Abstract
Objective
To determine circulating levels of adiponectin in preterm infants and examine possible associations with anthropometric measurements, weight gain, and leptin and insulin levels.
Design
Prospective study.
Setting
A university hospital neonatal care unit.
Study population
62 preterm (mean (SD) gestational age 32.0 (2.1) weeks) and 15 full‐term infants (reference group).
Interventions
Blood samples taken at discharge (40.9 (14.8) days of life) from the preterm infants and at a comparable postnatal age in full‐term infants. All infants were fed the same commercial formula, but in nine preterms the formula contained long‐chain polyunsaturated fatty acids (LCPUFAs).
Main outcome measures
Serum levels of adiponectin, leptin and insulin. Associations of adiponectin levels were tested only in the preterm group.
Results
Serum levels of adiponectin were lower in preterm (40.9 (14.8) μg/ml) than full‐term infants (53.1 (16.0) μg/ml, p<0.01). However, after adjustment for body weight, the influence of prematurity on adiponectin levels was no longer significant. In preterm infants, adiponectin levels independently correlated with being born small for gestational age (SGA) (β = −0.35, p = 0.01), weight gain (β = 0.28, p = 0.03) and LCPUFA‐supplemented formula (β = 0.34, p = 0.009). Serum adiponectin levels did not correlate with insulin or leptin levels. However, insulin levels were higher in preterm than in full‐term infants after adjustment for body weight.
Conclusions
Adiponectin levels are lower in preterm infants at discharge than full‐term infants probably due to decreased adiposity. The levels are influenced by being born SGA, weight gain and, possibly, by dietary LCPUFAs. The importance of these findings in the development of insulin or leptin resistance in children born prematurely needs to be further studied.
Keywords: adiponectin, leptin, insulin, preterm infants
Nutrition and growth during fetal life and early infancy have been linked to health and disease in later life.1,2,3 There is also evidence that insulin resistance is prevalent among children born prematurely, irrespective of whether they were appropriate or small for their gestational age.4 Adiponectin, the most abundant circulating adipose‐derived protein, has an insulin‐sensitising role, possibly protecting against the development of insulin resistance and metabolic syndrome X.5
In adults, circulating adiponectin levels are inversely related to the degree of adiposity and to serum leptin levels.5,6 There is evidence that, even in children 5–10 years of age, circulating adiponectin levels follow the adult pattern, correlating negatively with the degree of adiposity.7 In contrast, in full‐term neonates, during the first few days of life, serum or plasma adiponectin levels correlate positively with size at birth, neonatal adiposity and circulating levels of leptin.8,9,10,11,12,13,14 It has been suggested that the factors implicated in adiponectin inhibition, which are mainly related to the amount/distribution of body fat, are not operative in newborns.9 It has also been proposed that adiponectin has a role in fetal growth and development.13,14 However, others have not found such correlations.15,16,17
Studies of adiponectin levels in neonates have been conducted no later that the fifth day of life. Regarding preterm infants, there has been little research and only on day 1 of life.12 The present study aimed to determine the circulating levels of adiponectin in preterm infants and to examine possible associations with size, weight gain, and serum levels of leptin and insulin. To make a valid assessment of the relation between adiponectin levels and infant growth, adiponectin levels were obtained around the end of the neonatal period.
Subjects and methods
Our hospital's ethics committee approved the study. Informed parental consent was also obtained.
Subjects and study protocol
We included preterm infants admitted to our unit after birth provided that:
they did not have any congenital malformation or serious morbidity (necrotising enterocolitis, bronchopulmonary dysplasia, intraventricular haemorrhage greater than grade I according to Volpe's criteria18);
their mothers had chosen formula feeding;
they did not have an infection, at least during the past 10 days of hospitalisation.
We estimated the gestational age from the last menstrual period and this was supported by fetal ultrasound measurements and clinical examination of the neonate according to the New Ballard Score.19
The feeding protocol and the method of assessment of growth have been described previously in a report about serum levels of peptide YY and ghrelin in the same study population.20 Briefly, infants <1500 g, or not well during the first days of life, were initially given total parenteral nutrition, which was gradually replaced by oral feeds. Infants <32 weeks' postconceptional age and/or those with immature sucking were given two‐hourly bolus feeds through a nasogastric tube. After sucking feeds had been established, the infants were given formula every 3 h. Finally, all infants included in the study were bottle fed every 3 h, at least during the last week of hospitalisation. All infants were fed the same commercial formula (S‐26, Wyeth Nutritionals, Ireland). However, the formula given to nine preterm infants contained long‐chain polyunsaturated fatty acids (LCPUFAs) (arachidonic acid 12.0 mg/100 ml and docosahexaenoic acid 7.1 mg/100 ml of formula). This LCPUFA‐supplemented formula had just been introduced into the market by the manufacturer; however, apart from LCPUFAs, the nutrient composition of the new formula was identical to that of the supplement‐free formula. Both formulas offered 67 kcal/100 ml. The amount of formula consumed at each meal was recorded and the sum of kcal/kg/day over the last week of hospitalisation was calculated.
Body weight was measured daily, using a standard electronic scale. Recumbent length and head circumference were measured weekly by the same investigator. Measurements of birth weight and body weight at discharge were converted to z scores (SD scores from the mean after adjustment for gestational age and sex) with a software program using reference data for infants born at 24–44 weeks' gestation (http://www.biomedcentral.com/1471–2431/3/6). Small for gestational age (SGA) was defined as a birth weight z score less than −2.
Weight gain in g/kg/day was estimated since birth, as well as over the last week of hospitalisation. In addition, body weight balance was calculated by subtracting the birth weight z score from the weight z score at discharge. On the morning of the day of discharge and before feeding, venipuncture was carried out for routine blood tests, as well as for determining serum levels of adiponectin, leptin and insulin. A venous blood sample for measuring adiponectin, leptin and insulin levels was also taken before feeding from 15 healthy full‐term infants (the reference group). All full‐term infants were exclusively formula fed with the same commercial formula (S‐26) without LCPUFAs.
Hormone assays
Serum adiponectin levels were assessed with a human adiponectin radioimmunoassay (RIA) kit (Linco Research, St Charles, Missouri, USA). The interassay and intraassay coefficients of variation (CV) were less than 8%, and the sensitivity limit was 1.0 μg/ml. Serum leptin levels were measured by an enzyme‐linked immunosorbent “two‐step” sandwich‐type assay (ELISA) (Diagnostic Systems Laboratories, Texas, USA). The intraassay and interassay CVs were 3.2% and 4.0%, respectively; the sensitivity was 0.05 μg/l. Serum insulin levels were determined by chemiluminescence immunoassay (ASC:180, Bayer Diagnostics, Tarrytown, NY, USA) on an automated analyser.
Statistical analyses
Data are presented as mean (SD), unless otherwise noted. We used Student's t test or Mann–Whitney U test to evaluate intergroup differences in quantitative variables, as appropriate. Univariate and multiple linear regression analyses were carried out to examine relations between the variables of interest; associations were tested only in the preterm group. Variables that were not normally distributed (leptin and insulin levels) were log‐transformed before applying regression analyses. The level of statistical significance was set at p⩽0.05. All statistical analyses were carried out using SPSS Version 10.0 (SPSS, Chicago, IL, USA).
Results
A total of 62 preterm infants (28 (45.1%) boys); gestational age 28–36 weeks, birth weight 940–2100 g; discharged at 40.0 (14.8) days of life) were included in the study. Table 1 shows the anthropometric measurements, caloric intake and weight gain in the full‐term infant (reference) group (n = 15), the entire sample of preterm infants and the preterm infants fed the supplemented (with LCPUFAs) or non‐supplemented formula. Sex and postnatal age distributions were similar in the full‐term and preterm infant groups. As expected, all anthropometric measurements (body weight, length and head circumference) were significantly higher in full‐term than in preterm infants (see table 1 for p values). Caloric intake over the last week of the study did not differ significantly between full‐term and preterm infants. Weight gain was significantly higher in the preterm than in full‐term infants but was insufficient to cover the deficit in weight z score; their body weight z score at discharge was significantly lower than the birth weight z score, resulting in a negative body weight balance (Δ weight z score −0.92 (0.72), table 1). Related to this, the number of preterm infants with body weight at discharge below the lowest normal limit (z score less than −2; 39/62, 62.9%) was higher than the number of preterm infants who were born small for gestational age (7/62, 11.3%). We found no significant differences in any of the variables shown in table 1 between infants fed the LCPUFA‐free or supplemented formula, thus these two subgroups were combined for further statistical analyses.
Table 1 Clinical characteristics of the preterm and full term infants included in the study.
| Full‐term infants (n = 15) | Preterm infants | p Value† | |||
|---|---|---|---|---|---|
| Total (n = 62) | Fed LCPUFA‐supplemented formula (n = 9) | Fed LCPUFA‐free formula (n = 53) | |||
| Girls/boys (n) | 9/6 | 34/28 | 5/4 | 29/24 | 0.62 |
| Gestational age (weeks) | 39.0 (1.0)** | 32.0 (2.1) | 31.7 (1.5) | 32.1 (2.2) | 0.67 |
| Birth weight (g) | 3200 (498)** | 1542 (275) | 1554 (186) | 1540 (289) | 0.89 |
| Birth weight (z score) | −0.4 (1.1)* | −1.0 (0.7) | −0.9 (0.5) | −1.0 (0.8) | 0.55 |
| Ponderal index at birth | 26.1 (3.1)** | 21.8 (2.7) | 22.2 (1.3) | 21.7 (2.9) | 0.64 |
| Postnatal age (days) | 35.1 (15.3) | 40.9 (14.8) | 37.4 (9.2) | 41.5 (15.6) | 0.45 |
| At testing | |||||
| Body weight (g) | 3835 (706)** | 2269 (162) | 2294 (126) | 2267 (167) | 0.65 |
| Body weight (z score) | −0.6 (1.2)** | −2.0 (0.4) | −1.9 (0.2) | −2.1 (0.4) | 0.12 |
| Body length (cm) | 54.3 (3.3)** | 46.7 (0.7) | 46.6 (0.5) | 46.8 (0.8) | 0.42 |
| Head circumference (cm) | 36.7 (1.6)** | 33.2 (1.0) | 33.1 (1.2) | 33.3 (0.9) | 0.70 |
| Δ weight (z score) | −0.15 (0.63)** | −0.92 (0.72) | −0.94 (0.32) | −0.92 (0.77) | 0.93 |
| Caloric intake (last week of study) (Kcal/kg/day) | 155.4 (27.6) | 156.7 (20.7) | 155.4 (15.0) | 156.8 (21.4) | 0.87 |
| Weight gain (g/kg/day) | |||||
| Entire study period | 6.4 (2.7)* | 8.3 (2.8) | 8.4 (1.0) | 8.2 (3.0) | 0.89 |
| Last week of study | 12.8 (4.5)** | 16.1 (4.4) | 17.6 (3.6) | 15.9 (4.5) | 0.32 |
LCPUFA, long‐chain polyunsaturated fatty acid.
Values are mean (SD).
*p<0.05; **p⩽0.01 compared with preterm infants.
†p Value for comparison between preterm infants fed the LCPUFA‐supplemented formula and those fed the LCPUFA‐free formula.
Serum levels of adiponectin were significantly lower in preterm than in full‐term infants (40.8 (15.3) μg/ml and 53.1 (16.0) μg/ml, respectively; p = 0.007). Even in preterm infants who were appropriate for gestational age (AGA), adiponectin levels (42.0 (15.5) μg/ml) were significantly lower than those of full‐term infants (p = 0.01). However, after adjustment for body weight of the infants at testing, the influence of prematurity on adiponectin levels was no longer significant. We found no differences in the serum levels of leptin between preterm (range 0.1–15.6 μg/l; median 1.2 μg/l) and full‐term infants (range 0.1–9.7 μg/l; median 1.2 μg/l). Similarly, serum levels of insulin did not differ significantly between preterm (range 0.7–22.2 mU/l; median 2.3 mU/l) and full‐term infants (range 0.5–10.6 mU/l; median 3.1 mU/l). However, after adjustment for body weight, insulin levels tended to be significantly higher in preterm than full‐term infants (p = 0.07).
What is already known on this topic
Circulating levels of adiponectin are inversely related to the degree of adiposity in older children and adults, but correlate positively with size at birth and neonatal adiposity in full‐term infants.
A role for adiponectin in fetal growth has been proposed.
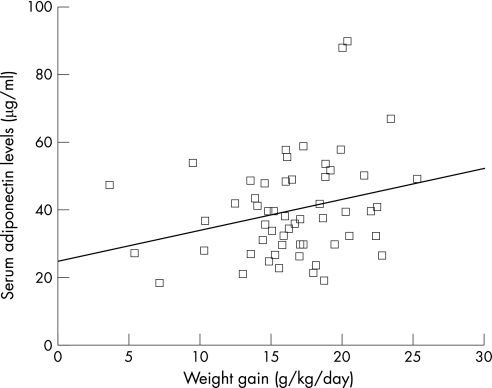
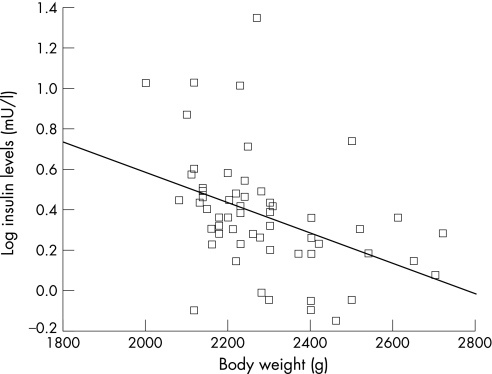
In the univariate regression analysis, serum levels of adiponectin among preterm infants were positively correlated with birthweight (β = 0.31, p = 0.01), ponderal index (kg/m3) at birth (β = 0.25, p<0.05), body weight z score at discharge (β = 0.28, p = 0.02), weight gain over the last week of the study (β = 0.26, p<0.05, fig 1) and feeding with a formula containing LCPUFAs (β = 0.33, p = 0.009). Being born SGA or having body weight below the lowest normal limit at discharge was negatively correlated with serum adiponectin concentrations (β = −0.26 and −0.25, respectively, p<0.05). We found no significant correlation between the serum levels of adiponectin in preterm infants and gestational age, sex, body length, head circumference, caloric intake and circulating levels of leptin or insulin. Serum levels of leptin were negatively correlated with weight gain over the last week of the study (β = −0.27, p<0.05), but not with anthropometric measures at birth or at discharge (body weight and length, head circumference). Serum levels of insulin were negatively correlated with the infants' body weight at discharge (β = −0.43, p<0.01, fig 2).
Figure 1 Correlation between serum levels of adiponectin in preterm infants and weight gain over the last week of the study. The line represents the regression slope (β = 0.26, p<0.05).
Figure 2 Correlation between serum levels of insulin in preterm infants and body weight at discharge. The lines represent the regression slopes (β = −0.43, p<0.01).
We used multiple regression analysis to control for potential confounding factors. The variables “birth weight or ponderal index at birth” and “being born small for gestational age” were not introduced in the same model, as well as the variables “body weight below the lowest normal limit at discharge” and “body weight z score at discharge”, due to colinearity. In multiple regression analysis, feeding with a formula containing LCPUFAs, weight gain over the last week of the study and being born SGA were the independent predictors of serum levels of adiponectin (table 2).
Table 2 Multiple regression analysis model for serum levels of adiponectin in preterm infants.
| Independent variables | β | B (95% CI for B) | p Value |
|---|---|---|---|
| Gestational age | 0.22 | 1.6 (−0.4 to 3.6) | 0.11 |
| Being born small for gestational age* | −0.35 | −18.3 (−33.0 to −3.5) | 0.01 |
| Body weight z score at discharge† | 0.05 | 1.6 (−8.6 to 11.8) | 0.75 |
| Weight gain (g/kg/day) | 0.28 | 1.0 (0.1 to 1.9) | 0.03 |
| Dietary LCPUFAs | 0.34 | 14.4 (3.8 to 25.0) | 0.009 |
| Insulin levels | −0.04 | −0.2 (−1.2 to 0.9) | 0.73 |
| Leptin levels | −0.01 | −0.08 (−1.3 to 1.4) | 0.89 |
LCPUFA, long‐chain polyunsaturated fatty acid.
β, Standardised regression coefficient; R2 (%) = 33.5; p = 0.005; dependent variable: serum adiponectin levels.
*If substituted by birth weight or ponderal index at birth, only weight gain and dietary LCPUFAs correlate independently with serum adiponectin levels.
†If substituted by “body weight at discharge below the lowest normal limit” (z score ⩽ 2), the results were similar to those shown in table.
To further explore the correlation between adiponectin levels and weight gain, we examined the associations between weight gain in preterm infants and several factors, including sex, gestational age, anthropometric parameters at birth and at discharge, dietary LCPUFAs, caloric intake and levels of the hormones studied. Apart from the levels of adiponectin and leptin, other factors shown by univariate analysis to correlate with weight gain over the last week of the study were the birthweight (β = −0.27, p = 0.04), body length at discharge (β = −0.36, p = 0.02) and caloric intake (β = 0.53, p<0.001). However, in the multiple regression analysis, caloric intake and adiponectin levels were the only variables that correlated independently with weight gain (table 3).
Table 3 Multiple regression analysis model for weight gain in preterm infants.
| Independent variables | β | B (95% CI for B) | p Value |
|---|---|---|---|
| Gestational age | 0.23 | 0.45 (−0.23 to 1.12) | 0.18 |
| Birth weight | −0.13 | −0.002 (−0.008 to 0.004) | 0.49 |
| Sex | 0.29 | 2.22 (−0.41 to 4.85) | 0.10 |
| Body weight at discharge | −0.03 | −0.001 (−0.01 to 0.008) | 0.86 |
| Body length at discharge | −0.26 | −1.28 (−3.15 to 0.59) | 0.17 |
| Dietary LCPUFAs | −0.003 | −0.02 (−2.95 to 2.90) | 0.98 |
| Caloric intake | 0.44 | 0.05 (0.01 to 0.09) | 0.01 |
| Adiponectin levels | 0.35 | 0.08 (0.009 to 0.16) | 0.03 |
| Insulin levels | 0.009 | 0.008 (−0.31 to 0.32) | 0.95 |
| Leptin levels | −0.07 | −0.15 (−0.79 to 0.49) | 0.63 |
LCPUFA, long‐chain polyunsaturated fatty acid.
β, standardised regression coefficient; R2 (%) = 57.6, p = 0.008; dependent variable: weight gain (g/kg/day) over the last week of the study.
Discussion
What this study adds
In preterm infants at discharge, serum adiponectin levels are lower than those in full‐term infants of a comparable postnatal age and correlate positively with body weight.
Being born small for gestational age, weight gain and, possibly, dietary long‐chain polyunsaturated fatty acids, are independent predictors of serum levels of adiponectin in preterm infants.
In the present study, preterm infants at discharge from the neonatal unit had lower serum levels of adiponectin than full‐term infants of comparable postnatal age. The lower levels were probably due to reduced synthesis/secretion of adiponectin as a result of decreased adiposity. In preterm and full‐term neonates, body weight provides a satisfactory estimate of body fat as it correlates positively with body fat mass and explains, with sex, 85% of fat mass and 72% of the variance in per cent body fat as assessed by dual‐energy x ray absorptiometry.21 After adjustment for body weight, the serum levels of adiponectin were no longer lower in our preterm compared with full‐term infants.
We found much higher levels of adiponectin in preterm infants than reported previously in older children and adults.9,10,11 However, our results are in accordance with previous studies of full‐term newborns of a much younger age.8,9,10,13,14,15,16 Abundance of adiponectin in newborns has been attributed to a deficiency of negative feedback for adiponectin production13 resulting from lack of adipocyte hypertrophy, low percentage of body fat and different distribution of fat depots, with almost 90% of total body fat being in the subcutaneous compartment and only 4% in the abdomen.22 Other possible explanations for the hyperadiponectinaemia in newborns are the presence of adiponectin‐producing brown adipose tissue, which progressively atrophies during childhood,23 and the production of adiponectin by a number of non‐adipose tissues, including skeletal muscle, smooth muscle cells of the small intestine, arterial walls, connective tissues and epidermis—production in these tissues declines as gestation progresses.24
We found being born SGA correlated independently with serum levels of adiponectin in our preterm infants. Its negative influence on serum adiponectin levels is consistent with Kamoda et al's study, which found lower levels of circulating adiponectin in SGA than in AGA full‐term newborns.11 Similar to that study, the influence of being born SGA on serum levels of adiponectin in our study could not be explained by the low adiposity alone. This is because the correlation between adiponectin levels and being born SGA remained significant after adjustment for body weight (data not shown) or body weight z score at discharge (table 2). Other factors associated with low adiponectin production, such as cortisol levels or visceral fat distribution,5 may have negatively influenced the serum adiponectin levels in our SGA preterm infants. Previous studies have shown that in SGA neonates, serum levels of cortisol are higher than those in AGA newborns25 and that the intra‐abdominal adipose tissue, unlike subcutaneous adipose tissue, is not reduced in amount.26
In the present study, weight gain in preterm infants positively influenced serum levels of adiponectin (table 2). This may be due to an increased number of differentiating or newly differentiated adipocytes that may lack the ability to inhibit adiponectin production.13 Alternatively, factors such as insulin‐like growth factor (IGF)‐1, growth hormone and insulin, which, on the one hand have been implicated in the regulation of infant growth, and on the other hand stimulate adiponectin gene expression,27,28 may be responsible for the correlation found between adiponectin and weight gain. We did not find any correlation between levels of insulin and adiponectin, but we did not evaluate the other factors mentioned above.
Previous studies have supported a role for adiponectin, similar to other adipocytokines, in fetal growth; this suggestion is mainly based on the positive correlations between levels of adiponectin in cord blood and neonatal size or adiposity at birth.8,12,13,14 Our finding of independent correlation between serum levels of adiponectin and weight gain in preterm infants (tables 2 and 3) is novel and, possibly provides stronger evidence for a role of adiponectin in growth regulation in preterm infants. Adiponectin may affect growth directly and/or by increasing the sensitivity of tissues to endocrine regulators of growth, such as insulin and the active components of the IGF system.29,30
Our finding of a positive influence of dietary LCPUFAs on serum levels of adiponectin independent of weight gain is a new observation in human beings and of considerable interest. There have been only a few relevant studies in animals so far, showing that dietary n‐3 LCPUFAs lead to rise in systemic levels of adiponectin.31,32 The potential mechanisms underlying this observation have not been fully elucidated. Flachs et al showed that dietary n‐3 LCPUFAs stimulate the expression of the gene encoding adiponectin and the release of adiponectin from adipose tissue relatively independent of food intake and body fat mass.31 However, Rossi et al did not observe modifications in adiponectin gene expression by dietary LCPUFAs and they attributed the increase in plasma levels of adiponectin levels by dietary LCPUFAs to normalisation of plasma free fatty acids and triglyceride levels, which in high concentrations exert an inhibitory effect on the release of adiponectin by adipocytes.32 A positive effect of dietary LCPUFAs on adiponectin production/release may explain, at least in part, the reported beneficial effect of dietary LCPUFAs on insulin resistance in animals and human adults.1 However, in our study, only a few preterm infants was fed the LCPUFA‐supplemented formula; thus, this finding needs to be confirmed by well‐designed case–control studies.
In adults, a low baseline level of adiponectin is an independent predictor for the later development of insulin resistance.33,34,35 In our preterm infants, serum levels of adiponectin did not correlate with levels of insulin or leptin, similar to previous studies in newborns.13,17 However, insulin levels were negatively correlated with body weight at discharge in preterm infants and they also tended to be significantly higher in preterm than full‐term infants after adjustment for body weight. In addition, contrary to expectations,36 leptin levels were not lower in preterm than full‐term infants, and a positive correlation between leptin levels and body weight was not observed; rather an inverse association between leptin levels and weight gain was noticed. As in previous studies,37 a high percentage of our preterm infants—even those not born SGA—had lower body weight at discharge than expected on the basis of “normal” intrauterine growth rates and, in a sense, they resembled the SGA model. A previous study had reported that levels of insulin were higher in SGA than in AGA infants at 19.6±12.1 days after birth, and insulin resistance was negatively related to body weight at the time of examination.38 In addition, leptin levels are also higher in SGA than in normal infants39 and do not correlate with body weight or body fat mass40; this has been attributed to leptin resistance in SGA infants. Follow‐up of our preterm infants will help elucidate whether the findings of the present study indicate that insulin and leptin resistance, which have been reported in childhood or later life after a premature birth,4,41 develop much earlier, in the neonatal period.
Abbreviations
AGA - appropriate for gestational age
SGA - small for gestational age
LCPUFA - long‐chain polyunsaturated fatty acid
Footnotes
Competing interests: None.
References
- 1.Das U N. Pathophysiology of metabolic syndrome X and its links to the perinatal period. Nutrition 200521762–773. [DOI] [PubMed] [Google Scholar]
- 2.Barker D J P, Osmond C, Forsen T J.et al Trajectories of growth among children who have coronary events as adults. N Engl J Med 20053531802–1809. [DOI] [PubMed] [Google Scholar]
- 3.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet 20043631642–1645. [DOI] [PubMed] [Google Scholar]
- 4.Hofman P L, Regan F, Jackson W E.et al Premature birth and later insulin resistance. N Engl J Med 20043512179–2186. [DOI] [PubMed] [Google Scholar]
- 5.Nedvidkova J, Smitka K, Kopsky V.et al Adiponectin, an adipocyte‐derived protein. Physiol Res 200554133–140. [PubMed] [Google Scholar]
- 6.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal‐weight and obese women. Eur J Endocrinol 2002147173–180. [DOI] [PubMed] [Google Scholar]
- 7.Stefan N, Bunt J C, Salbe A D.et al Plasma adiponectin concentrations in children: Relationships with obesity and insulinemia. J Clin Endocrinol Metab 2002874652–4656. [DOI] [PubMed] [Google Scholar]
- 8.Tsai P J, Yu C H, Hsu S P.et al Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol 20046188–93. [DOI] [PubMed] [Google Scholar]
- 9.Pardo I M C G, Geloneze B, Tambascia M A.et al Hyperadiponectinemia in newborns: Relationship with leptin levels and birth weight. Obes Res 200412521–524. [DOI] [PubMed] [Google Scholar]
- 10.Kotani Y, Yokota I, Kitamura S.et al Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clin Endocrinol 200461418–423. [DOI] [PubMed] [Google Scholar]
- 11.Kamoda T, Saitoh H, Saito M.et al Serum adiponectin concentrations in newborn infants in early postnatal life. Pediatr Res 200456690–693. [DOI] [PubMed] [Google Scholar]
- 12.Kajantie E, Hytinantti T, Hovi P.et al Cord plasma adiponectin: A 20‐fold rise between 24 weeks gestation and term. J Clin Endocrinol Metab 2004894031–4036. [DOI] [PubMed] [Google Scholar]
- 13.Sivan E, Mazaki‐Tovi S, Pariente C.et al Adiponectin in human cord blood: Relation to fetal birth weight and gender. J Clin Endocrinol Metab 2003885656–5660. [DOI] [PubMed] [Google Scholar]
- 14.Chan T F, Yuan S S, Chen H S.et al Correlations between umbilical and maternal serum adiponectin levels and neonatal birthweights. Acta Obstet Gynecol Scand 200483165–169. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay R S, Walker J D, Havel P J.et al Adiponectin is present in cord blood but is unrelated to birth weight. Diabetes Care 2003262244–2249. [DOI] [PubMed] [Google Scholar]
- 16.Mantzoros C, Petridou E, Alexe D M.et al Serum adiponectin concentrations in relation to maternal and perinatal characteristics in newborns. Eur J Endocrinol 2004151741–746. [DOI] [PubMed] [Google Scholar]
- 17.Petridou E, Mantzoros C, Belechri M.et al Neonatal leptin levels are strongly associated with female gender, birth length, IGF‐I levels and formula feeding. Clin Endocrinol 200562366–371. [DOI] [PubMed] [Google Scholar]
- 18.Volpe J J. Intracranial hemorrhage: germinal matrix‐intraventricular hemorrhage of the premature infant. In: Volpe JJ, ed. Neurology of the newborn. Philadelphia: WB Saunders, 2001428–493.
- 19.Ballard J L, Khoury J C, Wedig K.et al Νew Ballard Score, expanded to include extremely premature infants. J Pediatr 1991119417–423. [DOI] [PubMed] [Google Scholar]
- 20.Siahanidou T, Mandyla H, Vounatsou M.et al Circulating levels of PYY are higher in preterm than term infants and correlate negatively with body weight and positively with serum ghrelin levels. Clin Chem 2005512131–2137. [DOI] [PubMed] [Google Scholar]
- 21.Rigo J, Nyamugabo K, Picaud J C.et al Reference values of body composition obtained by dual energy x‐ray absorptiometry in preterm and term neonates. J Pediatr Gastroenterol Nutr 199827184–190. [DOI] [PubMed] [Google Scholar]
- 22.Dunger D, Ong K. Abundance of adiponectin in the newborn. Clin Endocrinol 200461416–417. [DOI] [PubMed] [Google Scholar]
- 23.Viengchareun S, Zennaro M C, Pascual‐Le Tallec L.et al Brown adipocytes are novel sites of expression and regulation of adiponectin and resistin. FEBS Lett 2002532345–350. [DOI] [PubMed] [Google Scholar]
- 24.Gorbetta S, Bulfamante G, Cortelazzi D.et al Adiponectin expression in human fetal tissues during mid‐ and late gestation. J Clin Endocrinol Metab 2005902397–2402. [DOI] [PubMed] [Google Scholar]
- 25.Heckmann M, Wudy S A, Haack D.et al Reference range for serum cortisol in well preterm infants. Arch Dis Child Fetal Neonatal Ed 199981F171–F174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington T A M, Thomas E L, Frost G.et al Distribution of adipose tissue in the neonate. Pediatr Res 200455437–441. [DOI] [PubMed] [Google Scholar]
- 27.Halleux C M, Takahashi M, Delporte M L.et al Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem Biophys Res Commun 20012881102–1107. [DOI] [PubMed] [Google Scholar]
- 28.Xu A, Wong L C, Wang Y.et al Chronic treatment with growth hormone stimulates adiponectin gene expression in 3T3‐L1 adipocytes. FEBS Lett 2004572129–134. [DOI] [PubMed] [Google Scholar]
- 29.Fu Y, Luo N, Klein R L.et al Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 2005461369–1379. [DOI] [PubMed] [Google Scholar]
- 30.Havel P J. Update on adipocyte hormones. Regulation of energy balance and carbohydrate/lipid metabolism. Diabetes 200453(Suppl 1)143–151. [DOI] [PubMed] [Google Scholar]
- 31.Flachs P, Mohamed‐Ali V, Horakova O.et al Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high‐fat diet. Diabetologia 200649394–397. [DOI] [PubMed] [Google Scholar]
- 32.Rossi A S, Lombardo Y B, Lacorte J M.et al Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrose‐fed, insulin‐resistant rats. Am J Physiol Regul Integr Comp Physiol 2005289R486–R494. [DOI] [PubMed] [Google Scholar]
- 33.Fumeron F, Aubert R, Siddiq A.et al Epidemiologic data on the insulin resistance syndrome (DESIR) study group: adiponectin gene polymorphism and adiponectin levels are independently associated with the development of hyperglycemia during a 3‐year period: the epidemiologic data on the insulin resistance syndrome prospective study. Diabetes 2004531150–1157. [DOI] [PubMed] [Google Scholar]
- 34.Spranger J, Kroke A, Mohlig M.et al Adiponectin and protection against type 2 diabetes mellitus. Lancet 2003361226–228. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Hirose H, Saito I.et al Adiponectin, an adipocyte‐derived protein, predicts future insulin resistance: two‐year follow‐up study in Japanese population. J Clin Endocrinol Metab 20048987–90. [DOI] [PubMed] [Google Scholar]
- 36.Ng P C, Lam C W K, Lee C H.et al Leptin and metabolic hormones in preterm newborns. Arch Dis Child Fetal Neonatal Ed 200083F198–F202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark R H, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics 2003111986–990. [DOI] [PubMed] [Google Scholar]
- 38.Gray I P, Cooper P A, Cory B J.et al The intrauterine environment is a strong determinant of glucose tolerance during the neonatal period, even in prematurity. J Clin Endocrinol Metab 2002874252–4256. [DOI] [PubMed] [Google Scholar]
- 39.Jaquet D, Leger J, Tabone M D.et al High serum leptin concentrations during catch‐up growth of children born with intrauterine growth retardation. J Clin Endocrinol Metab 1999841949–1953. [DOI] [PubMed] [Google Scholar]
- 40.Orbak Z, Coker M, Darcan S.et al Association between serum leptin and anthropometric parameters at birth and at 15th day of life in infants born asymmetrically small for gestational age. J Pediatr Endocrinol Metab 200114185–192. [DOI] [PubMed] [Google Scholar]
- 41.Singhal A, Farooqi I S, O'Rahilly S.et al Early nutrition and leptin concentrations in later life. Am J Clin Nutr 200275993–999. [DOI] [PubMed] [Google Scholar]