Abstract
Background
A patent ductus arteriosus (PDA) is common among preterms, and prophylactic medical treatment has been advocated as the first‐line approach. Conservative treatment may result in similar outcome, but without exposure to the harmful side effects of medication. A retrospective analysis revealed a ductal closure rate of 94% after conservative treatment with adjustment of ventilation (lowering the inspiratory time and increasing positive end expiratory pressure) and fluid restriction.
Objective
To study prospectively over one year the rate of PDA closure, and morbidity and mortality following conservative treatment.
Method
Prospective study (1 January 2005 – 31 December 2005) including 30 newborns ⩽30 weeks' gestation, all of whom were being ventilated and required surfactant. Echocardiography was performed 48–72 h after birth. Clinically important PDA was conservatively treated as described above. The percentage of children with PDA, ductal ligation and major complications was determined.
Results
Ten neonates (33%) developed a clinical important PDA. Following conservative treatment the duct closed in all neonates (100%), and none required ductal ligation or medical treatment. The rates of major complications were no higher than those reported by the Vermont Oxford Network and in the literature.
Conclusion
The managed care plan resulted in an overall ductal closure rate of 100%. These results suggest that conservative treatment of PDA is a worthy alternative to prophylactic medical treatment.
Keywords: PDA, preterm, outcome, conservative treatment, ibuprofen prophylaxis
The ductus arteriosus closes spontaneously in most full‐term infants during the first three days of life, but in preterm neonates it often fails to close. The incidence of patent ductus arteriosus (PDA) in preterm neonates varies from 40% to 60% on the third day of life, depending on estimated gestational age. Therefore, it continues to be one of the commonest problems in preterm neonates.1,2,3,4,5,6,7,8,9
It is important to make distinguish between a clinically significant and non‐significant PDA.1,2,5,10 A clinically important PDA is characterised by respiratory problems with ventilation difficulties, metabolic acidosis, and pulmonary congestion with tachycardia and bounding pulses. The consequence of this left‐to‐right shunt is an increased risk of complications, including intraventricular haemorrhage (IVH), necrotising enterocolitis (NEC), chronic lung disease (CLD) and death. Hence, PDA affects key outcome variables of early preterm life.3,5,11,12,13,14,15
Currently, many preterm care units implement systematic treatment of PDA with ibuprofen or indometacin. On the basis of studies comparing the efficacy and safety of both drugs, ibuprofen has been proposed as the drug of choice: the rate of closure of PDA was comparable with both drugs, but ibuprofen was associated with fewer side effects.1,4,12,16 However, controversy still exists about the optimal timing for starting ibuprofen (prophylactic or therapeutic).17 An important issue is the lack of documentation of side effects, especially long term side effects following its prophylactic use.6,17 Moreover, its value is still questionable, as data remain scanty on the outcome of PDA following conservative treatment according to current standards.5,6,7,8,9,10,11,15,16,17,18 Current conservative treatments include adjustment of ventilation by reducing inspiratory time and giving more positive end expiratory pressure (PEEP), and fluid restriction not exceeding 130 ml/kg a day beyond day 3.2 With the use of this procedure, we have noticed a high closure rate of PDA at our centre. Therefore, with the aim of establishing the best possible managed care plan, we prospectively quantified the outcome of PDA closure and its complications in our preterm population, and evaluated whether prophylactic ibuprofen is needed.
Methods
We retrospectively analysed all medical records in the neonatal intensive care unit at the hospital of Genk, Belgium, from 1 January 1999 to 31 December 2004 (fig 1; table 1). PDA was initially clinically diagnosed based on the detection of murmur, deterioration of respiratory function, metabolic acidosis and/or blood pressure problems. Treatment was started on a clinical basis, and the diagnosis was confirmed by echocardiography (DA diameter ⩾1.4 mm, completed with Doppler colour flow). After conservative treatment, the remaining patency of PDA was confirmed echocardiographically. If the duct had failed to close, it was ligated.
Figure 1 Occurrence of patent ductus arteriosus (PDA) in 109 conservatively managed preterm neonates ⩽30 weeks' gestation, requiring ventilation and surfactant treatment (retrospective analysis). PEEP, positive end expiratory pressure; Ti, inspiratory time.
Table 1 Complications in 109 conservatively managed preterm neonates ⩽30 weeks' gestation, requiring ventilation and surfactant treatment: comparison with data from the Vermont Oxford Network.
| Proportion (%) of neonates with complications | ||
|---|---|---|
| Conservative treatment* (N = 109) | Medical treatment† (reference database) | |
| Necrotising enterocolitis | 0 | 4–6 |
| Intraventricular haemorrhage grade 3 | 7 | 6–7 |
| Chronic lung disease | 8 | 18–28 |
| Death | 13 | 12 |
*See Methods: Adjusment of ventilation and fluid restriction, and if PDA still present, ductal ligation carried out
†Range based on data from the Vermont Oxford Network and data on outcome with ibuprofen and/or indometacin, as provided by Orphan Europe (http://www.Orphan‐europe.com).19,20,21,22,23,24,25,26
On the basis of the excellent results of the retrospective analysis a managed care plan was developed. We then undertook a prospective study in our neonatal unit from 1 January 2005 to 31 December 2005. Neonates were eligible if born at ⩽30 weeks' gestation, if they were being ventilated and required surfactant replacement. Echocardiography was carried out for every neonate 48–72 h after birth. All infants with PDA were treated following our centre's standard protocol as soon as a diagnosis of an haemodynamically important PDA was made (DA diameter ⩾1.4 mm, completed with Doppler colour flow): conservative treatment consisting of fluid restriction (maximum 130 ml/kg a day beyond day 3) and adjustment of ventilation by lowering inspiratory time to as low as 0.35 s, and giving higher PEEP (as high as 4.5 mbar). (Usual practice in our ward includes inspiratory time 0.4–0.45 s and PEEP 3.5–4.0 mbar.) For a PDA that did not show clinical improvement and/or deteriorated, and for continuing need for ventilatory support, ductal ligation was carried out. All other PDAs closed with conservative treatment. We did not use any medication for prophylactic or therapeutic treatment of PDA.
Outcome was assessed by analysing the percentage of children with PDA, ductal ligation and major complications. The rate of occurrence of NEC (Bell staging 2–3), IVH grade 3, CLD n‐continuous positive airways pressure (nCPAP) and/or oxygen need beyond 36 weeks' gestational age) and death were compared with data from the Vermont Oxford Network and the data on outcome with ibuprofen and/or indometacin, as provided by Orphan‐Europe (http://www.Orphan‐europe.com).19,20,21,22,23,24,25,26
Results
Patent ductus arteriosus
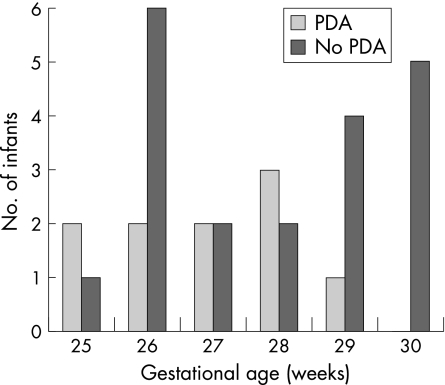
A total of 30 neonates (46% boys, 54% girls; mean gestational age 26.6 weeks (range 25–30 weeks); mean birth weight 994 g (600–1484 g)) were included in the analysis (fig 2). The infants with and without PDA did not differ significantly with regard to birth weight and gestational age (Mann Whitney U test, fig 3). The median gestational age of two groups was 27 (interquartile range 26–28) weeks and 28 (interquartile range 26–30) weeks, respectively. The median birth weight of the group with PDA was 1010 g (interquartile range 825–1425 g) and of the group without PDA was 926 g (interquartile range 785–1208 g). Figure 2 illustrates the outcome of the study population: 20 neonates (67%) had no clinical significant PDA and therefore received no extra treatment. Clinically important PDA was found in 10 neonates (33%). Adjustment of ventilation and fluid restriction led to closure of all PDAs. Ductal ligation was not needed.
Figure 2 Flow chart of prospective study of occurrence of patent ductus arteriosus (PDA) in 30 conservatively managed preterm neonates ⩽30 weeks' gestation, requiring ventilation and surfactant treatment. PEEP, positive end expiratory pressure; Ti, inspiratory time.
Figure 3 Distribution of infants with and without a patent ductus arteriosus (PDA) according to gestational age.
Complications
Overall, none of the infants in this series developed NEC; 2% developed IVH and 7% developed CLD (Table 2). According to the records, total mortality (any cause) added up to 12% during stay in the unit.
Table 2 Occurrence of complications in 30 conservatively managed preterm neonates ⩽30 weeks' gestation, requiring ventilation and surfactant treatment: comparison with data from the Vermont Oxford Network.
| Proportion (%) of neonates developing complications | ||
|---|---|---|
| Conservative treatment* (N = 30) | Medical treatment† (reference database) | |
| Necrotising enterocolitis | 0 | 4–6 |
| Intraventricular haemorrhage grade 3 | 2 | 6–7 |
| Chronic lung disease | 7 | 18–28 |
| Death | 12 | 12 |
*See Methods: Adjustment of ventilation and fluid restriction, and if PDA still present, ductal ligation.
†Range based on the data from the Vermont Oxford Network and data on outcome with ibuprofen and/or indometacin, as provided by Orphan Europe (http://www.Orphan‐europe.com).19,20,21,22,23,24,25,26
Discussion
What is already known on this topic
Many preterm care units implement systematic treatment of patent ductus arteriosus (PDA) with ibuprofen or indometacin. Ibuprofen has been proposed as the drug of choice as closure rates of PDA are comparable with both but ibuprofen is associated with fewer side effects.
Controversy still exits about the optimal timing for starting this treatment (prophylactic or therapeutic).
There is a lack of documentation of side effects following prophylactic use of ibuprofen, especially in the long term.
Its value remains questionable, as data are scanty on the outcome of PDA following conservative treatment according to current standards.
What this study adds
The results of this study do not support the use of pharmacological treatment with ibuprofen, as proposed by some centres.
Conservative treatment avoids exposure of preterm infants to potential side effects of medication.
This findings of this study are therefore relevant to the international medical community.
PDA continues to be a common problem among preterm infants. Although many studies have been published on medical and surgical treatment, only few studies have evaluated the outcome of current conservative treatment that includes adjusting ventilation and fluid restriction. Moreover, ibuprofen appears to have been mainly evaluated against indometacin, and prophylactic studies seem to have defined success by the status of ductal closure on day 3 of life, rather than considering the overall outcome.17
In our population of preterm babies of ⩽30 weeks' gestational age, 72% had spontaneous closure of PDA. The children who had a clinically important PDA (28%) (echocardiographically confirmed) were all conservatively treated as soon as diagnosis was made. With fluid restriction to a maximum of 130 ml/kg/day beyond day 3 and adjustment of ventilation by decreasing inspiratory time and increasing PEEP, the PDA closed in another 22%, resulting in a total closure rate of 94%. This rate compares well with the rates reported in literature following medical treatment (80–92%).1,2,6,13,15,16,18
Given that our retrospective analysis revealed a PDA closure rate of 94% after conservative treatment, we wondered whether prophylactic ibuprofen, the currently recommended drug of choice, was indicated. We postulated that a high rate of PDA closure could be achieved with conservative treatment, thereby avoiding potential side effects of medical treatment. Our excellent retrospective results were confirmed in the prospective study with even more convincing results (an overall closure rate of 100%).
Ibuprofen is widely used for prophylaxis. Compared with indometacin, it is associated with a lower risk of oliguria.12 Prophylactic use of ibuprofen has no major influence on reducing morbidity or the need of surgical PDA closure.11 Importantly, one study showed an increased risk of pulmonary hypertension and had to be terminated early.6 An increased incidence of NEC was also reported in the treatment group in that study.6 Hammerman and Kaplan recently observed that “ibuprofen is not as benign as implied by much of the PDA literature”: early postnatal administration in small premature neonates may be associated with more complications than later therapeutic use, after further postnatal maturation.17 The 2003 Cochrane systematic review on ibuprofen prophylaxis concluded that although prophylactic ibuprofen use reduces the incidence of PDA on day 3, the potential adverse effects should be further addressed, along with neurodevelopmental outcomes.11
Our results indicate that ibuprofen prophylaxis would have unnecessarily exposed the majority of our preterm neonates to the risk of side effects. At least for the acute treatment of PDA, a Cochrane review in 2003 concluded that the data on net benefit/harm were insufficient to conclude whether surgical ligation or medical treatment is preferred as initial treatment for symptomatic PDA in preterm infants.3 As the rate of complications in our study population compared well with the currently established reference rates using medication (table 1 and 2), our findings further support our approach (figs 1 and 2) as a favourable alternative to medication prophylaxis and a valid managed care plan.
In conclusion, the rate of PDA closure achieved with conservative treatment at our centre was comparable to the rates previously reported with drug prophylaxis. Although our approach resulted in a similar risk profile for major complications, it did so without exposing the neonates to potential side effects of drug treatment. The results of our retrospective analysis were confirmed prospectively with even better results. Therefore, we postulate that prophylactic use of ibuprofen is not indicated and that conservative treatment by means of adjusting ventilation (inspiratory time as low as 0.35 s and PEEP as high 4.5 mbar) and fluid restriction (130 ml/kg/day beyond day 3) is a more favourable alternative, following the first law of medicine “primum non nocere”.
To confirm our results, we recommend carrying out prospective multicentre randomised controlled trials with larger patient samples comparing conservative treatment (with placebo) with pharmacological treatment.
Acknowledgement
We thank Suzy Huijghebaert for assisting us in preparing and submitting this manuscript.
Abbreviations
CLD - chronic lung disease
IVH - intraventricular haemorrhage
NEC - necrotising enterocolitis
PDA - patent ductus arteriosus
PEEP - positive end expiratory pressure
Footnotes
Competing interests: None.
Ethics committee approval and patient consent: Not needed (analysis of outcome of standard procedure in our unit).
References
- 1.Van Overmeire B, Follens I, Hartman S.et al Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child Fetal Neonatal Ed 199776F179–F184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wyllie J. Treatment of patent ductus arteriosus. Semin Neonatol 20038425–432. [DOI] [PubMed] [Google Scholar]
- 3.Malviya M, Ohlsson A, Shah S. Surgical versus medical treatment with cyclooxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev 20033CD003951. [DOI] [PubMed] [Google Scholar]
- 4.Flores M. Ibuprofen: alternative treatment for patent ductus arteriosus. Neonatal Netw 20032227–31. [PubMed] [Google Scholar]
- 5.Knight D B. The treatment of patent ductus arteriosus in preterm infants. A review and overview of randomized trials. Semin Neonatol 2001663–73. [DOI] [PubMed] [Google Scholar]
- 6.Gournay V, Roze J C, Kuster A.et al Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double‐blind, placebo‐controlled trial. Lancet 20043641939–1944. [DOI] [PubMed] [Google Scholar]
- 7.Furzan J A, Reisch J, Tyson J E.et al Incidence and risk factors for symptomatic patent ductus arteriosus among inborn very‐low‐birth‐weight infants. Early Hum Dev 19851239–48. [DOI] [PubMed] [Google Scholar]
- 8.Mouzinho A I, Rosenfeld C R, Risser R. Symptomatic patent ductus arteriosus in very‐low‐birth‐weight infants: 1987–1989. Early Hum Dev 19912765–77. [DOI] [PubMed] [Google Scholar]
- 9.Koch J, Hensley G H, Roy L.et al Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics 20061171113–1121. [DOI] [PubMed] [Google Scholar]
- 10.Hammerman C, Strates E, Valaitis S. The silent ductus: its precursors and its afterdeath. Pediatr Cardiol 19867121–127. [DOI] [PubMed] [Google Scholar]
- 11.Shah S S, Ohlsson A. Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev 20032CD004213. [DOI] [PubMed] [Google Scholar]
- 12.Ohlsson A, Walia R, Shah S. Ibuprofen for the treatment of a patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev 20032CD003481. [DOI] [PubMed] [Google Scholar]
- 13.Koehne P S, Bein G, Alexi‐Meskhishvilli V.et al Patent ductus arteriosus in very low birthweight infants: complications of pharmacological and surgical treatment. J Perinat Med 200129327–334. [DOI] [PubMed] [Google Scholar]
- 14.Brooks J M, Travadi J N, Patole S K.et al Is surgical ligation of patent ductus arteriosus necessary? The Western Australian experience of conservative management. Arch Dis Child Fetal Neonatal Ed 200590F190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowlie P W, Davis P G. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev 20023CD000174. [DOI] [PubMed] [Google Scholar]
- 16.Van Overmeire B, Allegaert K, Casaer A.et al Prophylactic ibuprofen in premature infants: a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 20043641945–1949. [DOI] [PubMed] [Google Scholar]
- 17.Hammerman C, Kaplan M. Primum non nocere: prophylactic versus curative ibuprofen. Lancet 20043641920–1922. [DOI] [PubMed] [Google Scholar]
- 18.Cooke L, Steer P, Woodgate P. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev 20032CD003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermont Oxford Network http://www.vrtoxford.org
- 20.Varvarigou A, Bardin C L, Beharry K.et al Early ibuprofen administration to prevent patent ductus arteriosus in premature newborn infants. JAMA 1996275539–544. [PubMed] [Google Scholar]
- 21.Mosca F, Bray M, Lattanzio M.et al Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr 1997131549–554. [DOI] [PubMed] [Google Scholar]
- 22.Pezzati M, Vangi V, Biagiotti R.et al Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. J Pediatr 1999135733–738. [DOI] [PubMed] [Google Scholar]
- 23.Dani C, Bertini G, Reali M F.et al Prophylaxis of patent ductus arteriosus with ibuprofen in preterm infants. Acta Paediatr 2000891369–1374. [DOI] [PubMed] [Google Scholar]
- 24.De Carolis M P, Romagnoli C, Polimeni V.et al Prophylactic ibuprofen therapy of patent ductus arteriosus in preterm infants. Eur J Pediatr 2000159364–368. [DOI] [PubMed] [Google Scholar]
- 25.Patel J, Roberts I, Azzopardi D.et al Randomized double‐blind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatr Res 20004736–42. [DOI] [PubMed] [Google Scholar]
- 26.Van Overmeire B, Smets K, Lecoutere D.et al A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med 2000343674–681. [DOI] [PubMed] [Google Scholar]