus arteriosus should quantify these benefits and risks. In addition, they have the potential to provide insights about the contribution of the PDA to morbidity. If the PDA is related to morbidity in a causal relationship, closure should result in a reduction in the incidence of the morbidity. In this section, we examine the benefits of medical treatment and surgical ligation in relation to reduction in morbidities associated with PDA.
COX inhibitors: indomethacin and ibuprofen
The use of the COX inhibitor, indomethacin, to promote closure of the ductus arteriosus was first reported over three decades ago.22 Since then, the efficacy of this drug, as well as another COX inhibitor, ibuprofen, has been investigated extensively. Three strategies of treatment have been described:
prophylactic treatment of infants at risk for persistent ductal patency, usually initiated during the first day of life;
treatment of infants with PDA accompanied by signs and symptoms consistent with a major ductal shunt;
treatment of infants who have echocardiographic evidence of PDA but lack these signs and symptoms.
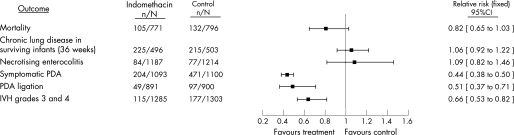
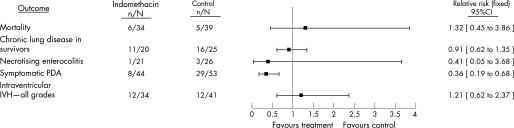
The Cochrane Database of Systematic Reviews includes reviews of two of these strategies of therapy.23,24 The review of prophylactic indomethacin for preventing mortality and morbidity in preterm infants suggests that there are several short‐term benefits of this treatment, including a reduction in the incidence of symptomatic PDA and serious intraventricular haemorrhage (IVH) (fig 1). In addition, fewer surgical ligations of the PDA are performed following this treatment. However, despite improvements in these outcomes, mortality and other morbidities (eg, CLD and NEC) are not reduced, and the reduction in severe IVH does not translate into improved developmental outcome.25 Treatment with indomethacin of infants with asymptomatic PDA seems to result in a similar profile of benefits with one notable exception24 (fig 2). Use of indomethacin in this strategy does not reduce the likelihood of IVH. This finding is not surprising because the treatment usually begins after the postnatal age at which IVH most probably will occur. There have been no reports of large, controlled trials of indomethacin for the treatment of symptomatic PDA conducted in the post‐surfactant era. A comprehensive report of older trials suggests a similar profile of risks and lack of long‐term benefits using this strategy.26 Treatment with indomethacin results in closure of approximately two‐thirds of symptomatic PDAs but does not reduce mortality or a serious morbidity.
Figure 1 A comparison of selected outcomes generated by the Cochrane Database of Systematic Reviews meta‐analysis of randomised controlled trials of prophylactic intravenous indomethacin for the prevention of mortality and morbidity in preterm infants with a patent ductus arteriosus (PDA). The point estimates with 95% confidence intervals are generated by combining the data from each trial, and the number of infants with the outcome of interest in each group is in the numerator with the total number of infants in the denominator.27 IVH, intraventricular haemorrhage. (Adapted from Laughon et al with permission from Lippincott Williams & Wilkins.)
Figure 2 A comparison of selected outcomes generated by the Cochrane Database of Systematic Reviews meta‐analysis of randomised controlled trials of indomethacin for asymptomatic patent ductus arteriosus (PDA) in preterm infants. The point estimates with 95% confidence intervals are generated by combining the data from each trial, and the number of infants with the outcome of interest in each group is in the numerator with the total number of infants in the denominator.27 IVH, intraventricular haemorrhage. (Adapted from Laughon et al with permission from Lippincott Williams & Wilkins.)
There has been considerable recent interest in ibuprofen as an alternative to indomethacin because it seems to have less effect on renal and cerebral blood flow. This potential advantage does translate into fewer infants with impaired renal function during treatment with ibuprofen compared with indomethacin.28 Ibuprofen seems to be as effective at promoting ductal closure, but like indomethacin, does not appear to reduce mortality or any serious morbidity; it may increase the likelihood of CLD when compared with indomethacin. Although a non‐significant reduction in serious IVH was observed in a recent study of prophylactic ibuprofen,29 this finding was not observed in the Cochrane review that included the results of four trials.30
Although no demonstrable benefit seems to result from closure of the PDA, one might still conclude that treatments to promote closure are indicated for the singular benefit of elimination of the ductus arteriosus if these therapies were entirely benign. Unfortunately, COX inhibitors do have adverse effects, and some of these may have long‐term consequences. They have potent effects on vasculature and organ perfusion.31 Decreased urine output and raised creatinine levels are frequent occurrences during treatment with indomethacin and ibuprofen, although this adverse effect seems to be less common with ibuprofen and is reversible with both drugs. Recent data indicate that indomethacin, especially in conjunction with corticosteroids, may cause intestinal perforation.32,33 A potential adverse effect was suggested from results of the largest trial to date of the effects of the prophylactic use of indomethacin.25 Infants without persistent ductal patency who were exposed to indomethacin were at increased likelihood of developing BPD compared with infants without PDA not exposed to the drug.34 Although this phenomenon may occur as a result of fluid retention during indomethacin treatment, and its negative effect on lung function, an alternative explanation is that indomethacin has a direct toxic effect on the lung.
Surgical closure of the ductus arteriosus
Surgical closure of the ductus arteriosus is usually reserved for infants in whom medical treatment has failed. Infants who undergo ligation are often critically ill. Therefore, it is not surprising that outcome following surgery is poor.35 In addition to high morbidity and mortality inherent to the population, there are complications related to the procedure, such as recurrent laryngeal nerve damage and pneumothorax. Recent reports suggest that there is a period of left ventricular dysfunction immediately following ligation,36 and that infants whose ductus arteriosus is ligated are at greater risk for poor developmental outcome compared with infants treated medically.37 Unfortunately, there have been no recent controlled trials comparing outcomes following ligation with outcomes following either placebo or medical treatment. Therefore, the risks and benefits of surgical ligation of the PDA are unknown.
It is discouraging that after the conduct of numerous trials of therapies for closure of the ductus arteriosus over several decades, we have little knowledge about their benefits and risks. This lack of knowledge has resulted, in part, from limitations imposed by study designs. Under the assumption that closure of a PDA is beneficial, almost all clinical trials in the modern era have focused on the most expeditious way in which to close a PDA. None have looked at the more fundamental question of whether closing the PDA improves outcome. Those trials that have included control groups treated with a placebo have permitted treatment of PDA that persisted after reaching a defined study endpoint, often just days after enrolment. This study design has resulted in high rates of treatment in the “placebo” group (usually in the range of 40%) and has markedly handicapped our ability to answer the fundamental question of whether closure of the PDA influences outcome. In addition, our ability to detect adverse effects of treatment is equally compromised. Despite these handicaps, the message from clinical trials is not encouraging. There is no evidence that the use of medical treatments for the prevention and treatment of PDA decreases mortality or serious morbidity, despite success in closure of the PDA.25,26,29,38,39
Commentary
Treatments to promote closure of the PDA cannot be justified on the basis of proven benefit, unless one finds the reduction in severe IVH with the prophylactic administration of indomethacin a sufficiently compelling benefit to justify its use. Therefore, the debate regarding the use of these therapies is in reality philosophical in nature. How should treatment decisions be made for individual infants in the absence of knowledge about benefits and risks of a treatment? Some would argue that biological plausibility of these treatments and the association between patency of the ductus arteriosus and morbidity is sufficient support for treatment. However, many commonly accepted therapies, whose use was supported by plausibility and an improvement in short‐term surrogate outcomes, were ultimately proved to be ineffective, hazardous and occasionally catastrophic.40
We argue that the converse approach should be undertaken. Therapies to prevent patency or effect closure of the ductus arteriosus should not be used unless there is irrefutable evidence of harm resulting from a PDA. Qualifying circumstances might include intractable hypotension or refractory congestive heart failure attributable to large aortopulmonary ductal shunts. Further, we urge the medical community to conduct trials that will provide clinicians with a precise understanding of the benefits and risks of treatments designed to close the ductus arteriosus. These trials would of course be randomised and placebo controlled. Besides evaluation of mortality and morbidity during the initial hospitalisation, outcomes of particular interest are: cardiopulmonary function in infants with persistent patency beyond the neonatal period; and adverse events associated with medications and procedures that may be required for treatment or closure of the PDA later in infancy or childhood. Some might argue that such trials would be unethical given the widespread acceptance and use of these treatments. We argue the contrary—that is, the medical community has an ethical obligation to conduct such trials rather than continue to use treatments with uncertain benefits and risks.
Abbreviations
BPD - bronchopulmonary dysplasia
CLD - chronic lung disease
COX - cyclo‐oxygenase
IVH - intraventricular haemorrhage
NEC - necrotising enterocolitis
PDA - patent ductus arteriosus
Footnotes
Competing interests: None.
References
- 1.Costeloe K, Hennessy E, Gibson A T.et al The EPICure study: outcomes to discharge from hospital for infants born at the threshold of viability. Pediatrics 2000106659–671. [DOI] [PubMed] [Google Scholar]
- 2.Marshall D D, Kotelchuck M, Young T E.et al Risk factors for chronic lung disease in the surfactant era: a North Carolina population‐based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics 19991041345–1350. [DOI] [PubMed] [Google Scholar]
- 3.Rojas M A, Gonzalez A, Bancalari E.et al Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr 1995126605–610. [DOI] [PubMed] [Google Scholar]
- 4.Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population‐based study. J Pediatr Gastroenterol Nutr 200540184–188. [DOI] [PubMed] [Google Scholar]
- 5.Clyman R I. Recommendations for the postnatal use of indomethacin: an analysis of four separate treatment strategies. J Pediatr 1996128(5 Pt 1)601–607. [DOI] [PubMed] [Google Scholar]
- 6.Gentile R, Stevenson G, Dooley T.et al Pulsed Doppler echocardiographic determination of time of ductal closure in normal newborn infants. J Pediatr 198198443–448. [DOI] [PubMed] [Google Scholar]
- 7.Van Overmeire B, Van de Broek H, Van Laer P.et al Early versus late indomethacin treatment for patent ductus arteriosus in premature infants with respiratory distress syndrome. J Pediatr 2001138205–211. [DOI] [PubMed] [Google Scholar]
- 8.Narayanan M, Cooper B, Weiss H.et al Prophylactic indomethacin: factors determining permanent ductus arteriosus closure. J Pediatr 2000136330–337. [DOI] [PubMed] [Google Scholar]
- 9.Koch J, Hensley G, Roy L.et al Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics 20061171113–1121. [DOI] [PubMed] [Google Scholar]
- 10.Nemerofsky S, Parravicini E, Kleinman C.et al The natural course of the ductus arteriosus in very low birthweight infants. E‐PAS 200659542 [Google Scholar]
- 11.Stefano J L, Abbasi S, Pearlman S A.et al Closure of the ductus arteriosus with indomethacin in ventilated neonates with respiratory distress syndrome. Effects of pulmonary compliance and ventilation. Am Rev Respir Dis 1991143236–239. [DOI] [PubMed] [Google Scholar]
- 12.Yeh T F, Luken J A, Thalji A.et al Intravenous indomethacin therapy in premature infants with persistent ductus arteriosus—a double‐blind controlled study. J Pediatr 198198137–145. [DOI] [PubMed] [Google Scholar]
- 13.Krauss A N, Fatica N, Lewis B S.et al Pulmonary function in preterm infants following treatment with intravenous indomethacin. Am J Dis Child 198914378–81. [DOI] [PubMed] [Google Scholar]
- 14.Farstad T, Bratlid D. Pulmonary effects of closure of patent ductus arteriosus in premature infants with severe respiratory distress syndrome. Eur J Pediatr 1994153903–905. [DOI] [PubMed] [Google Scholar]
- 15.Van Marter L J, Pagano M, Allred E N.et al Rate of bronchopulmonary dysplasia as a function of neonatal intensive care practices. J Pediatr 1992120938–946. [DOI] [PubMed] [Google Scholar]
- 16.Oh W, Poindexter B B, Perritt R.et al Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J Pediatr 2005147786–790. [DOI] [PubMed] [Google Scholar]
- 17.Shimada S, Kasai T, Konishi M.et al Effects of patent ductus arteriosus on left ventricular output and organ blood flows in preterm infants with respiratory distress syndrome treated with surfactant. J Pediatr 1994125270–277. [DOI] [PubMed] [Google Scholar]
- 18.Shimada S, Kasai T, Hoshi A.et al Cardiocirculatory effects of patent ductus arteriosus in extremely low‐birth‐weight infants with respiratory distress syndrome. Pediatr Int 200345255–262. [DOI] [PubMed] [Google Scholar]
- 19.Campbell M. Patent ductus arteriosus; some notes on prognosis and on pulmonary hypertension. Br Heart J 195517511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb G, Gatzoulis M A. Atrial septal defects in the adult: recent progress and overview. Circulation 20061141645–1653. [DOI] [PubMed] [Google Scholar]
- 21.Campbell M. Natural history of persistent ductus arteriosus. Br Heart J 1968304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heymann M A, Rudolph A M, Silverman N H. Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. N Engl J Med 1976295530–533. [DOI] [PubMed] [Google Scholar]
- 23.Fowlie P W. Intravenous indomethacin for preventing mortality and morbidity in very low birth weight infants. Cochrane Database Syst Rev 20002CD000174. [DOI] [PubMed] [Google Scholar]
- 24.Cooke L, Steer P, Woodgate P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev 20032CD003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt B, Davis P, Moddemann D.et al Long‐term effects of indomethacin prophylaxis in extremely‐low‐birth‐weight infants. N Engl J Med 20013441966–1972. [DOI] [PubMed] [Google Scholar]
- 26.Knight D B. The treatment of patent ductus arteriosus in preterm infants. A review and overview of randomized trials. Semin Neonatol 2001663–73. [DOI] [PubMed] [Google Scholar]
- 27.Laughon M M, Simmons M A, Bose C L. Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated? Curr Opin Pediatr 200416146–151. [DOI] [PubMed] [Google Scholar]
- 28.Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of a patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev 20032CD003481. [DOI] [PubMed] [Google Scholar]
- 29.Gournay V, Roze J C, Kuster A.et al Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double‐blind, placebo‐controlled trial. Lancet 20043641939–44 [DOI] [PubMed] [Google Scholar]
- 30.Shah S S, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev 20061CD004213. [DOI] [PubMed] [Google Scholar]
- 31.Christmann V, Liem K D, Semmekrot B A.et al Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin. Acta Paediatr 200291440–446. [DOI] [PubMed] [Google Scholar]
- 32.Watterberg K L, Gerdes J S, Cole C H.et al Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 20041141649–1657. [DOI] [PubMed] [Google Scholar]
- 33.Attridge J T, Clark R, Walker M W.et al New insights into spontaneous intestinal perforation using a national data set: SIP is associated with early indomethacin exposure. J Perinatol 20062693–99. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt B, Roberts R S, Fanaroff A.et al Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP). J Pediatr 2006148730–734. [DOI] [PubMed] [Google Scholar]
- 35.Lee L C, Tillett A, Tulloh R.et al Outcome following patent ductus arteriosus ligation in premature infants: a retrospective cohort analysis. BMC Pediatr 2006615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moin F, Kennedy K A, Moya F R. Risk factors predicting vasopressor use after patent ductus arteriosus ligation. Am J Perinatol 200320313–320. [DOI] [PubMed] [Google Scholar]
- 37.Kabra N, Schmidt B, Roberts R.et al Surgical closure of a patent ductus arteriosus (PDA) is associated with increased neurosensory impairment in extremely low birth weight (ELBW) infants. PAS 200557591 [Google Scholar]
- 38.Fowlie P W, Davis P G. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev 20023CD000174. [DOI] [PubMed] [Google Scholar]
- 39.Van Overmeire B, Allegaert K, Casaer A.et al Prophylactic ibuprofen in premature infants: a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 20043641945–1949. [DOI] [PubMed] [Google Scholar]
- 40.Silverman W.Retrolental fibroplasia: a modern parable. New York: Grune & Stratton, Inc, 1980