Abstract
Background
Serratia marcescens is an opportunistic gram‐negative rod which typically infects compromised hosts.
Objectives
To identify risk factors, signs, and outcomes associated with non‐epidemic S marcescens bacteremia in a neonatal intensive care unit (NICU).
Methods
The records of infants with S marcescens bacteremia while in the Yale‐New Haven Hospital NICU from 1980–2004 were reviewed. A matched case‐control study was performed by comparing each case of S marcescens to 2 uninfected controls and 2 cases of Escherichia coli bacteremia.
Results
Twenty‐five sporadic cases of S marcescens bacteremia were identified. Eleven available isolates were determined to be different strains by pulse field gel electrophoresis. Infants with S marcescens bacteremia had median gestational age and birth weight of 28 weeks and 1235 grams, respectively. Compared to matched, uninfected controls, infants with S marcescens bacteremia were more likely to have had a central vascular catheter (OR = 4.33; 95% CI (1.41 to 13.36)) and surgery (OR = 5.67; 95% CI (1.81 to 17.37)), and had a higher overall mortality (44% vs 2%; OR = 38.50; 95% CI (4.57 to 324.47)). Compared to E coli matched controls, infants with S marcescens bacteremia had later onset of infection (median of 33 days of life vs 10; p<0.001), prolonged intubation (OR = 5.76; 95% CI (1.80 to 18.42)), and a higher rate of CVC (OR = 7.77; 95% CI (2.48 to 24.31)) use at the time of infection. A higher rate of meningitis (24% vs 7%; OR = 3.98; 95% CI (1.09 to 14.50)) was observed with S marcescens bacteremia compared to E coli.
Conclusions
S marcescens bacteremia occurs sporadically in the NICU, primarily in premature infants requiring support apparatus late in their hospital course. Associated meningitis is common and mortality high.
Serratia marcescens is a pathogenic and opportunistic gram‐negative rod (GNR) of the Enterobacteriaceae family with a proclivity toward infecting immunocompromised hosts. The newborn patient population is particularly at risk secondary to deficits in, and the immature function of, several components of the natural and acquired immune systems. The preterm neonate is further compromised by an ineffective skin barrier and increased need for instrumentation to facilitate survival. The escalating frequency of premature births1 coupled with therapeutic advances in their care has thereby resulted in an increase in the population at risk.
S marcescens bacteremia in neonatal intensive care units (NICU) has typically been observed as hospital‐acquired, epidemic outbreaks.2,3,4,5,6,7,8,9,10,11,12 These outbreaks have traditionally been linked to environmental sources2,3,4,5,6,7,9,10,11,12 such as contaminated medical devices.2,4,9,10,12 We have periodically observed non‐epidemic cases of S marcescens bacteremia in our NICU. The purpose of this case‐control analysis was to identify and compare cases of endemic S marcescens bacteremia in an NICU over a 25‐year period with matched uninfected and Escherichia coli‐infected controls in an attempt to identify risk factors, presenting signs and symptoms, and outcomes specific to S marcescens that may facilitate and expedite their diagnosis and intervention.
Methods
The Yale‐New Haven Hospital (Y‐NHH) newborn special care unit is a 46‐bed, level IIID tertiary care referral center for infants with complex medical and surgical conditions.
Case selection
A matched case‐control study was performed by comparing each case of S marcescens to 2 uninfected controls and 2 cases of Escherichia coli bacteremia.
Given the relatively small number of cases, two controls were utilized to improve the precision of the statistical estimate. To identify cases, the medical records of all infants with blood cultures positive for S marcescens obtained while inpatients in the NICU at Y‐NHH from January 1, 1980, to December 31, 2004, were reviewed and data pertaining to demographics, potential risk factors for infection, and outcomes were extracted.
Controls
Each case of S marcescens was matched and compared to two uninfected controls in an attempt to isolate potential risk factors associated with demographics and clinical care variables. Next, to compare S marcescens bacteremia to the most common GNR pathogen in our NICU, each case was matched and compared with two cases of Escherichia coli (E coli) bacteremia. Due to the observation that many cases of E coli sepsis, unlike those caused by S marcescens, occur during the first week of life, in the final part of this study, we compared cases of S marcescens sepsis with cases of E coli sepsis that occurred after day of life 7.
Matching criteria
Two uninfected controls were matched to each case of S marcescens bacteremia using birth weight (BW; ±250 grams), gender, and date of birth (±1 year). Two cases of E coli bacteremia were identified for each case of Serratia and also matched according to BW (±350 grams), gender, and date of birth (±3 years). Matching criteria for BW and date of birth differed between control groups because there were fewer E coli‐bacteremic subjects available. No matching could be performed for cases of E coli at >7 days of life due to the small data pool.
Definitions
Sepsis
Sepsis was defined as described.13 A repeat positive blood culture was defined as a positive blood culture with the same species and identical antibiotic susceptibility pattern obtained after⩾24 hours of appropriate antibiotic therapy. Multiple positive repeat blood cultures from a single patient obtained within one week were considered a single episode of sepsis.
Meningitis
Meningitis was documented if culture‐proven or if pleocytosis (>100 white blood cells/mm3) in the cerebrospinal fluid was detected.
Presumptive primary source of infection
This was defined as a positive culture from a source other than blood with an identical antibiotic susceptibility pattern which predated (⩽7 days) or, in the case of a central vascular catheter (CVC) culture, coincided with the onset of bacteremia
Risk factors and signs
Preterm labor was defined as onset of labor prior to 37 weeks' gestation. Maternal fever was defined as core temperature >38°C prior to delivery. Prolonged rupture of membranes was defined as rupture of membranes ⩾18 hours prior to delivery. Choriomanionitis was identified by the isolation, under sterile conditions, of a pathogenic organism from the amniotic fluid or maternal blood and/or the presence of intrapartum fever in conjunction with two or more of the following: fetal tachycardia; uterine tenderness; malodorous vaginal discharge; or maternal leukocytosis (>15 000 leukocytes/mm3).14
The presence of a CVC, including umbilical venous and arterial catheters, percutaneous central venous catheters, Arrow® (Arrow International, Reading, PA, USA), and Broviac Silastic® (Bard Access Systems, Salt Lake City, UT, USA) catheters, (author, please include the place and country of origin) was included as a risk factor only if placed before the onset of sepsis and in place at the time of the positive blood culture. Surgery was included only if the procedure occurred ⩽7 days prior to the onset of bacteremia. Prolonged mechanical ventilation was defined as the need for mechanical ventilation for >14 days prior to infection. Other potential risk factors for sepsis (i.e. urinary catheter, total parenteral nutrition) had to be in place or in use at the time a positive culture was obtained.
Apnea and bradycardia were classified as a clinical sign of sepsis only if the episodes were new in onset, or more frequent, prolonged, or severe than previously observed. Hypoglycemia was defined as blood glucose<40 mg/dl not coinciding with reduction in the glucose infusion rate or volume of enteral intake. Hyperglycemia was defined as blood glucose of >140 mg/dl that did not coincide with an increase in the glucose infusion rate or volume of enteral intake. Hypothermia was defined as a core body temperature<36.5°C. Hyperthermia was defined as a core body temperature >38°C. The absolute neutrophil count was obtained at the same time as the positive blood culture and was defined as the fraction of the sum of the segmented and band forms, or the automated neutrophil count, multiplied by the total white blood cell count. Neutropenia was defined as an absolute neutrophil count of <1500 cells/mm3.
Death
Death was considered related to sepsis if it occurred within 7 days of the positive blood culture or if clinical signs and symptoms of sepsis were documented in the medical record as the direct cause of death. Sepsis‐related death was calculated with the numerator representing the number of episodes of sepsis with death and the denominator as the total number of episodes of sepsis.
Laboratory
Blood cultures were assessed using a fluorescent‐detection system for the presence of CO2 (Bactec II or 9240®, Becton Dickinson, USA). Antibiotic susceptibility patterns were tested using the disk diffusion method.15
Strain relatedness of S marcescens was determined by pulse‐field gel electrophoresis. Genomic DNA was digested with SpeI and XbaI (Boehringer Mannheim) and separated by electrophoresis with a CHEF‐DR III apparatus (Bio‐Rad, Hercules, CA, USA). The following parameters were used: temperature of 15°C with a run time of 18 h, switch time of 1–20 s, and voltage of 5.6 V/cm.
The pattern of DNA fragments for each S marcescens isolate was compared with others of the same species independently by 2 investigators. Patterns that were distinctly different from each other (>3 bands different) in the same subspecies were considered to be unrelated and were defined as unique. Patterns that were identical or differed from each other by ⩽3 fragments were considered to be the same strains and were defined as shared.16,17
Statistical analysis
The SPSS v13.0 statistical software package (SPSS Inc., Chicago, IL, USA) was utilized for data analyses. Continuous data from cases and controls were analyzed using the Wilcoxon Rank Sum test. Dichotomous variables were analyzed using odds ratio and bivariable logistic regression analysis. Despite the relatively small sample size and risk of model breakdown, independent covariates with a p‐value of <0.10 as identified by bivariable analysis were subjected to multivariable logistic regression analysis. A p‐value of <0.05 was considered statistically significant.
Research ethics committee approval
This study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Results
Patient characteristics, risk factors, and outcome
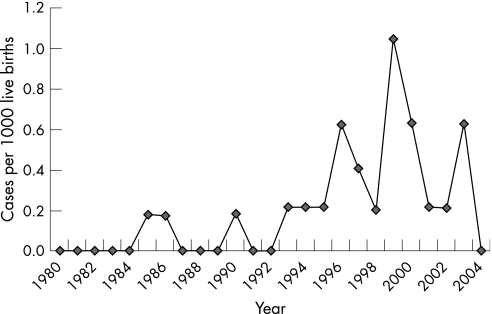
Twenty‐five cases of S marcescens bacteremia occurred in infants hospitalized in the NICU at Y‐NHH between January 1, 1980 and December 31, 2004 (fig 1). Median gestational age (GA) was 28 weeks, with a body weight (BW) of 1235 grams. Infection presented at a median of 33 days of life (table 1). At the time of infection, more than 50% of infants were mechanically ventilated and receiving total parenteral nutrition (TPN) through a CVC (table 2). Meningitis occurred with 24% of cases of S marcescens bacteremia (table 3). The overall mortality was 44% (table 2). Death from sepsis occurred in 24% of infants (table 3).
Figure 1 Cases of S marcescens per 1,000 live births in the Y‐NHH NICU from 1980 to 2004.
Table 1 Characteristics of cases of S marcescens sepsis versus uninfected, sepsis with E Coli, and sepsis with E Coli>7 days of life controls.
| Characteristic | Median (Range) | |||
|---|---|---|---|---|
| S marcescens (n = 25) | Uninfected controls (n = 50) | E Coli (n = 50) | E Coli>7 days (n = 68) | |
| BW (g) | 1235(470–3350) | 1250(590–3360) | 1129(570–3830) | 1470(545–4610) |
| GA (wks) | 28(23–40) | 31(23–40) | 28(24–42) | 30(23–42) |
| Day of life at positive culture | 33(13–146) | N/A | 10(1–117)* | 22(8–117)** |
| Number of days from surgery to onset of infection | 15(1–65) | N/A | 27(1–61) | 19(1–74) |
| Number of days with central line¶ | 16(1–43) | N/A | 8(2–49) | 13(1–38) |
| Number of days receiving antibiotics¶ | 11(0–50) | N/A | 2(0–58)* | 3(0–62)** |
| Platelet count | 220(5–511) | N/A | 238(10–504) | 207(8–504) |
| White blood cell count | 13.5(3–62) | N/A | 11.3(2–57) | 11.8(0.2–56) |
¶Prior to onset of infection
*P<0.001
**P = 0.001
Table 2 Bivariable logistic regression analysis of risk factors and outcomes associated with cases of S marcescens sepsis versus uninfected controls.
| Characteristic | S marcescens (n = 25) | Uninfected controls (n = 50) | OR (95% CI)* | p† |
|---|---|---|---|---|
| Host and perinatal factors | ||||
| Vaginal delivery | 10 (40) | 19 (38) | 1.09 (0.41 to 2.91) | 0.87 |
| Maternal antibiotic use | 9 (36) | 15 (30) | 1.31 (0.48 to 3.63) | 0.60 |
| Preterm labor | 16 (64) | 24 (48) | 1.93 (0.72 to 5.17) | 0.25 |
| Prolonged rupture of membranes | 2 (8) | 5 (10) | 0.78 (0.14 to 4.35) | 0.80 |
| Maternal fever | 1 (4) | 2 (4) | 1.00 (0.09 to 11.59) | 0.96 |
| Chorioamnionitis | 3 (12) | 0 | 0.03 | |
| Procedures and conditions | ||||
| Intubated | 13 (52) | 22 (44) | 1.38 (0.53 to 3.61) | 0.51 |
| Prolonged intubation | 11 (44) | 15 (30) | 1.83 (0.68 to 4.96) | 0.23 |
| Surgery | 12 (48) | 7 (14) | 5.67 (1.81 to 17.37) | <0.01 |
| Urinary catheter | 1 (4) | 1 (2) | 2.04 (0.12 to 34.07) | 0.61 |
| CVC | 20 (80) | 24 (48) | 4.33 (1.41 to 13.36) | 0.01 |
| TPN | 14 (56) | 31 (62) | 0.78 (0.29 to 2.07) | 0.62 |
| Intralipids | 13 (52) | 31 (62) | 0.66 (0.25 to 1.75) | 0.41 |
| H2‐blockers | 11 (44) | 31 (62) | 0.48 (0.18 to 1.28) | 0.14 |
| Clinical outcome | ||||
| Death | 11 (44) | 1 (2) | 38.50 (4.57 to 324.47) | 0.001 |
†Bivariable logistic regression
*OR: odds ratio; CI: confidence interval
Table 3 Bivariable logistic regression analysis of risk factors and outcomes associated with cases of S marcescens bacteremia versus E Coli‐bacteremic controls.
| Characteristic | S marcescens (n = 25) | E Coli (n = 50) | OR (95% CI)* | p† |
|---|---|---|---|---|
| Host and perinatal factors | ||||
| Vaginal delivery | 10 (40) | 14 (28) | 1.40 (0.82 to 2.40) | 0.19 |
| Maternal antibiotic use | 9 (36) | 27 (54) | 0.48 (0.18 to 1.29) | 0.14 |
| Preterm labor | 16 (64) | 36 (72) | 0.69 (0.25 to 1.92) | 0.48 |
| Prolonged rupture of membranes | 2 (8) | 13 (26) | 0.25 (0.05 to 1.20) | 0.06 |
| Maternal fever | 1 (4) | 7 (14) | 0.26 (0.03 to 2.21) | 0.21 |
| Chorioamnionitis | 3 (12) | 10 (20) | 0.55 (0.14 to 2.19) | 0.39 |
| Procedures and conditions | ||||
| Intubated | 13 (52) | 21 (42) | 1.50 (0.57 to 3.93) | 0.41 |
| Prolonged intubation | 11 (44) | 6 (12) | 5.76 (1.80 to 18.42) | <0.01 |
| Surgery | 12 (48) | 13 (26) | 2.63 (0.96 to 7.20) | 0.06 |
| Urinary catheter | 1 (4) | 1 (2) | 2.04 (0.12 to 34.07) | 0.61 |
| CVC | 20 (80) | 17 (34) | 7.77 (2.48 to 24.31) | <0.001 |
| TPN | 14 (56) | 14 (28) | 3.27 (1.20 to 8.92) | 0.02 |
| Intralipids | 13 (52) | 13 (26) | 3.08 (1.13 to 8.44) | 0.03 |
| H2‐blockers | 11 (44) | 10 (20) | 3.14 (1.10 to 8.99) | 0.03 |
| Repeat positive blood culture | 13 (52) | 12 (24) | 3.43 (1.24 to 9.50) | 0.02 |
| Presenting signs and symptoms | ||||
| Apnea | 9 (36) | 23 (46) | 0.66 (0.25 to 1.77) | 0.41 |
| Bradycardia | 7 (28) | 16 (32) | 0.83 (0.29 to 2.38) | 0.72 |
| Hypoglycemia | 3 (12) | 5 (10) | 1.23 (0.27 to 5.61) | 0.79 |
| Hyperglycemia | 8 (32) | 18 (36) | 0.84 (0.30 to 2.32) | 0.73 |
| Hypothermia | 11 (44) | 17 (34) | 1.53 (0.57 to 4.08) | 0.40 |
| Hyperthermia | 8 (32) | 7 (14) | 2.89 (0.91 to 9.22) | 0.07 |
| Neutropenia | 1 (4) | 6 (12) | 0.31 (0.04 to 2.69) | 0.29 |
| Clinical outcome | ||||
| Meningitis | 6 (24) | 8 (16) | 1.66 (0.51 to 5.45) | 0.41 |
| Death | 11(44) | 14(28) | 2.02 (0.74 to 5.51) | 0.17 |
| Sepsis‐related death | 6 (24) | 11 (22) | 1.12 (0.36 to 3.49) | 0.85 |
†Bivariable logistic regression
*OR: odds ratio; CI: confidence interval
Organism characteristics and antibiotic susceptibility patterns
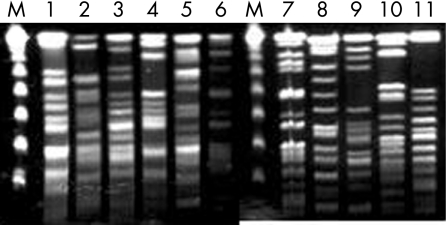
Cases occurred sporadically over a 25‐year period with a minimum of 41 days between episodes and no more than five cases in a 12‐month period (1999). Eleven isolates were available for comparison analysis of organism DNA using pulse field gel electrophoresis. All 11 were determined to be different strains, including isolates from four cases in 1999 (fig 2).
Figure 2 Pulse field gel electrophoresis of S marcescens blood isolates. DNA was prepared from 11 blood isolates of S marcescens, digested with SpeI and XbaI, subjected to pulse field gel electrophoresis, and banding patterns compared. M denotes a λ DNA molecular weight marker. The numbers represent individual isolates. Isolate 1 was from 1998, isolates 2 through 5 were from 1999, isolates 6 and 7 were from 2000, isolate 8 was from 2001, isolate 9 was from 2002, and isolates 10 and 11 were from 2003.
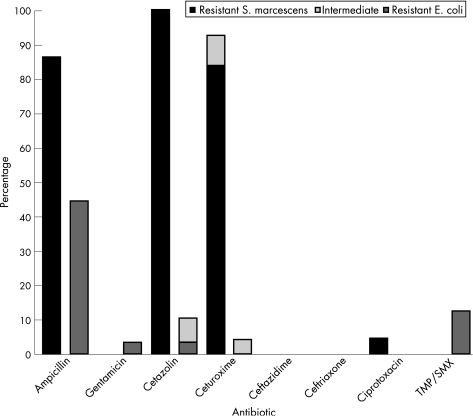
Eighty‐six percent of S marcescens isolates were resistant to ampicillin. None was resistant to gentamicin or 3rd generation cephalosporins (fig 3).
Figure 3 Percentage of non‐susceptible (resistant and intermediate) isolates of S marcescens and E coli cultured at >7 days of life for each antibiotic tested.
A presumptive primary source of infection was identified in 21 of 25 (84%) cases of S marcescens bacteremia (table 4). A CVC catheter was the believed to be the primary source of infection in 8 (32%) cases, while the conjunctiva, gastrointestinal, genitourinary, and respiratory tracts were the presumed source in 3 (12%) cases each.
Table 4 Presumptive primary source of infection in 25 cases of S marcescens bacteremia.
| Source | Number of Cases (%) |
|---|---|
| Central vascular catheter | 8 (32) |
| Respiratory | 3 (12) |
| Genitourinary | 3 (12) |
| Conjunctiva | 3 (12) |
| Gastrointestinal | 3 (12) |
| Ventriculo‐peritoneal shunt | 1 (4) |
| Unknown | 4 (16) |
Cases of S marcescens and uninfected controls
The overall mortality was higher among infected patients (44% vs 2%, OR = 38.50; 95% CI (4.57 to 324.47); table 2). Bivariable logistic regression analysis revealed that patients with S marcescens bacteremia were more likely to have been born to mothers with chorioamnionitis, to have an indwelling CVC (OR = 4.33; 95% CI (1.41 to 13.36)), and to have had surgery (OR = 5.67; 95% CI (1.81 to 17.37); table 2). Multivariable logistic regression confirmed the presence of a CVC (adjusted OR = 3.25; 95% CI (1.12 to 10.65); p = 0.04) and surgery (adjusted OR = 4.07; 95% CI (1.26 to 13.10); p = 0.01) as significant independent risk factors for S marcescens bacteremia.
Cases of S marcescens and E coli controls
S marcescens bacteremia (median, 33 days) presented later in onset than E coli (median, 10 days, p<0.001; table 1). Infants with S marcescens bacteremia received more total days of antibiotics prior to infection (p<0.001; table 1) and mechanical ventilation (OR = 5.76; 95% CI (1.80 to 18.42)), and were more likely to have a CVC in place (OR = 7.77; 95% CI (2.48 to 24.31)) and be receiving TPN (OR = 3.27; 95% CI (1.20 to 8.92)), intralipids (OR = 3.08; 95% CI (1.13 to 8.44)), and H2‐blockers at the time of infection (OR = 3.14; 95% CI (1.10 to 8.99); table 3). Infants with S marcescens bacteremia were also more likely to have a positive repeat culture, despite appropriate antibiotic therapy for >24 hours, than those with E coli (OR = 3.43; 95% CI (1.24 to 9.50); table 3). There were no differences in perinatal risk factors, presenting signs, or mortality (table 3). Despite relatively small numbers of cases and controls for analysis, a multivariable logistic regression was performed (with day of life at onset of infection included) and the presence of a CVC determined to be a significant independent covariate for S marcescens bacteremia as compared with E coli (adjusted OR = 4.94; 95% CI (1.20 to 20.41); p<0.01).
Cases of S marcescens and E Coli controls presenting at >7 days of life
Data analyses revealed no significant differences in body weight, gestation age, or gender (tables 1 and 5). Subjects with S marcescens sepsis still presented with later onset infection (p = 0.001; table 1), received more total days of antibiotic therapy prior to infection (p = 0.001; table 1), were more likely to require prolonged mechanical ventilation, (OR = 5.15; 95% CI (1.79 to 14.81); table 5), and to have an indwelling CVC in place at the time of infection (OR = 3.35; 95% CI (1.13 to 9.97); table 5). A higher rate of meningitis was observed with S marcescens bacteremia as compared with later onset E coli (24% vs 7%, OR = 3.98; 95% CI (1.09 to 14.50); table 5). Multivariable logistic regression confirmed prolonged intubation (adjusted OR = 3.45; 95% CI (1.11 to 10.91); p = 0.02) and the presence of a CVC (adjusted OR = 3.54; 95% CI (1.01 to 12.37); p = 0.048) as independent risk factors for S marcescens bacteremia.
Table 5 Bivariable logistic regression analysis of risk factors and outcomes associated with cases of S marcescens bacteremia versus E Coli‐bacteremic controls at >7 days of life.
| Characteristic | S marcescens (n = 25) | E Coli (n = 68) | OR (95% CI) | P† |
|---|---|---|---|---|
| Host and perinatal factors | ||||
| Male sex | 16 (64) | 45 (66) | 0.91 (0.35 to 2.37) | 0.85 |
| Vaginal delivery | 10 (40) | 46 (68) | 0.31 (0.15 to 1.06) | 0.06 |
| Maternal antibiotic use | 9 (36) | 29 (43) | 0.76 (0.29 to 1.95) | 0.56 |
| Preterm labor | 16 (64) | 41 (60) | 1.17 (0.45 to 3.03) | 0.75 |
| Prolonged rupture of membranes | 2 (8) | 9 (13) | 0.57 (0.11 to 2.84) | 0.49 |
| Maternal fever | 1 (4) | 5 (7) | 0.53 (0.06 to 4.73) | 0.57 |
| Chorioamnionitis | 3 (12) | 5 (7) | 1.72 (0.38 to 7.79) | 0.48 |
| Procedures and conditions | ||||
| Intubated | 13 (52) | 23 (34) | 2.10 (0.84 to 5.38) | 0.11 |
| Prolonged intubation | 11 (44) | 9 (13) | 5.15 (1.79 to 14.81) | <0.01 |
| Surgery | 12 (48) | 19 (28) | 2.36 (0.92 to 6.14) | 0.07 |
| Urinary catheter | 1 (4) | 1 (2) | 2.79 (0.17 to 46.40) | 0.47 |
| CVC | 20 (80) | 37 (54) | 3.35 (1.13 to 9.97) | 0.03 |
| TPN | 14 (56) | 29 (43) | 1.71 (0.68 to 4.31) | 0.26 |
| Intralipids | 13 (52) | 27 (40) | 1.65 (0.65 to 4.14) | 0.29 |
| H2‐blockers | 11 (44) | 20 (29) | 1.89 (0.73 to 4.86) | 0.19 |
| Presenting signs and symptoms | ||||
| Apnea | 9 (36) | 28 (41) | 0.80 (0.31 to 2.08) | 0.65 |
| Bradycardia | 7 (28) | 21 (31) | 0.87 (0.31 to 2.35) | 0.79 |
| Hypoglycemia | 3 (12) | 3 (4) | 3.27 (0.44 to 12.63) | 0.30 |
| Hyperglycemia | 8 (32) | 27 (40) | 0.72 (0.27 to 1.89) | 0.50 |
| Hypothermia | 11 (44) | 22 (32) | 1.64 (0.64 to 4.20) | 0.30 |
| Hyperthermia | 8 (32) | 19 (28) | 1.21 (0.45 to 3.28) | 0.70 |
| Neutropenia | 1 (4) | 3 (4) | 0.90 (0.09 to 9.11) | 0.93 |
| Clinical outcome | ||||
| Meningitis | 6 (24) | 5 (7) | 3.98 (1.09 to 14.50) | 0.04 |
| Death | 11 (44) | 16 (24) | 2.55 (0.97 to 6.73) | 0.09 |
| Sepsis‐related death | 6 (24) | 9 (13) | 2.07 (0.65 to 6.57) | 0.22 |
CV: central vascular catheter; TPN: total parenteral nutrition.
†Bivariable logistic regression
Antibiotic susceptibility pattern differences between cases of S marcescens and E Coli
Antibiotic susceptibility patterns were compared between cases of S marcescens and E coli presenting at >7 days of life. Both organisms were almost uniformly sensitive to gentamicin and 3rd generation cephalosporins (fig 3). Although not typically used in the treatment of S marcescens, a higher percentage of ampicillin (p<0.01), cefazolin (p<0.001), and cefuroxime (p<0.001) resistance was observed in S marcescens compared with E coli.
Discussion
S marcescens is an opportunistic organism which, in adults, predominantly infects immunocompromised and debilitated hosts including postoperative patients18,19 and hospitalized patients with endotracheal tubes,20,21,22,23 and CVC.20,21,24 These findings are mirrored in our population. S marcescens sepsis in the Y‐NHH NICU occurred more commonly in premature, very low birth weight infants and was more likely to occur in infants who had surgery and a CVC in place at the time of infection. Presumptive primary sources of infection included support apparatus and the gastrointestinal, genitourinary, and respiratory tracts.
S marcescens sepsis in the NICU was associated with a significant risk of morbidity and mortality. Previous reports have documented an association between S marcescens bacteremia and meningoencephalitis and brain abscess formation.7,8,25,26 Central nervous system infection with S marcescens is aggressive and may advance despite appropriate antibiotic therapy.7,25,26 This is comparable to other neurotropic pathogens associated with neonatal sepsis and meningoencephalitis and brain abscess formation including K1‐serotype E Coli, Citrobacter spp. and Enterobacter sakazakii.27 In our case series, the prevalence of meningitis among infants with S marcescens bacteremia was 24% (as compared with a 6% overall prevalence of meningitis with all documented cases of sepsis in our NICU). This is significantly higher than observed in 68 cases of E Coli bacteremia occurring after 7 days of life (7% prevalence).
What is already known on this topic
S marcescens bacteremia in the NICU is typically epidemic, occurring as outbreaks in association with contaminated medical devices.
S marcescens bacteremia is associated with significant morbidity and mortality in the preterm patient population
Mortality after S marcescens bacteremia has been reported to be 10% to 20%.28,29,30 In our study, S marcescens bacteremia had a 24% associated mortality (as compared with an overall prevalence of sepsis‐related death in our NICU of 12%).13 In cases of S marcescens bacteremia with meningitis, the prevalence of sepsis‐related mortality increased to 50%.
Episodes of S marcescens sepsis in the NICU have traditionally been reported as clustered epidemics with contamination of a common source leading to colonization and subsequent infection.2,3,4,5,6,7,9,10,11,12 At Y‐NHH, cases have occurred sporadically with no predominant strain or definable outbreak over the past quarter‐century. The epidemiology of S marcescens bacteremia in the NICU at Y‐NHH appears comparable to that observed in a study of endemic, antibiotic‐resistant Gram‐negative bacilli in a NICU.31 This report suggested that there is gradual, but temporary, acquisition of these organisms from the NICU environment into the changing neonatal microflora with little cross‐colonization.31 A recent study of NICU Gram‐negative bacilli infection demonstrated that contact from the hands of providers is an unlikely cause of NICU‐associated GNR bacteremia.32 Together, these data indicate that the source of GNR in NICU‐related bacteremia is environmental and/or associated with noncutaneous reservoirs such as the gastrointestinal or respiratory tracts. As a result, prevention strategies should focus on identification and manipulation of these potential sources as well as attention to traditional hygienic issues such as hand hygiene and environmental cleanliness.
Antibiotic susceptibility patterns for S marcescens exhibit institutional and chronologic variability. Some centers report high rates of resistance to aminoglycosides and 3rd generation cephalosporins,23,33 while others report sensitivity to these agents.34,35S marcescens isolates from our NICU are universally sensitive to gentamicin and 3rd generation cephalosporins, similar to the susceptibility patterns of S marcescens observed throughout Y‐NHH.36 Despite this finding, a high percentage (52%) of infants were found to have a repeat positive blood culture after >24 hours of appropriate antibiotic therapy. This may be due to factors related to the organism, to the debilitated nature of the host population, or other factors. Although the data represent a small number of cases and no differences in outcome were observed among infants with and without persistently positive cultures, antibiotic therapy with both an aminoglycoside and a 3rd generation cephalosporin should be considered if S marcescens bacteremia is confirmed, particularly when central nervous system infection cannot be excluded. Therapy with a 3rd generation cephalosporin should also be considered when S marcescens is isolated from a patient in an institution where gentamicin‐resistant S marcescens has been observed.
What this study adds
S marcescens bacteremia can occur sporadically in the NICU without a definable strain or outbreak.
Compared to E Coli, S marcescens bacteremia typically occurs late in the NICU hospitalization and tends to infect more debilitated infants.
Acknowledgement
We acknowledge the limitations of our study, given its retrospective nature and relatively small sample size. We attempted to address some of these issues with our study design. A matched case‐control study was chosen in an attempt to control for the potential confounding effects of prematurity and gender on the susceptibility to infection. In addition, matching by date of birth was performed to control for the potential confounding effects of the differences in treatment protocols and practices over the 25‐year study period. Finally, a 2:1 control‐to‐case ratio was performed in an attempt to improve the precision of the statistical model given the small number of available cases.37 Multivariable logisitic regression, where appropriate, was also employed in an attempt to identify significant independent risk factors for each desired outcome of interest and the data analyzed and presented accordingly.
Abbreviations
CVC - central vascular catheter
GNR - gram‐negative rod
NICU - neonatal intensive care unit
TPN - total parenteral nutrition
Y‐NHH - Yale‐New Haven Hospital
Footnotes
Funding: Supported in part by National Institute of Child Health and Human Development Training Grant T32 HD 07094 (M.J.B.).
Competing interests: None.
References
- 1.Hoyert D L, Mathews T J, Menacker F.et al Annual summary of vital statistics: 2004. Pediatrics 2006117168–183. [DOI] [PubMed] [Google Scholar]
- 2.Stamm W E, Kolff C A, Munoz Dones E.et al A nursery outbreak caused by Serratia marcescens‐scalp‐vein needles as a portal of entry. J Pediatr 19768996–99. [DOI] [PubMed] [Google Scholar]
- 3.Berthelot P, Grattard F, Amerger C.et al Investigation of a nosocomial outbreak due to Serratia marcescens in a maternity hospital. Infect Control Hosp Epidemiol 199920233–236. [DOI] [PubMed] [Google Scholar]
- 4.Neal T J, Corkill J E, Bennett K J.et alSerratia marcescens pseudobacteraemia in neonates associated with a contaminated blood glucose/lactate analyzer confirmed by molecular typing. J Hosp Infect 199941219–222. [DOI] [PubMed] [Google Scholar]
- 5.Jang T N, Fung C P, Yang T L.et al Use of pulsed‐field electrophoresis to investigate an outbreak of Serratia marcescens infection in a neonatal intensive care unit. J Hosp Infect 20014813–19. [DOI] [PubMed] [Google Scholar]
- 6.Uduman S A, Farrukh A S, Nath K N R.et al An outbreak of Serratia marcescens in a special‐care baby unit of a community hospital in United Arab Emirates: the importance of the air conditioner duct as a nosocomial reservoir. J Hosp Infect 200252175–180. [DOI] [PubMed] [Google Scholar]
- 7.Berger A, Rohrmeister K, Haiden N.et alSerratia marcescens in the neonatal intensive care unit: re‐emphasis of the potentially devastating sequelae. Wien Klin Wochenschr 20021141017–1022. [PubMed] [Google Scholar]
- 8.Assadian O, Berger A, Aspöck C.et al Nosocomial outbreak of Serratia marcescens in a neonatal intensive care unit. Infect Control Hosp Epidemiol 200223457–461. [DOI] [PubMed] [Google Scholar]
- 9.Sarvikivi E, Lyytikäinen O, Salmenlinna S.et al Clustering of Serratia marcescens infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol 200425723–729. [DOI] [PubMed] [Google Scholar]
- 10.Cullen M M, Trail A, Robinson M.et alSerratia marcescens outbreak in a neonatal intensive care unit prompting review of decontamination of laryngoscopes. J Hosp Infect 20055968–70. [DOI] [PubMed] [Google Scholar]
- 11.Youssef R F, Darcy E, Barone A.et al Expressed breast milk as a source of neonatal sepsis. Ped Inf Dis J 200221888–889. [DOI] [PubMed] [Google Scholar]
- 12.Milisavvljevic V, Wu F, Larson E.et al Molecular epidemiology of Serratia marcescens outbreaks in two neonatal intensive care units. Infect Control Hosp Epidemiol 200425719–721. [DOI] [PubMed] [Google Scholar]
- 13.Bizzarro M J, Raskind C, Baltimore R S.et al Seventy‐five years of neonatal sepsis at Yale: 1928–2003. Pediatrics 2005116595–602. [DOI] [PubMed] [Google Scholar]
- 14.Shah S S, Ehrenkranz R A, Gallagher P G. Increasing incidence of Gram‐negative rod bacteremia in a newborn intensive care unit. Pediatr Infect Dis J 199918591–595. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards ( N C C L S.Performance standards for antimicrobial disk susceptibility tests. 5th ed. Villanova, PA: NCCLS, 1993
- 16.Almuneef M A, Baltimore R S, Farrel P A.et al Molecular typing demonstrating transmission of gram‐negative rods in a neonatal intensive care unit in the absence of a recognized epidemic. Clin Infect Dis 200132220–227. [DOI] [PubMed] [Google Scholar]
- 17.Tenover F C, Arbeit R D, Goering R V.et al Interpreting chromosomal DNA restriction patterns produced by pulsed‐field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995332233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowder J G, Gilkey G H, White A C.Serratia marcescens bacteremia. Ach Intern Med 1971128247–253. [DOI] [PubMed] [Google Scholar]
- 19.Kwitko A O, Hamra L K, Atkinson J M.Serratia: opportunistic pathogen of increasing clinical importance. Med J Aust 19772119–121. [DOI] [PubMed] [Google Scholar]
- 20.Yu V L, Oakes C A, Axnick K J.et al Patient factors contributing to the emergence of gentamicin‐resistant serratia marcescens. Am J Med 197966468–472. [DOI] [PubMed] [Google Scholar]
- 21.Fry D E, Fry R V, Shlaes D M. Serratial bacteremia in the surgical patient. Am Surg 198753438–441. [PubMed] [Google Scholar]
- 22.Bouza E, García de la Torre M, Erice A.et alSerratia bacteremia. Diagn Microbiol Infect Dis 19877237–247. [DOI] [PubMed] [Google Scholar]
- 23.Choi S ‐ H, Kim Y S, Chung J ‐ W.et alSerratia bacteremia in a large university hospital: trends in antibiotic resistance during 10 years and implications for antibiotic use. Infect Control and Hosp Epidemiol 200223740–747. [DOI] [PubMed] [Google Scholar]
- 24.Saito H, Elting L, Bodey G P.et al Serratia bacteremia: review of 118 cases. Reviews of Infect Dis 198911912–920. [DOI] [PubMed] [Google Scholar]
- 25.Campbell J R, Diacovo T, Baker C J. Serratia marcescens meningitis in neonates. Pediatr Infect Dis J 199211881–888. [DOI] [PubMed] [Google Scholar]
- 26.Ries M, Deeg K H, Heininger U.et al Brain abcesses in neonates‐report of three cases. Eur J Pediatr 1993152745–746. [DOI] [PubMed] [Google Scholar]
- 27.Kimberlin D W. Meningitis in the neonate. Curr Treat Options Neurol 20024239–248. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin D K, DeLong E R, Cotton C M.et al Postconception age and other risk factors associated with mortality following gram‐negative rod bacteremia. J Perinatol 200424169–174. [DOI] [PubMed] [Google Scholar]
- 29.Makhoul I R, Sujov P, Smolkin T.et al Pathogen‐specific early mortality in very low birth weight infants with late‐onset sepsis: a national survey. Clin Infect Dis 200540218–224. [DOI] [PubMed] [Google Scholar]
- 30.Cordero L, Rau R, Taylor D.et al Enteric gram‐negative bacilli bloodstream infections: 17 years' experience in a neonatal intensive care unit. Am J Infect Control 200432189–195. [DOI] [PubMed] [Google Scholar]
- 31.Toltzis P, Dul M J, Hoyen C.et al Molecular epidemiology of antibiotic‐resistant gram‐negative bacilli in a neonatal intensive care unit during a nonoutbreak period. Pediatrics 20011081143–1148. [DOI] [PubMed] [Google Scholar]
- 32.Larson E L, Cimiotti J P, Haas J.et al Gram negative bacilli associated with catheter‐associated and non‐catheter‐associated bloodstream infections and hand carriage by healthcare workers in neonatal intensive care units. Pediatr Crit Care Med 20056457–461. [DOI] [PubMed] [Google Scholar]
- 33.Hsueh P R, Chen W H, Luh K T. Relationships between antimicrobial use and antimicrobial resistance in Gram‐negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan. Int J Antimicrob Agents 200526463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisplinghoff H, Bischoff T, Tallent S M.et al Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 200439309–317. [DOI] [PubMed] [Google Scholar]
- 35.Wenzel R P, Sahm D F, Thornsberry C.et al In vitro susceptibilities of gram‐negative bacteria isolated from hospitalized patients in four European countries, Canada, and the United States in 2000–2001 to expanded‐spectrum cephalosporins and comparator antimicrobials: implications for therapy. Antimicrob Agents Chemother 2003473089–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yale‐New Haven Hospital Clinical Microbiology Laboratory, Department of Pharmacy Services, Hospital Epidemiology and Infection Control 2004 Antibiotic susceptibility report: susceptibility summary of isolates obtained from January 1, 2004–December 31, 2004. 2005; Department of Pharmacy Services, Yale‐New Haven Hospital, New Haven, Connecticut.
- 37.Ury H K. Efficiency of case‐control studies with multiple controls per case: continuous or dichotomous data. Biometrics 197531643–649. [PubMed] [Google Scholar]