Abstract
Background
When medical treatment of a symptomatic arterial duct in a preterm infant fails, management is surgical.
Patients and Methods
10 preterm neonates referred to a tertiary cardiac centre for treatment of a symptomatic patent arterial duct who underwent cardiac catheterisation with the intention of device closure.
Results
Successful catheter device closure of the arterial duct in nine preterm infants is described.
Conclusion
In selected cases, catheter device closure may offer an alternative to thoracotomy and surgical ligation.
Keywords: arterial duct, preterm, device closure
When a significant arterial duct is diagnosed in a preterm infant with the attendant multiple problems of prematurity, the treatment strategy is correction of anaemia, fluid restriction and diuretics. If these measures fail, a course of a non‐steroidal anti‐inflammatory drug (NSAID) is followed by surgical ligation if this medical treatment is unsuccessful.1 In a proportion of infants, the use of an NSAID is contraindicated.1,2 The current UK surgical Central Cardiac Audit Database for infants weighing < 2.5 kg and undergoing surgical ligation of the arterial duct documents a 30 day mortality of 8% for this group of patients.3 Avoiding a thoracotomy in an infant with chronic lung disease is clearly a worthwhile goal.
Patients and methods
Ten infants underwent cardiac catheterisation with the intention of device closure of the arterial duct. All patients were referred from other neonatal units with the opinion of the referring neonatologist that the arterial duct was a possible contributor to the infant's lack of progress. Table 1 summarises the details of the patients. All were symptomatic requiring various levels of ongoing respiratory support including intermittent positive pressure ventilation, nasal continuous positive airway pressure, nasal oxygen and diuretics. Seven patients had received between one and three courses of indomethacin and/or ibuprofen. In one patient (case 1) the use of an NSAID was contraindicated because of an associated cerebral bleed, necrotising enterocolitis and thrombocytopenia. In all patients, there was echocardiographic evidence of a significant left to right shunt (table 2).
Table 1 Summary of patient details.
| Case | Gestation (weeks) | Post‐conceptual age at procedure (weeks) | Birth weight (g) | Procedure weight (g) | Respiratory support | Courses of NSAIDs |
|---|---|---|---|---|---|---|
| 1 | 26 | 38 | 712 | 1600 | CPAP | 0 |
| 2 | 25 | 36 | 760 | 1993 | CPAP | 2 |
| 3 | 26 | 43 | 660 | 2200 | CPAP | 0 |
| 4 | 33 | 36 | 2460 | 2500 | Nasal O2 | 0 |
| 5 | 24 | 40 | 695 | 2610 | Nasal O2 | 3 |
| 6 | 24 | 40 | 696 | 2650 | Nasal O2 | 1 |
| 7 | 28 | 40 | 1200 | 2100 | IPPV | 1 |
| 8 | 27 | 41 | 645 | 2200 | CPAP | 3 |
| 9 | 25 | 36 | 670 | 1777 | Nasal O2 | 3 |
| 10 | 30 | 39 | 960 | 2300 | Nasal O2 | 3 |
CPAP, continuous positive airway pressure; IPPV, intermittent positive pressure ventilation; NSAID, non‐steroidal anti‐inflammatory drug.
Table 2 Summary of echocardiographic findings.
| Case | Arterial duct size (mm) | LA/aortic ratio | Subjective left heart volume loading | PFO or ASD present | LPA Doppler velocity after device deployment (m/s) |
|---|---|---|---|---|---|
| 1 | 2 | 2 | Yes | No | 2.3 |
| 2 | 2.4 | 1.9 | Yes | PFO | 1.4 |
| 3 | 2 | 1.5 | Yes | PFO | 1.6 |
| 4 | 3.6 | 2.3 | Yes | PFO | 1.8 |
| 5 | 2.7 | 1.8 | Yes | PFO | < 1 |
| 6 | 2.8 | 1.9 | Yes | PFO | Surgical ligation |
| 7 | 2 | 1 | Yes | ASD | 2.2 |
| 8 | 2.7 | 1.6 | Yes | PFO | 0.8 |
| 9 | 2.6 | 1.6 | Yes | PFO | 0.9 |
| 10 | 3 | 1.7 | Yes | PFO | <1 |
PFO, patent foramen ovale; ASD, atrial septal defect.
Results
The pulmonary artery pressures ranged between half systemic and systemic in all patients. Deployment of a device was achieved in 9/10 cases. In one patient (case 6), the largest available coil (6.5 mm) was unstable, thus it was removed and the patient referred for surgical ligation. In total, eight flipper‐controlled release coils (Cook) ranging from 3 to 6.5 mm diameter and one Amplatzer duct occluder were used. On‐table closure was achieved in two cases on angiography and in 7/9 by day 2 on echocardiography and Doppler measurement. Two infants had haemodynamically insignificant residual flow at the time of transfer back to their referral units. In both cases, the ducts have since closed completely. Procedure time, which includes anaesthesia administration and recovery, ranged from 80 to 180 min (mean 113), fluoroscopy time from 8.2 to 27.3 min (mean 16), and fluoroscopy dose from 56 to 183 cGy/cm2 (mean 97). There have been no deaths with a maximum follow up period of 21 months.
Figure 1 Aortograms before and after release of a coil in a 1.6 kg infant.
Immediate objective evidence of improvement after duct closure was difficult to assess in these patients because of the complex problems of prematurity, in particular chronic lung disease. Two patients (cases 4 and 5) discontinued receiving diuretics immediately, and one (case 4) was out of oxygen by the following day. With time, most of the extremely preterm infants had a reduction in oxygen requirements and ventilatory support. One infant (patient 7) with severe congenital muscular dystrophy was successfully extubated 1 month after duct occlusion, multiple previous attempts having failed.
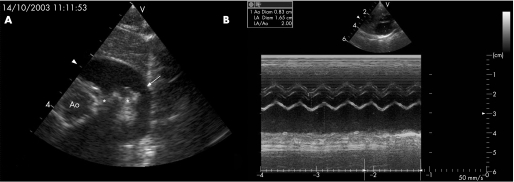
Figure 2 (A) Parasternal short axis echocardiogram showing a large arterial duct (case 4). The arrow indicates the arterial duct and the asterisk the origins of the left and right pulmonary arteries. Ao, aorta. (B) Left artery (LA)/aortic ratio of 2 (case 1).
Complications included catheter‐induced transient hypotension during device deployment in two patients. Two patients received intravenous heparin overnight after the procedure and subsequent low‐dose aspirin for temporary femoral artery insufficiency. Doppler evidence of left pulmonary artery (LPA) stenosis was noted in 5/9 infants before discharge but with the velocity only >1.6 m/s in three cases. This is resolving spontaneously in all cases. No aortic gradient was present in any infant on pull‐back measurements after device release.
Discussion
The preterm infant with complications of prematurity and an arterial duct is usually sick and a poor candidate for any intervention, surgical or otherwise. The 30‐day surgical mortality obtained from the UK Central Cardiac Audit Database probably reflects a smaller and sicker group of infants than those in our series. However, this is the only nationally validated data available that we are aware of and are broken down as infants ⩾2.5 kg and <2.5 kg. Until recently, data on the outcome of infants with a significant arterial duct pre‐dates the use of surfactant, prostaglandin synthetase inhibitors and more recent ventilatory strategies.4,5 In a retrospective study of 252 infants (gestation <28 weeks), Brooks and colleagues found that there was an increased mortality in the presence of a persistent arterial duct, but no difference with respect to morbidity from chronic lung disease, necrotising enterocolitis, intracranial haemorrhage, duration of oxygen therapy, and duration of hospital stay.6 The precise contribution of a patent arterial duct to the clinical condition can be difficult to quantify, but has been implicated in contributing to lung disease, necrotising enterocolitis and intracerebral haemorrhage.1,4 Two patients had clear early evidence of improvement after closure of the duct (oxygen and diuretics discontinued), but in the remaining infants no benefit was immediately evident. In general, the more preterm the infants with worse lung disease and complications of prematurity, the more difficult it was to demonstrate clear benefit after duct closure. Avoiding a thoracotomy is desirable to avoid both the acute effects and long‐term sequelae with scoliosis.7,8 A short surgical procedure needs to be weighed against a potentially longer cardiac catheter procedure. Both approaches have their risks.
Table 3 Summary of catheter data.
| Case | Device deployed | Procedure time (min) | Fluoroscopy time (min) | Fluoroscopy dose (cGy/m2) |
|---|---|---|---|---|
| 1 | 5 mm coil | 180 | 19.3 | 61 |
| 2 | 5 mm coil | 123 | 20.4 | 183 |
| 3 | 3 mm coil | 121 | 8.6 | 56 |
| 4 | 5/4 ADO | 111 | 16.4 | 127 |
| 5 | 5 mm coil | 86 | 8.2 | 84 |
| 6 | Failed | 122 | 27 | 201 |
| 7 | 5 mm coil | 86 | 13.9 | 94 |
| 8 | 5 mm coil | 73 | 11.3 | 85 |
| 9 | 6.5 mm coil | 127 | 27.3 | 93 |
| 10 | 3 mm coil | 101 | 11.7 | 44 |
The most common finding after device closure of the arterial duct was pulmonary branch stenosis evident by flow acceleration and increased velocity on Doppler insonation. In all cases, the LPA stenosis is improving or has resolved completely as it does in physiological pulmonary branch stenosis with spontaneous duct closure in infants.9,10 The mechanism for LPA stenosis after device closure is likely to be different; however, instrumentation of the arterial duct does induce spasm in some cases and therefore LPA stenosis may not solely be due to mechanical obstruction by the device but may also involve some spasm at the LPA end of the arterial duct. We did not think that any long‐term or problematic ventilation/perfusion mismatch was induced. However, no formal data to support this were obtained.
What is already known on this topic
Transcatheter device closure of the arterial duct is a well‐established technique in older children.
What this study adds
Transcatheter device closure of the arterial duct in infants below 2.5 kg is technically possible and in selected preterm infants in whom medical treatment has failed may offer an alternative strategy to thoracotomy and surgical closure.
Current practice in the UK is that catheter device closure of the arterial duct is generally reserved for infants who weigh 5 kg or more. Device closure of the arterial duct is well established in children and was first performed 38 years ago.11,12,13 We believe that this is the first report of transcatheter device closure in a series of preterm infants.
Limitations
Pressure measurement of LPA stenosis after device release was not recorded to avoid prolongation of the procedure and because of concerns about device displacement on manipulating the catheter in the LPA. In the presence of a normal right pulmonary artery, both Doppler and pressure measurements provide limited quantification of the degree of narrowing because of preferential flow down the right pulmonary artery. Shunt size was not measured during catheterisation because of time, the potential for catheter‐induced haemodynamic instability, and the potential for blood loss.
Conclusions
In selected cases, device closure of the arterial duct in preterm infants can be considered as an alternative to thoracotomy and surgical closure.
Abbreviations
LPA - left pulmonary artery
NSAID - non‐steroidal anti‐inflammatory drug
Footnotes
Sponsorship: None
Competing interests: None.
References
- 1.Archer N. Patent arterial duct in the newborn. Arch Dis Child 199369529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer N. Drug induced closure of the patent arterial duct. Heart 199676384–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham D. NHS Information Authority
- 4.Ellison R C, Peckham G J, Lang P.et al Evaluation of the preterm infant for patent arterial duct. Paediatrics 198371364–372. [PubMed] [Google Scholar]
- 5.Jones R N A, Pickering D. Persistent arterial duct complicating the respiratory distress syndrome. Arch Dis Child 197752274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks J M, Travadi J N, Patole S K.et al Is surgical ligation of the patent ductus arteriosus necessary? The Western Australian experience of conservative management. Arch Dis Child Fetal Neonatal Ed 200590F235–F239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Biezen F C, Bakx P A, Villeneuve V H.et al Scoliosis in children after thoracotomy for aortic coarctation. J Bone Joint Surg Am 199375(4)514–518. [DOI] [PubMed] [Google Scholar]
- 8.Bal S, Elshershari H, Celiker R.et al Thoracic sequels after thoracotomies in children with congenital cardiac disease. Cardiol Young 200313264–267. [PubMed] [Google Scholar]
- 9.Arlettaz R, Archer N, Wilkinson A. Natural history of innocent murmurs in newborn babies: controlled echocardiographic study. Arch Dis Child Fetal Neonatal Ed 199878166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arlettaz R, Archer N, Wilkinson A. Closure of the ductus arteriosus and development of pulmonary branch stenosis in babies of less than 32 weeks gestation. Arch Dis Child Fetal Neonatal Ed 200185197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porstmann W, Wierney L, Warnke H. Closure of the persistent arterial duct without thoracotomy. Ger Med Mon 196712259–261. [PubMed] [Google Scholar]
- 12.Faella H J, Hijazi Z M. Closure of the patent arterial duct with the Amplatzer PDA device: immediate results of the international clinical trial. Catheter Cardiovasc Interv 20005150–54. [DOI] [PubMed] [Google Scholar]
- 13.Butera G, De G Rosa, Chessa M.et al Transcatheter closure of the persistent arterial duct with the Amplatzer duct occluder in very young symptomatic children. Heart 2004901467–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]