Abstract
Oxygen is the most commonly used therapy in neonatal nurseries as an integral part of respiratory support. The goal of oxygen therapy is to achieve adequate delivery of oxygen to the tissue without creating oxygen toxicity. Oxygen must have been given to newborn preterm babies more than any other medicinal product in the past 60 years. Despite this, we still know very little about how much oxygen these babies actually need, or how much oxygen is safe to give, especially in the first few weeks of life. Recent observational studies have raised concerns that giving oxygen to target the saturation at “physiological” levels in newborn preterm babies may do more harm than good, but to date, clinicians have not been able to resolve the uncertainties surrounding optimum oxygen therapy.
Priestley,1 along with Scheele and Lavoisier,1a,1b contributed to the discovery that the air we breathe is really a mixture of dephlogisticated or “vital” air and “gas azote”. Although the use of oxygen as a medicinal product has a long history,2 the “routine” use of supplemental oxygen in the care of preterm infants originated from the observation in the 1940s by Wilson et al3 that the irregular pattern of periodic breathing commonly seen in babies of short gestation was largely overcome when they were given ⩾70% oxygen to breathe. This observation eventually led to the widespread practice of unrestricted oxygen supplementation for small or sick infants in the early 1950s, and the ensuing epidemic of severe eye disease and blindness is well documented.
Campbell4 from Australia was the first person to suggest in 1951, that oxygen could be responsible for the rising epidemic of severe retinopathy of prematurity (ROP), and she concluded that “normal oxygen environment of the newborn full‐term infant is abnormal for the premature infant”. More convincing evidence came within a year from Crosse and Evans5 in England, and from a randomised trial by Patz et al6 in the USA, followed by a cooperative trial by Kinsey et al7 in 1956. This centrally randomised trial allocated babies of weight ⩽1500 g who survived until 48 h of age into “routine‐oxygen group” (received oxygen at a concentration of >50% for 28 days) or “curtailed‐oxygen group” (received added oxygen at the clinician's discretion, but not >40–50%). The results of this trial were widely interpreted at the time as suggesting that oxygen therapy was safe as long as the inspired oxygen concentration was not >40%.8 The fact that babies in one arm of the trial had not only had more oxygen but also had it for much longer was almost entirely overlooked. Even more seriously, it took a long time for clinicians to realise that a policy of restricting exposure to oxygen rather than restricting arterial oxygen levels was almost certainly causing a rise in the number of early neonatal deaths.9 Once it became clear from the Cooperative Trial that, although excess exposure to oxygen was at least one of the causes of retinopathy, but there was no clarity as to how oxygen administration could be optimised, clinicians started to look for ways of monitoring arterial oxygen levels.
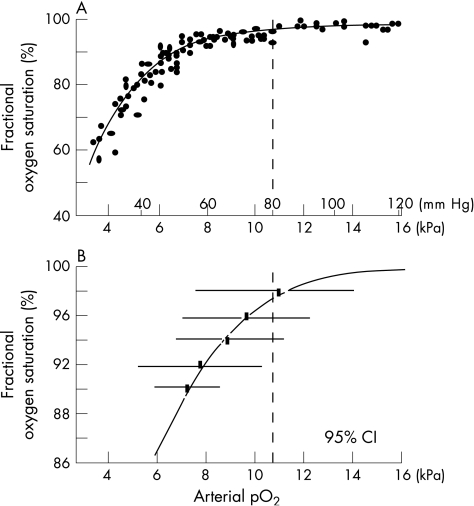
Indwelling arterial lines were soon being widely used to monitor arterial oxygen tension, but no controlled trial has ever shown that their use reduces the risk of permanent retinal damage.10 One attempt was made when it first became possible to monitor oxygen levels continuously and non‐invasively to see whether transcutaneous monitoring could reduce the risk of oxygen exposure. There was no evidence that it did, but later analysis of some of the information collected during that trial suggested that retinopathy occurred more often when the transcutaneous reading reached or exceeded 80 mm Hg (10.7 kPa) in the first 4 weeks of life.11 No comparable studies have been attempted as it became commonplace to use pulse oximetry to monitor oxygen saturation. Evidence from non‐randomised studies suggests that pulse oximetry is a reliable measure of oxygenation in infants with chronic lung disease and prolonged oxygen dependency, particularly at lower arterial oxygen pressure (PaO2) levels.12,13 However, the ability of pulse oximeters to reliably detect hyperoxia remains controversial, and it has been shown that fractional oxygen saturations of more than 92% can often be associated with hyperoxia, as defined by an arterial oxygen tension of more than 80 mm Hg14 (fig 1).
Figure 1 (A) The relationship between fractional oxygen saturation measured with a pulse oximeter and arterial partial pressure of oxygen (pO2) in mm Hg and kPa. (B) The bars show the range within which 95% of all measures of pO2 varied when the oximeter read 90%, 92%, 94%, 96% and 98% in the study reported by Brockway and Hay14. The dashed lines in A and B mark the transcutaenous pO2 above which there was an increased risk of retinopathy in the study reported by Flynn in 1992.11 Reproduced with permission from Neonatal Formulary.15
Following the lessons learnt from the oxygen‐induced blindness epidemic in the 1950s and the development of oxygen monitoring devices, many studies have tried to define what constitutes a safe level of oxygenation for newborn preterm babies over the past 50 years. Unfortunately, few studies in this field of medicine have used the method known to be the best way of reliably assessing the effects of interventions—“the randomised controlled trial”.
Oxygen and neonatal morbidities in a preterm infant
Oxygen and the eye
Although the aetiology and pathogenesis of ROP is considered multifactorial, it is primarily affected by the immaturity of the retina itself and by the levels of retinal arterial oxygenation. Development of the retinal vasculature is affected by oxygen‐regulated vascular endothelial growth factor and non‐oxygen‐regulated insulin‐like growth factor‐1. Treatment with supplemental oxygen in preterm infants with incompletely vascularised retina may cause hyperoxia and vasoconstriction. This in turn may lead to local hypoxia, up‐regulation of vascular endothelial growth factor, and excessive proliferation of new vessels and fibrous tissue that invades the vitreous. Contraction of fibrous tissue may then result in retinal detachment.16 It was observed in animal studies on rat pups that fluctuations of inspired oxygen around higher fractional inspired oxygen levels (mean 24%) lead to more severe vascular abnormalities than around lower fractional inspired oxygen levels (means of 21% and 17%).17
Oxygen and the brain
The effects of oxygen on the brain may be, to some extent, similar to its effect on the retina, as the retina is a specialised part of the central nervous system and thus may share the same pathophysiological processes; cerebral and retinal damage have been suggested to share a common vasoreactive vascular origin.18 The effect of exposure to pure oxygen on the brain was described as early as in 1959 by Gyllensten,19 who reported that rearing mice in pure oxygen for 20–30 days had a marked deleterious effect on subsequent cortical vascularisation and cellular differentiation. In humans, development of an extensive form of periventricular leucomalacia is also strongly correlated with sustained hyperoxia. More recently, Haynes et al20 described oxidative damage to premyelinating oligodendrocytes in cerebral white matter as a mechanism of development of periventricular leucomalacia. In a large clinical study on 1105 low birthweight infants by Collins et al,21 the risk of disabling cerebral palsy was observed to be doubled in those exposed to hyperoxia. The adjusted odds for risk of cerebral palsy increased further to eightfold in infants with the highest quintiles compared with the lowest quintiles of exposure to oxygen.
Oxygen and the lung
When bronchopulmonary dysplasia (BPD) was first described in preterm babies, it was widely believed that oxygen toxicity was a major contributing factor.22 Oxygen itself, rather than the effect of providing artificial ventilatory support, can cause rapidly lethal damage to the previously normal lung, as was shown by an elegant small experiment involving 18‐week‐old lambs in 1969.23 In preterm babies, the effect of high inspired oxygen concentration as an important cause of BPD is well documented.24 Even if the inspired oxygen concentration is not high, oxidative stress can still occur and cause lung injury, and high levels of biochemical markers of oxidative stress have been identified in the pulmonary lavage in the first few days of life in preterm babies who later develop BPD. Premature babies are also more prone to oxidative stress, as they have a higher risk of exposure to high oxygen concentrations, reduced antioxidant defence, and more free iron, leading to production of hydroxyl radicals. However, studies using antioxidants such as superoxide dismutase, vitamin E and vitamin A for the prevention of BPD have so far shown conflicting results.25
“Normal” levels of oxygenation
Preterm babies can maintain their growth in utero, with a mean PaO2 of 3.2 kPa, equivalent to arterial oxygen saturations of about 70%.26 However, clinical practice in neonatal units has generally been to attempt to keep oxygen levels in “all” newborns in line with those of term and non‐compromised preterm infants. Several studies27,28 have shown a relatively narrow range of normal baseline values of oxygen saturation during the regular breathing state of preterm infants in their neonatal period, which is 93–100%. What is also known is that preterm infants with prolonged dependency on supplemental oxygen have lower baseline saturation levels29 and greater risk of pulmonary hypertension,30 compared with preterm infants without chronic oxygen dependency or infants born at term. What is not known, however, is whether these associations are causal. It remains unclear whether attempts to ameliorate the “non‐normal” states described above do more harm than good, and whether oxygen‐related interventions designed to reduce desaturation episodes and decrease PaO2 variability actually make any material difference to meaningful long‐term outcomes such as improving growth and development or reducing serious adverse pulmonary complications or death.
Optimum levels of oxygenation in preterm infants
Neonatal period
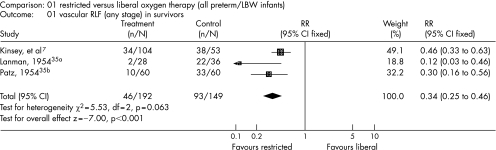
To date, there is not sufficient evidence to suggest what the optimal oxygen saturation is or PaO2 values to aim for in preterm infants who receive oxygen therapy to avoid potential oxygen toxicity while ensuring adequate oxygen delivery to tissues. Three trials published >50 years ago showed the effect of unrestricted, high levels of ambient oxygen in causing severe eye disease in premature infants (fig 2). Recent evidence from observational studies31,32,33,34 has suggested that this hypothesis of a “restrictive oxygen approach” is worth exploring.
Figure 2 Forest plot showing the meta‐analysis of three trials included in the review (reproduced with permission from J Wiley and sons).35 LBW, low birthweight; RLF, retrolental fibroplasia.
A prospective observational study by Tin et al31 reported some provocative findings in 2001. All the babies in this study were born in, or referred to, one of five neonatal intensive care units, where the policy towards monitoring the oxygen saturation varied, but other care policies were fairly similar. Two of the five neonatal units shared the same oxygen saturation monitoring policy, hence there were four different practices of oxygen monitoring during the study period. Survival rates and survivors without evidence of cerebral palsy at 1 year were almost identical in the five units in babies of 23–27 weeks gestation still alive 1 year after birth (table 1). In one unit, target fractional oxygen saturation was 80–90% with the lower alarm limit set to operate only if saturation fell below 70% (restrictive approach). Such a policy was sustained for all babies thought to need supplemental oxygen until retinal vascularisation was complete. In another unit, target functional oxygen saturation was 94–98%, with the lower alarm set to operate at 88% (liberal approach). The other three units had intermediate policies. Careful, uniform, ophthalmic review of all the survivors showed that retinopathy severe enough to merit treatment with cryotherapy occurred in 6.3% (95% confidence interval (CI) 1.7% to 15.0%) of the babies cared for with the restrictive approach, and 27.7% (95% CI 17.3% to 40.2%) with the liberal approach. No child from those cared for with the restrictive approach, but four from those cared for with the liberal approach, became blind. The three units using intermediate policies for target oxygen saturation had “threshold” retinopathy rates in the middle of this range. In the unit that used the restrictive approach, half of the 64 long‐term survivors were managing without endotracheal intubation and mechanical ventilation by 7 days, and without supplemental oxygen by 30 days. By contrast, these milestones were achieved by 21 and 72 days, respectively, for survivors cared for with the liberal approach. There was no evidence that targeting much lower oxygen saturation in these preterm babies had an adverse effect on growth between birth and their discharge. The neurodevelopmental outcome was similar at 1 year (no child having been lost to follow‐up).
Table 1 Outcome at 1 year in all babies of 23–27 weeks gestation born during 1990–1994 and its relationship to minimum and maximum pulse oximeter alarm settings31.
| Oximeter alarm settings (%) | Number of babies admitted | Number of survivors (%) | 1 year survivors | ||
|---|---|---|---|---|---|
| Median number of days ventilated, n | Cerebral palsy, n (%) | Threshold retinopathy, n (%) | |||
| 88–98* | 123 | 65 (52.8) | 21 | 11 (16.9) | 18 (27.7) |
| 85–95 | 235 | 128 (54.5) | 16 | 20 (15.6) | 20 (15.6) |
| 84–94 | 84 | 37 (44.0) | 15 | 6 (16.2) | 5 (13.5) |
| 70–90 | 126 | 64 (50.8) | 7 | 10 (15.6) | 4 (6.3) |
*Nellcor pulse oximeter measurements (functional saturation). Other measurements are fractional saturation measurements.
Target saturation was in the upper half of the accepted range.
Sun32 collated data from the Vermont Oxford Network, and compared the survival, chronic lung disease and ROP of 1544 extremely low birthweight babies who were cared for in units that aimed to keep oxygen saturation ⩽95% and those >95% while these babies were treated with supplemental oxygen. They reported the significantly lower incidences of chronic lung disease (27% v 53%) as well as stage 3/4 ROP (10% v 29%) among babies cared for with targeted oxygen saturations of ⩽95%. The survival rate was marginally higher in the low‐saturation group, but this was not significant. Chow et al33 showed that implementation of clinical practices of oxygen management and monitoring was associated with a significant decrease in the incidence of stage 3/4 ROP from 12% to 2.5%, and a need for retinal surgery from 4.5% to 0% in infants with birth weight of 500–1500 g. A national survey by Anderson et al34 also showed significantly less stage 3/4 ROP (2.4% v 5.5%) and less retinal surgery (1.3% v 3.3%) in babies of weight <1501 g at birth, in neonatal units where the upper alarm limit of pulse oxygen saturation in babies >2 weeks old was ⩽92% v >92%.
In contrast with the report of these observational studies, Poets et al36 reported their observation on 891 babies of <30 weeks gestation and admitted to two neonatal units using different oxygen saturation limits (80–92% v 92–97%). A retrospective analysis of their data showed that the incidence of ROP (more than stage 2) was significantly higher in the unit that used a lower alarm limit (13% v 6%), although no difference was seen in the incidence of ROP that required surgery. The difficulty with all these observational studies, however, is that the association between target oxygen saturation and improved outcomes cannot be deemed causal, and as no randomised controlled trials exist to answer this question comprehensively, the uncertainty remains.
Postneonatal period
Two randomised trials have recently been conducted to see whether it is better to maintain high oxygen saturation in very preterm babies when they are more than a few weeks old. The Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP‐ROP) trial,37 which recruited 649 babies with pre‐threshold ROP born between 1990 and 1994 with a mean birth gestation of 25.4 weeks and a mean postmenstrual age at trial entry of 35 weeks, showed that keeping fractional oxygen saturation >95% (96–99% v 89–94%) slightly reduced the number of babies with pre‐threshold retinopathy who went on to develop disease severe enough to require retinal surgery. However, benefit was only seen in those without evidence of “plus disease” at recruitment (32% v 46%). More unexpectedly, targeting the higher oxygen saturation significantly increased the number of infants who remained in hospital, receiving supplemental oxygen, and receiving diuretics at a postmenstrual age of 50 weeks. Among babies with evidence of chronic lung disease at trial entry, targeting high oxygen saturation was also associated with more common pulmonary deterioration (13.2% v 8.5%). The higher oxygenation target did not improve growth or the eventual retinal outcome as assessed 3 months after the expected date of delivery.
The result of the Benefit of Oxygen Saturation Targeting (BOOST) Trial was published in 2003.38 The aim of this randomised, double‐blind study was to see whether maintaining higher oxygen saturations versus standard levels in oxygen‐dependent preterm babies improves their growth and development. This study recruited 358 babies of <30 weeks gestation who continued receiving supplemental oxygen at 32 weeks postmenstrual age. Collaborating units had different policies with regard to optimum oxygenation in the period immediately after birth, but specified pulse oximeters were used after recruitment for as long as supplemental oxygen was deemed necessary. Trial oximeters were modified to keep the functional saturation in the range 91–94% or 95–98% depending on allocation at trial entry, while displaying a figure in the range 93–96%. This study showed no evidence that the growth and developmental outcome of the oxygen‐dependent preterm infant was improved by keeping their oxygen saturation in the high range. In keeping with the observation in the STOP‐ROP study, the BOOST study also showed that infants in the higher oxygen saturation range had greater use of postnatal steroids (58% v 50%) and diuretics (52% v 44%), more readmissions (54% v 48%) and more pulmonary‐related deaths (6% v 1%). However, neither trial was designed to look at these pulmonary events as primary outcome measures.
Approaches to oxygen therapy and clinical outcome
Neonatal morbidities related to oxygen therapy have already been described. The prognostic effect of severe ROP, BPD and brain injury is additive, and was shown to be independently correlated to poor outcome at 18 months of age.39 An observational study by Tin et al31 is the only published study that has provided data on neurodevelopmental outcome of “all” surviving children who received their early neonatal care under different oxygen monitoring policies. Their initial finding of no difference in the rate of cerebral palsy among survivors, although important, is not entirely reassuring considering that the use of the “restrictive” oxygen therapy approach in neonatal life may still cause a negative effect on cognitive function, adaptive skills and behaviour. The second follow‐up of the same cohort40 included a total of 124 surviving children who had neonatal care under “liberal” and “restrictive” oxygen therapy approaches. Children were seen at about 10 years of age, and the follow‐up rate in this cohort was 96%. Mean score for full‐scale IQ of all the 119 children assessed was about one standard deviation below the population mean. There were no differences in the proportion of children with behavioural problems or poor adaptive skills between the two groups. More children cared for with the liberal approach had cognitive disability compared with those cared for with the restrictive approach (35% v 23%). Mean full‐scale IQ of children in the liberal group was eight points lower than that in the restricted group, and a similar trend was seen for literacy and numeracy skills. The scores varied widely and showed a skewed distribution, and the difference between two mean IQ scores was not significant, but these findings provide reassurance to clinicians that restrictive oxygen therapy with an aim to keep oxygen saturations between 80% and 90% in babies of <28 weeks gestation in their neonatal period is not associated with any disadvantages in terms of intellectual skills, academic achievements, adaptive functioning and behaviour.
Resolving the uncertainty
The lack of knowledge and ongoing uncertainty for >50 years on what is optimal oxygenation for the very preterm infants making the transition to extrauterine life has led to wide variations in policies on oxygen monitoring and therapy in neonatal nurseries. Observational studies over the past 50 years have got us nowhere, and the only way to resolve the uncertainties and controversies of oxygen monitoring and therapy is to conduct well‐designed randomised controlled trials.41,42
Multicentre randomised controlled trials—the oxygen trials
In response to the growing demand to resolve the controversy on oxygen therapy in very preterm babies, an international collaborative effort has been mounted since 2003 to conduct large, multicentre, blinded randomised trials to answer the question “What oxygen saturation level should be aimed for in very premature infants?” Randomised trials are being planned—namely, BOOST 2 (Australia), BOOST 2 (New Zealand), BOOST 2 (UK), Canadian Oxygen Trial, Pulse Oximetry Saturation Trial to prevent Retinopathy of Prematurity and the Surfactant Positive Airway Pressure and Pulse Oximetry Trial in the US. Infants of <28 weeks gestation are eligible for these studies if they are <24 h of age. BOOST 2 UK, funded by The Medical Research Council UK, will recruit 1200 babies over a 4‐year period. However, with the international collaborative effort, it is envisaged that a total of about 5000 babies will be enrolled into the “oxygen trials”.
All these trials will deal with the principal research question of whether varying the concentration of inspired oxygen to maintain a “low” oxygen saturation range of 85–89% versus a “high” range of 91–95% in babies of <28 weeks gestation from the day of birth until they are breathing air or until 36 weeks postmenstrual age affects the incidence of (1) death or severe neurosensory disability at a corrected age of 2 years, (2) retinal surgery for ROP, (3) the need for supplemental oxygen therapy or respiratory support at 36 weeks postmenstrual age, (4) patent ductus arteriosus and necrotising enterocolitis, and (5) poor growth at 36 weeks postmenstrual age and at 2 years. There has already been a prospective agreement to combine the individual patient data from all the trials to increase the ability to detect much smaller differences in the important outcomes, and this controlled trial strategy of prospective meta‐analysis is likely to be established for the first time in neonatal medicine from “the oxygen trials”.
Conclusion
Collaboration across three continents helped Campbell4 to identify the cause of retinopathy in the preterm baby >50 years ago. A similar collaboration to mount well‐designed randomised trials and the strategy of prospective meta‐analysis will provide important information to confirm or refute the fact that the “restrictive” oxygen approach does more good than harm in preterm babies vulnerable to oxygen toxicity. However, clinicians should be aware that the current oxygen trials may not end the controversies on “oxygen”—the powerful and most commonly used “drug” in neonatal medicine.
Abbreviations
BOOST - Benefit of Oxygen Saturation Targeting
BPD - bronchopulmonary dysplasia
PaO2 - arterial oxygen pressure
pO2 - partial pressure of oxygen
ROP - retinopathy of prematurity
Footnotes
Competing interests: None declared.
References
- 1.Priestley J.Experiments and observations on different kinds of air. London: J Johnson, 1775101
- 1a. Scheele K W.Chemical observations and experiments an air and fire.. Introduction by Torben Bergman. London: J Johnson, 1780
- 1b. Lavoisier A‐L. Experiences sur la respiration des animaux. Mém Soc Sci Paris 17802185 [Google Scholar]
- 2.Tin W, Hey E. The medical use of oxygen: a century of research in animals and humans. NeoReviews 20034e340–e349. [Google Scholar]
- 3.Wilson J L, Long S B, Howard P J. Respiration of premature infants: response to variations of oxygen and to increased carbon dioxide in inspired air. Am J Dis Child 1942631080–1085. [Google Scholar]
- 4.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia: a clinical approach. Med J Aust 1951ii48–50. [PubMed] [Google Scholar]
- 5.Crosse V M, Evans P J. Prevention of retrolental fibroplasia. Arch Ophthalmol 19524883–87. [DOI] [PubMed] [Google Scholar]
- 6.Patz A, Hoeck L E, de la Cruz E. Studies on the effect of high oxygen administration in retrolental fibroplasia. 1. Nursery observations. Am J Ophthalmol 1952351248–1253. [DOI] [PubMed] [Google Scholar]
- 7.Kinsey V E, Jacobus J T, Hemphill F. Retrolental fibroplasia: cooperative study of retrolental fibroplasia and the use of oxygen. Arch Ophthalmol 195656481–543. [PubMed] [Google Scholar]
- 8.Guy L P, Lanman T J, Dancis J. The possibility of total elimination of retrolental fibroplasia by oxygen restriction. Pediatrics 195617247–249. [PubMed] [Google Scholar]
- 9.Cross K W. Cost of preventing retrolental fibroplasia? Lancet 1973ii954–956. [DOI] [PubMed] [Google Scholar]
- 10.Duc G, Sinclair J C. Oxygen administration. In Sinclair JC, Bracken MB, eds. Effective care of the newborn infant. Oxford: Oxford University Press, 1992178–199.
- 11.Flynn J T, Bancalari E, Snyder E S.et al A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med 19923261050–1054. [DOI] [PubMed] [Google Scholar]
- 12.Walsh M C, Noble L M, Carlo W A.et al Relationship of pulse oximetry to arterial oxygen tension in infants. Crit Care Med 1987151102–1105. [DOI] [PubMed] [Google Scholar]
- 13.Southall D P, Bignall S, Stebbens V A.et al Pulse oximeter and transcutaneous arterial oxygen measurements in neonatal and paediatric intensive care. Arch Dis Child 198762882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brockway J, Hay W W. Prediction of arterial partial pressure of oxygen with pulse oxygen saturation measurements. J Pediatr 199813363–66. [DOI] [PubMed] [Google Scholar]
- 15.Hey E.Neonatal formulary, vol. 4, London: BMJ Books, 2003187
- 16.Smith L E H. Pathogenesis of retinopathy of prematurity. Semin Neonatol 20038469–473. [DOI] [PubMed] [Google Scholar]
- 17.McColm J R, Cunningham S, Wade J.et al Hypoxic oxygen fluctuations produce less severe retinopathy than hyperoxic fluctuations in a rat model of retinopathy of prematurity. Pediatr Res 200455107–113. [DOI] [PubMed] [Google Scholar]
- 18.Fledelius H C. Central nervous system damage and retinopathy of prematurity—an ophthalmic follow‐up of prematures born in 1982–84. Acta Paediatr 1996851186–1191. [DOI] [PubMed] [Google Scholar]
- 19.Gyllensten L. Influence of oxygen exposure on the differentiation of the cerebral cortex of growing mice. Acta Morphol Neerl Scand 19592311–330. [PubMed] [Google Scholar]
- 20.Haynes R L, Folkerth R D, Keefe R J.et al Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol 200362441–450. [DOI] [PubMed] [Google Scholar]
- 21.Collins M P, Lorenz J M, Jetton J R.et al Hypocapnia and other ventilation related risk factors for cerebral palsy in low birth weight infants. Pediatr Res 200150712–719. [DOI] [PubMed] [Google Scholar]
- 22.Northway W H, Rosan R C, Porter D Y. Pulmonary disease following respirator therapy of hyaline membrane disease—bronchopulmonary dysplasia. N Engl J Med 1967267357–368. [DOI] [PubMed] [Google Scholar]
- 23.deLemos R, Wolfsdorf J, Nachman R.et al Lung injury from oxygen in lambs. The role of artificial ventilation. Anesthesiology 196930609–618. [DOI] [PubMed] [Google Scholar]
- 24.Jobe A H, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 20011631723–1729. [DOI] [PubMed] [Google Scholar]
- 25.Saugstad O D. Brochopulmonary dysplasia—oxidative stress and oxidants. Semin Neonatol 2003839–49. [DOI] [PubMed] [Google Scholar]
- 26.Nicolini U, Nicolaidis P, Fisk N M.et al Limited role of fetal blood sampling in prediction of outcome in intrauterine growth retardation. Lancet 1990336768–772. [DOI] [PubMed] [Google Scholar]
- 27.Richard D, Poets C F, Neale S.et al Arterial oxygen saturation in preterm neonates without respiratory failure. J Pediatr 1993123963–968. [DOI] [PubMed] [Google Scholar]
- 28.Ng A, Subhedar N, Primhak R A.et al Arterial oxygen saturation profiles in healthy preterm infants. Arch Dis Child 199879F64–F66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekar K, Duke J C. Sleep apnoea and hypoxaemia in recently weaned premature infants with and without bronchopulmonary dysplasia. Pediatr Pulmonol 199110112–116. [DOI] [PubMed] [Google Scholar]
- 30.Subhedar N V, Shaw N J. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch Dis Child 200082F243–F247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tin W, Milligan D W A, Pennefather P.et al Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child 200184F106–F110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun S C. Relation of target SpO2 levels and clinical outcome in ELBW infants on supplemental oxygen. Pediatr Res 200251A350 [Google Scholar]
- 33.Chow L, Wright K W, Sola S. Can changes in clinical practice decrease the incidence of severe retinopathy in very low birth weight infants? Pediatrics 2003111339–345. [DOI] [PubMed] [Google Scholar]
- 34.Anderson C G, Benitz W E, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. J Perinatol 200424164–168. [DOI] [PubMed] [Google Scholar]
- 35.Askie L, Henderson‐Smart D J. Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birthweight infants. Cochrane Library, Issue 3. Oxford: Update Software, 2003
- 31a. Lanman J T, Guy L P, Dancis J. Retrolental fibroplasia and oxygen therapy. JAMA 1954155223–226. [DOI] [PubMed] [Google Scholar]
- 31b. Patz A. Oxygen studies in retrolental fibroplasia. IV. Clinical and experimental observations. Am J Ophthalmol 195438291–308. [DOI] [PubMed] [Google Scholar]
- 36.Poets C, Arand J, Hummler H.et al Retinopathy of prematurity: a comparison between two centers aiming for different pulse oximetry saturation levels. Biol Neonate 200384A267 [Google Scholar]
- 37.The STOP‐ROP Multicenter Study Group Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP‐ROP), a randomized, controlled trial. I: Primary outcomes, Pediatrics 2000105295–310. [DOI] [PubMed] [Google Scholar]
- 38.Askie L M, Henderson‐Smart D J, Irwig L.et al Oxygen‐saturation targets and outcomes in extremely preterm infants. N Engl J Med 2003349953–961. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt B, Asztalos E V, Roberts R S.et al Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low‐birth‐weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterm. JAMA 20032891124–1129. [DOI] [PubMed] [Google Scholar]
- 40.Bradley S, Anderson K, Tin W.et al Early oxygen exposure and outcome at 10 years in babies of less than 28 weeks. Pediatr Res 200455A373 [Google Scholar]
- 41.Tin W, Wariyar U. Giving small babies oxygen: 50 years of uncertainty. Semin Neonatol 20027361–367. [DOI] [PubMed] [Google Scholar]
- 42.Silverman W A. A cautionary tale about supplemental oxygen: the albatross of neonatal medicine. Pediatrics 2004113394–396. [DOI] [PubMed] [Google Scholar]