Abstract
Objective
To examine the auditory perception of maternal utterances by neonates using near‐infrared spectroscopy (NIRS).
Methods
Twenty full‐term, healthy neonates were included in this study. The neonates were tested in their cribs while they slept in a silent room. First, two probe holders were placed on the left and right sides of the forehead over the eyebrows using double‐sided adhesive tape. The neonates were then exposed to auditory stimuli in the form of infant‐directed speech (IDS) or adult‐directed speech (ADS), sampled from each of the mothers, through an external auditory speaker.
Results
A 2 (stimulus: IDS and ADS) × 2 (recording site: channel 1 (right side) and channel 2 (left side)) analysis of variance for these relative oxygenated haemoglobin values showed that IDS (Mean = 0.25) increased brain function significantly (F = 3.51) more than ADS (Mean = −0.26).
Conclusions
IDS significantly increased brain function compared with ADS. These results suggest that the emotional tone of maternal utterances could have a role in activating the brains of neonates to attend to the utterances, even while sleeping.
Several behavioural studies have suggested that infants can recognise human voices irrespective of what they say.1 In particular, an infant can discriminate his or her own mother's voice from a different voice2,3 because he or she has heard the maternal voice in utero.4,5 Infants also prefer maternal utterances that are directed towards them—that is, infants prefer infant‐directed speech (IDS) to adult‐directed speech (ADS).6,7
Although previous studies have examined infant perception of IDS/ADS mainly using behavioural measures, Casey and de Haan8 have pioneered a future direction for infant studies using new methods for brain imaging. These techniques are expected to enhance our understanding of perceptual abilities of infants at the brain function level. In this study, we used near‐infrared spectroscopy (NIRS) brain imaging techniques. The use of NIRS is advantageous in infant studies because linguistic or behavioural skills are not required. Also, NIRS techniques are robust and reliable for use with human infants.9 Neonatal brain processing of acoustic stimulation is measurable using NIRS.10 Thus, we applied this new, non‐invasive brain‐imaging technique to neonates just after birth to elucidate not only perceptual discrimination between IDS and ADS, but also the neuro‐psychological process of infant speech perception.
Several previous brain imaging studies on neonatal speech perception have focused on examining the ability of neonates to process the linguistic components of IDS.10,11,12,13 This study focuses on the ability of neonates to process the emotional components of IDS and ADS, in terms of blood flow to the frontal lobe area, to elucidate whether and how different tonal features of maternal utterances are reflected in the blood flow to the frontal lobe area in neonates.
The prefrontal cortex plays a unique part in linking the sensory areas of the cortex with emotion‐oriented areas and survival‐oriented areas of the subcortex, which could be the functional aspect of this phenomenon.14 Previous research has suggested that frontal lobe abilities could be affected by the emotional environment experienced during the first few years of life, when the frontal lobe undergoes a rapid period of growth and development.15,16 The second purpose of this study was to elucidate whether the activation of the frontal lobe area in neonates could be related to the infant's perception of IDS in terms of emotional function on the basis of neurobiological studies of emotional development in infants.
Method
Participants
Twenty full‐term, healthy, Japanese neonates (eight boys and 12 girls) ranging in age from 2 to 9 days (mean 4.4 days) participated in this study. The neonates were born in 2005 and were admitted at birth to the Maternal and Perinatal Centre at the Paediatric Department of Hiroshima University Hospital, (Hiroshima, Japan). The mean gestational age was 38.9 weeks (36 weeks 5 days–41 weeks 3 days) and the average birth weight was 3028.6 g (2286–3662 g). Each neonate was clinically evaluated according to the procedure developed by Apgar,17 and all had Apgar scores of at least 9 (out of 10), 1 and 5 min after birth. Their auditory ability at birth was also assessed as normal by means of an automated auditory brain‐stem response. This research was endorsed by the ethics committee of the University of Hiroshima Hospital before conduction of the test. All parents received and understood the relevant research information and signed a consent form.
Stimuli and apparatus
The stimuli consisted of IDS and ADS in Japanese. We recorded speech samples from each of the mothers whose neonates participated in the experiment. They read the first scene of “Little Red Riding Hood,” the well‐known children's fairy tale, in Japanese. The text involved four sentences of 40 words, with 12 pauses. Before recording their speech, mothers were instructed to tell the story either to their baby or to the experimenter so that when recording the IDS version they were looking at their babies, and on recording the ADS version they were looking at the experimenter. The speech elements of the two versions such as total duration, tone intensity, speaking speed, tone volume and pause duration, differed among the mothers. The total duration ranged from 15 to 28 s; the mean duration of IDS was 22.4 s (SD = 2.9), whereas that of ADS was 19.4 s (SD = 2.2). Because Watterson and Riccillo18 found that white noise is particularly effective in soothing crying infants, computer‐generated white noise was used as a control auditory stimulus while baseline oxygenation levels were established, before exposure to IDS and ADS and during a relaxation period after exposure. All auditory stimuli were recorded by a stereo digital voice recorder (ICR‐S300RM, Sanyo K.K., Osaka, Japan), formatted as MP3 files, and treated with a low‐pass filter to about 16 kHz.
To measure the cerebral blood flow in the frontal area in infants, a two‐channel NIRS system (NIRO‐200, Hamamatsu Photonics K.K., Hamamatsu City, Japan) was used, which was able to detect concentration changes in oxygenated haemoglobin (oxy‐Hb), deoxygenated haemoglobin (deoxy‐Hb) and their sum (total‐Hb) by using near infrared light (wavelength of about 700–950 nm). These values were calculated every 1/6 s.
Procedure
The neonates were tested in their cribs while they slept in a silent room. The temperature and light intensity of the room were kept constant, and the noise level was reduced as much as possible. Firstly, two probe holders were placed on the left and right sides of the forehead over the eyebrows using double‐sided adhesive tape, corresponding to an Fp1 or an Fp2 placement of a 10/20 EEG system. To prevent ambient light from reaching the optode, dark felt was bandaged over the neonate's head. After fitting the optode sets on the forehead, a few minutes were required to check whether the sensors were making good contact. The experimenter did not touch or talk to the neonates during the test.
The neonates were then exposed to the auditory stimuli in the form of IDS, ADS or white noise through an external auditory speaker (Acoustic Bass Duct; Sony, Tokyo, Japan) set placed 15 cm away from their faces, through a portable storage device and multi‐code jukebox with 64‐kbit rates. The speaker sound had a 60–70‐dB sound‐pressure level (SPL) as measured with a sound‐level meter (NL‐05A; Rion, Tokyo, Japan).
Each test consisted of three sequential periods: a baseline period of 60 s, a speech stimulation period involving either IDS or ADS, and a relaxation period of 60 s, during which time oxygenation levels were checked to ensure that they returned to the baseline level. The tests were run in two blocks: one block involved IDS and the other involved ADS. Each block was conducted according to an event‐related design in which the NIRO‐200 was triggered by “on” and “off” signals when the neonates were exposed to auditory stimuli; oxygenation levels in the blood were scanned during each period. For half of the neonates the ADS stimulus was given first and the IDS stimulus was presented second. The presentation order was reversed for the other half of the group. The total procedure, including the placement of probes, took approximately 10 min.
Results
Oxy‐Hb is believed to be the most sensitive indicator of changes in cerebral blood flow in NIRS measurement.19 Accordingly, we focused our analyses on changes in oxy‐Hb. However, as body movements may also influence oxy‐Hb concentrations, we excluded data that were over two standard deviations (SDs) from the mean for each participant.
Because the oxy‐Hb concentration measured by NIRS is a relative value taken from a baseline value, we treated the quantity of change for each item as an interval value, by calculating the difference in oxy‐Hb 10 s before the onset of the stimulus. Accordingly, we used the differences between baseline stimulation relative values in the following statistical analyses. The means for the two different stimuli were calculated for each neonate (table 1).
Table 1 Change in oxy‐Hb concentration (mean).
| Participant | IDS | ADS | ||
|---|---|---|---|---|
| Ch1 | Ch2 | Ch1 | Ch2 | |
| 1 | −0.93 | −1.65 | 0.01 | 0.25 |
| 2 | 1.12 | 0.26 | −0.67 | −0.40 |
| 3 | 0.23 | 0.51 | −0.22 | 0.65 |
| 4 | 0.63 | −0.89 | 0.07 | 0.31 |
| 5 | −0.30 | −0.75 | −0.95 | −0.67 |
| 6 | 0.24 | 0.20 | −0.25 | −0.58 |
| 7 | 0.54 | 0.50 | −1.10 | −1.13 |
| 8 | 1.08 | 0.66 | −0.91 | −1.15 |
| 9 | 0.56 | 0.07 | 0.58 | −0.33 |
| 10 | −1.36 | −1.34 | −0.10 | −0.27 |
| 11 | −0.45 | −0.22 | 1.12 | 0.75 |
| 12 | 0.11 | 0.19 | 0.27 | 0.60 |
| 13 | 1.59 | 1.15 | 0.94 | 3.40 |
| 14 | 0.56 | 1.00 | −0.87 | −0.88 |
| 15 | −0.03 | 0.02 | 0.10 | −0.37 |
| 16 | 0.29 | 0.66 | −0.63 | −1.33 |
| 17 | 0.53 | 0.86 | −0.95 | −1.40 |
| 18 | 2.41 | 2.18 | −0.35 | 0.25 |
| 19 | 0.18 | −0.23 | −1.14 | −1.22 |
| 20 | −0.06 | −0.10 | −0.23 | −0.33 |
| Mean | 0.35 | 0.15 | −0.26 | −0.19 |
| (SD) | (0.81) | (0.86) | (0.64) | (1.06) |
ADS, adult‐directed speech; ch, channel; IDS, infant‐directed speech; oxy‐Hb, oxyhaemoglobin.
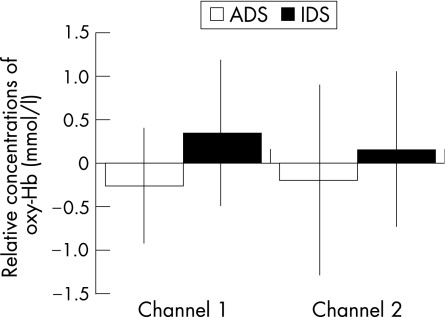
We conducted a 2 (stimulus: IDS and ADS) × 2 (recording site: channel 1 (right side) and channel 2 (left side)) analysis of variance for these relative oxy‐Hb values, which showed a statistical tendency for the stimulus effect (F(1, 19) = 3.51, p = 0.07). The results indicated that IDS (mean (SD) 0.25 (0.10)) produced a greater increase in oxygenated blood to the frontal area of the brain than did ADS (mean (SD) −0.23 (0.04)). Because the SDs for channel 1 (IDS = 0.83, ADS = 0.66) were smaller than those for channel 2 (IDS = 0.88, ADS = 1.08), a t test for the difference between the two stimuli was carried out only for the relative means of oxy‐Hb for channel 1, where a significant effect was found (t (19) = −2.45, p = 0.02). Figure 1 shows increased brain function caused by IDS (mean 0.25) compared with ADS (mean −0.26).
Figure 1 Mean relative values of oxyhaemoglobin (oxy‐Hb) for infant‐directed speech (IDS) and adult‐directed speech (ADS).
Discussion
We examined blood flow to the frontal area of the brain in neonates using NIRS, as they perceived different types of tonal features in their mothers' vocalisations—that is, IDS or ADS. Large individual differences were observed in the NIRS data; nevertheless, t tests of the results from channel 1 showed a significant difference in the amount of oxy‐Hb present in the IDS and ADS conditions. These results show that IDS affects blood flow to the frontal area of the brain in neonates more than ADS. Neonates seem to not only discriminate between differences in the prosodic patterns of IDS and ADS but also attend more to IDS than to ADS, in terms of their brain activation.
What is already known on this topic
Infants prefer infant‐directed speech (IDS) to adult‐directed speech (ADS)
Several previous brain imaging studies on neonatal speech perception have focused on examining the ability of neonates to process the linguistic components of IDS
The increase in oxy‐Hb in the frontal area of neonates caused by IDS is interesting. Our results show that the brain activation level due to IDS was higher than that due to white noise, which is also effective in soothing infants.18 Our results suggest that IDS did not have a soothing characteristic. Another study has suggested that IDS may have features that differentially elicit and maintain infant attention.20 Accordingly, the IDS from the mothers in this study could be presumed to have aroused the neonates rather than soothed them, when considered in terms of the emotional contour of the tonal stimuli.
Mehler21 proposed two interpretations for infant responses to maternal voices: one is related to the very rapid bonding that occurs between mother and offspring after birth, and the other is related to the effective transmission of the mother's voice in utero. IDS may provoke an evolutionarily important response in neonates to bond with their mothers after birth, which would link the close relationship between emotion due to IDS and frontal brain function in neonates. The maturation of the orbitofrontal region depends on socioaffective experience.16 The activation associated with IDS in the frontal area, including the orbitofrontal region, suggests that IDS has an emotional stimulation characteristic. On the other hand, ADS did not activate the frontal area, implying that ADS may not provide emotional stimulation for an infant. Lichty et al22 suggested that deactivation may be caused by the “stealing” of activation by a neighbouring area. These findings suggest that IDS provides positive sociostimulation that promotes healthy development and brain maturation in infants. In contrast, ADS does not relate to development of the frontal area of infants.
In our study, the marked difference between IDS and ADS on the right recording site suggests a hemispheric asymmetry, in which the right prefrontal area in neonates processes IDS more than ADS. However, we cannot conclude that there is hemispheric asymmetry in neonates because of no significant main effect of the two channels. Generally, hemispheric asymmetry of a function is found in the frontal area. However, the synaptic growth as well as differentiation of structure in the prefrontal cortex of the right hemisphere starts at the end of the first year,16 so that there may be no hemispheric asymmetry in the neonatal period.
Recent clinical studies on infant–mother relationships have found that children who have been taken care of by depressed mothers are themselves at higher risk of depression and other developmental problems at later developmental stages. This could be related to the mothers' emotional care for their children. Bettes23 showed that depressed mothers respond more slowly to their infants' vocalisations than non‐depressed mothers, and that their utterances are not only more variable but also contain many pauses and lack the characteristics of “motherese”, particularly the exaggerated intonation contour. With this in mind, we are cautious about interpreting our results, although they do indicate that the emotional tone of maternal utterances plays a part in activating the brains of infants to attend to their mothers, even while sleeping, and that maternal vocalisations may play an important part in the development of emotional function in infants.
What this study adds
We found that IDS affected the frontal area even in neonates
We suggest that IDS functions as a positive stimulation for the emotional development of infants
In summary, previous studies have shown the ability of infants to process the linguistic components of IDS but have not focused on the emotional components of IDS expressed during infant–care giver interactions. Using brain imaging, we found that IDS affected the frontal area even in neonates. We suggest that IDS functions as a positive stimulation for the emotional development of infants.
Acknowledgements
We thank the nurses at Hiroshima University Hospital for their invaluable assistance in the recruitment of neonates for this study. We also thank the neonates and their parents for their interested participation.
Abbreviations
ADS - adult‐directed speech
F0 - fundamental frequency
IDS - infant‐directed speech
NIRS - near‐infrared spectroscopy
oxy‐Hb - oxygenated haemoglobin
SPL - sound‐pressure level
Footnotes
This work was supported by a Grant‐in‐Aid for Scientific Research to T Toshima from the Japanese Society for the Promotion of Science (A: 14201012).
Competing interests: None declared.
References
- 1.Demany L, Mckensie B, Vurpillot E. Rhythm perception in early infancy. Nature 1977266718–719. [DOI] [PubMed] [Google Scholar]
- 2.DeCasper A J, Fifer W P. Of human bonding: newborns prefer their mother's voices. Science 19802081174–1176. [DOI] [PubMed] [Google Scholar]
- 3.DeRegnier R, Nelson C A, Thomas K M.et al Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr 2000137777–784. [DOI] [PubMed] [Google Scholar]
- 4.Hepper P G, Shahidullah B S. Development of fetal hearing. Arch Dis Child 199471F81–F87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisilevsky B S, Muir D W. Human fetal and subsequent newborn responses to sound and vibration. Inf Behav Dev 1991141–26. [Google Scholar]
- 6.Cooper R P, Aslin R N. Preference for infant‐directed speech in the first month after birth. Child Dev 1990611584–1595. [PubMed] [Google Scholar]
- 7.Pegg J E, Werker J F, McLead P J. Preference for infant‐directed over adult‐directed speech: evidence from 7‐week‐old infants. Inf Behav Dev 199215325–345. [Google Scholar]
- 8.Casey B J, de Haan M. Introduction: new methods in developmental science. Dev Sci 20025265–267. [Google Scholar]
- 9.Aslin R N, Mehler J. Near‐infrared spectroscopy for functional studies of brain activity in human infants: promise, prospects, and challenges. J Biomed Opt 200510011009–011013. [DOI] [PubMed] [Google Scholar]
- 10.Pena M, Maki A, Kovacic D.et al Sounds and silence: an optical topography study of language recognition at birth. Natl Acad Sci 2003100702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehaene‐Lambertz G, Dehaene S, Hertz‐Pannier L. Functional neuroimaging of speech perception in infants. Science 20022982013–2015. [DOI] [PubMed] [Google Scholar]
- 12.Mehler J, Jusczyk P, Lambertz G.et al A precursor of language acquisition in young infants. Cognition 198829143–178. [DOI] [PubMed] [Google Scholar]
- 13.Dehaene‐Lambertz G, Houston D. Faster orientation latencies toward native language in two‐month‐old infants. Lang Speech 19984121–43. [Google Scholar]
- 14.Ledoux J.The emotional brain. New York: Simon & Schuster, 1996
- 15.Dawson G, Ashman S B. The effect of early adversity in neurobehavioral development. In: Nelson CA, ed. The Minnesota symposia on child development. Vol 31. Mahwah: Lawrence Erlbaum Associates, 2000245–279.
- 16.Schore A N.Affect regulation and the origin of the self. Hillsdale: Lawrence Erlbaum Associates, 1994
- 17.Apgar V. The newborn (Apgar) scoring system. Ped Clin North Am 195213645–698. [DOI] [PubMed] [Google Scholar]
- 18.Watterson T, Riccillo S C. Vocal suppression as a neonatal response to auditory stimuli. J Audit Res 198323205–214. [PubMed] [Google Scholar]
- 19.Hoshi Y, Kobayashi N, Tamura M. Interpretation of near‐infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J Appl Physiol 2001901657–1662. [DOI] [PubMed] [Google Scholar]
- 20.Fernald A, Kulh P. Acoustic determinant of infant preference for motherese speech. Inf Behav Dev 1987101497–1510. [Google Scholar]
- 21.Mehler J.Neonate cognition: beyond the blooming buzzing confusion. In: Mehler J, Fox R, eds. Hilsdale: Lawrence Erlbaum Associates, 19857–28.
- 22.Lichty W, Sakatani K, Xie Y.et al Application of near‐infrared spectroscopy to investigate brain activity: clinical research. Proc SPIE 2000408234–39. [Google Scholar]
- 23.Bettes B A. Maternal depression and motherese: temporal and intonational features. Child Dev 1988591089–1096. [DOI] [PubMed] [Google Scholar]