Abstract
European water frog hybrids Rana esculenta (Rana ridibunda × Rana lessonae) reproduce hemiclonally, transmitting only their ridibunda genome to gametes. We compared fitness-related larval life-history traits of natural R. esculenta from Poland with those of the two sympatric parental species and of newly generated F1 hybrids. Compared with either parental species, F1 hybrid offspring had higher survival, higher early growth rates, a more advanced developmental stage by day 49, and earlier metamorphosis, but similar mass at metamorphosis. R. esculenta from natural lineages had trait values intermediate between those of F1 offspring and of the two parental species. The data support earlier observations on natural R. esculenta that had faster larval growth, earlier metamorphosis, and higher resistance to hypoxic conditions compared with either parental species. Observing larval heterosis in F1 hybrids in survival, growth rate, and time to metamorphosis, however, at an even higher degree than in hybrids from natural lineages, demonstrates that heterosis is spontaneous and results from hybridity per se rather than from subsequent interclonal selection; in natural lineages the effects of hybridity and of clonal history are confounded. This is compelling evidence for spontaneous heterosis in hybrid clonals. Results on hemiclonal fish hybrids (Poeciliopsis) showed no spontaneous heterosis; thus, our frog data are not applicable to all hybrid clonals. Our data do show, however, that heterosis is an important potential source for the extensively observed ecological success of hybrid clonals. We suggest that heterosis and interclonal selection together shape fitness of natural R. esculenta lineages.
Keywords: clonal reproduction, fitness, growth, interclonal selection, Rana esculenta
A small fraction (0.1%) of vertebrate taxa reproduce without genetic recombination (1–3). Features typically shared by these taxa with derived clonal reproduction include interspecies hybrid origin, local abundance, wide geographic range, and use of harsh or disturbed environments (4). Because the large amount of genic heterozygosity characterizing interspecies hybrids is thought to buffer against environmental variation and to increase developmental stability (e.g. refs. 5–8), spontaneous heterosis directly caused by hybridity has been hypothesized to explain the ecological success extensively observed in hybrid clonals (e.g. refs. 9–14). There are two important caveats, however, for conclusions based on studying natural lineages of clonals. (i) The observed broad environmental tolerance could either be the property of one or several constituent clones (either heterosis or the General-Purpose Genotype model based on interclonal selection promoting a highly generalized genotype; refs. 15–18) or could result from a wide array of clones that each have different tolerances and exploit different narrow ranges of resources along the environmental gradient (Frozen Niche Variation model; refs. 19–22). (ii) In natural clonal lineages, the effects of hybridity are confounded with those of clonal history (23, 24): superior fitness components observed in hybrid clonals may be either the result of interclonal selection or a preadapted general property among clones resulting from their hybridity (spontaneous heterosis). No evidence supporting widespread spontaneous heterosis among clones was found in a test using hybridogenetic Poeciliopsis fish hybrids (23). Thus, because the data demonstrating the ecological and demographic success of hybrid clonals are almost exclusively based on observations of natural lineages, heterosis has been dismissed as a major cause of such success (e.g. refs. 23, 24). We have tested the hypothesis of spontaneous heterosis in a system of hemiclonal frogs.
European water frogs Rana esculenta are natural hybrids between Rana ridibunda and Rana lessonae that occur in both sexes and reproduce hemiclonally, by hybridogenesis (reviewed in ref. 25). In the germ line of these hybrids, the R. lessonae chromosomes are excluded before meiosis, the remaining R. ridibunda chromosomes undergo a premeiotic or occasionally a prediplotene meiotic endoreduplication (26), and two apparently normal meiotic divisions result in functional, genetically identical haploid gametes containing an unrecombined R. ridibunda genome. Hybridity in these lineages is restored each generation through fertilization of these gametes by gametes from syntopic R. lessonae. Such mixed populations of the parental species R. lessonae and its sexual parasite R. esculenta form the widespread L–E system (27). In these frogs, hemiclonal F1 lineages can readily be generated in the laboratory: when crossing R. ridibunda from central Poland with sympatric R. lessonae in both reciprocal combinations, all F1 hybrids of both sexes tested have consistently evidenced hybridogenetic reproduction (14 R. ridibunda parents and 61 F1 hybrids tested; ref. 28).
The hybrid R. esculenta is widespread, occurring in almost the entire range of its sexual host R. lessonae (29). It is abundant in most breeding populations, although its proportion to R. lessonae can vary from 7% in forest ponds or natural marshes to 98% in gravel pits (30–32). The proportion of successfully metamorphosing R. lessonae and R. esculenta depends on the environment: the hybrid R. esculenta produces more metamorphs under more severe conditions of pond drying and interspecific competition that limit food availability, whereas the parental species R. lessonae does better in favorable growth environments (33). The success of R. esculenta in natural populations is probably in part a result of its ability to metamorphose earlier than R. lessonae under resource-limited conditions (34). A shorter time to metamorphosis in R. esculenta tadpoles relative to either parental species is regularly observed under a variety of conditions in central Poland (e.g., ref. 35). The higher growth rate of R. esculenta tadpoles apparently is underlain by their behavior: tadpoles of R. esculenta spend more time feeding and consume more food than either parental species rather than having higher physiological efficiency (36). Metamorphosed R. esculenta are more tolerant to hypoxic conditions than either parental species (37). In addition, R. esculenta tadpoles are more tolerant to a common agricultural fungicide (TPT, triphenyltin chloride) than R. lessonae tadpoles (38). These findings together suggest a broader environmental tolerance of the hybrid R. esculenta relative to its parental species, yet in most of these studies, R. esculenta was treated collectively, without resolving hemiclone identity. Recent results, however, have revealed large amounts of variation in various fitness-related larval life-history traits among coexisting R. esculenta hemiclones (attributable to their clonally transmitted ridibunda genome), which differed from each other by at least as much as hybrids differed from their parental species (39, 40). Moreover, using R. esculenta lineages from natural populations does not permit one to decide whether superior performance of hybrid genotypes reflects interclonal selection after the formation of hybrid lineages or spontaneous heterosis as a direct consequence of hybridity.
To test the hypothesis of spontaneous heterosis, we compared fitness-related larval life-history traits in progeny of four cross types: F1 hybrids generated by primary hybridizations of the sympatric parental species; hybrids R. esculenta from natural hemiclonal lineages of Poland; and the two sympatric parental species (R. lessonae, R. ridibunda). We present data from a laboratory experiment in which tadpoles, reared under controlled environmental conditions at two food levels, were tested for early larval growth rate, survival to metamorphosis, body mass at metamorphosis, and time to metamorphosis as surrogates of fitness. We then asked (i) Is there phenotypic variation among the cross types in fitness-related larval traits? (ii) Do the R. esculenta hybrids (wild-type and F1) perform better than either of the parental species? (iii) Is hybrid performance dependent on environmental condition, that is, food level? (iv) Do the primary F1 hybrids perform better than either of the parental species, thus providing evidence for spontaneous heterosis? (v) Do the natural hybrid lineages perform differently from the F1 hybrids, thus providing evidence for effects of clonal history such as the occurrence of interclonal selection?
MATERIALS AND METHODS
Source of Frogs.
Adult frogs used for the artificial crosses were collected from natural populations near Poznan in central Poland. We used seven females (three R. ridibunda, two R. esculenta, and two R. lessonae) and seven males (three R. ridibunda and four R. lessonae) in the crosses. Before crosses were made, the taxon identification of each frog was ascertained with protein electrophoresis (27, 41), by using tissue from clipped toes. As far as can be resolved by examining five loci that vary in R. ridibunda, the two R. esculenta parents used for the crosses belong to the same hemiclone (nonrecombining ridibunda genome haplotype). Because of the triploid hybrids found in some Polish populations (27, 42), diploidy of each hybrid was confirmed by erythrocyte size (27, 43).
Experimental Design and Artificial Crosses.
We generated progeny of four types of crosses: the two parental species inter se (R. lessonae, designated LL; R. ridibunda, RR), hybrids R. esculenta from natural hybridogenetic lineages (EL), and primary hybrids generated by hybridizations of the sympatric parental species (F1). We used a total of 14 families (3 LL, 5 RR, 4 EL, and 2 F1). The 2 families of F1 hybrids were independent full-sibling families from four randomly selected parents. Male R. lessonae were crossed with both female R. esculenta and R. ridibunda, rather than the reciprocal, because these are the usual mating combinations in natural L–E systems (25, 44, 45; L. Berger, personal communication) and in natural primary hybridizations (46–48; L. Berger, personal communication), respectively.
Artificial fertilization followed standard procedures (49, 50). On 23 May 1993, each female was injected with a fish luteinizing hormone-releasing hormone (LHRH; H-7525, Bachem) to induce ovulation. After females initiated ovulation, males were anesthetized with 3-aminobenzoic acid ethyl ester (MS-222) and both testes were crushed in pond water in a Petri dish. Eggs of one female at a time were then stripped into the resulting sperm suspension, cycling between males to avoid confounding ovulation order with paternal effects. After ≈3–5 min, sperm suspensions were rinsed into new Petri dishes and the fertilized eggs covered with fresh pond water. Eggs of the next female were fertilized in the same manner, rotating between taxa of females to prevent any bias in order of fertilization. All crosses were made within 6 h on 25 May; tadpoles hatched between 3–4 June and were then transferred to larger containers with 1.0 liters of pond water.
Rearing the Tadpoles.
On reaching stage 25 (ref. 51; free-swimming) on 7 June 1993 (day 0), tadpoles were reared in the laboratory in a temperature-controlled room with a 12 h light period at 20°C at two food levels. For each family, 20 tadpoles were haphazardly selected and counted into individual beakers, and 10 were randomly assigned to the high and 10 to the low food treatment (except for one EL cross family, for which only 6 tadpoles per treatment were available). A total of 272 tadpoles were reared individually in plastic dishpans (20 cm × 11.5 cm × 7.5 cm deep) filled with 1.0 liters of aged tap water. Dishpans were arranged into 10 randomized complete blocks according to a temperature and light gradient related to shelf height. Each block contained one replicate of each family at each food level. After the first 5 days, water was changed in each container every 3 days.
Food level was used as the environmental gradient on which to measure phenotypic variation. The high food level was a ration that was the maximum a tadpole could eat without excess food remaining in the water, and the low food level was always one-third of the high (49, 52). On day 1 of the experiment, food rations were set at 15 mg for high food and 5 mg for low and subsequently were increased in a stepwise manner according to the mass increase of tadpoles and the amount of uneaten food, to a maximum (day 134 and later) of 180 mg for high food and 60 mg for low food. Tadpoles were fed every 3 days, synchronized with changing water. The food consisted of a finely ground, seived mixture (2:1 by mass) of rabbit chow (Vitakraft) and fish flakes (Tetramin) that was vacuum-dried and weighed to the nearest 0.5 mg.
Measures of Performance.
Early larval growth rate, survival to metamorphosis, body mass at metamorphosis, and time to metamorphosis were used to measure the performance of individual tadpoles. Mass of each tadpole was measured, to the nearest 0.1 mg, on day 49 by blotting excess water with paper towels and placing it onto a tared weighing boat on a digital electronic analytical balance (Mettler AT200). Initial mass at stage 25 was determined for each family as the mean mass of 10 tadpoles at stage 25, or the average of families of the same cross type when the number of tadpoles was insufficient. To calculate the growth rate per day, the difference between the mass at day 49 and the initial mass was divided by 49 (linear growth during this early larval period was demonstrated in Hyla tadpoles; ref. 53). Also at day 49, the developmental stage of tadpoles (51) was recorded. Metamorphosis was defined as emergence of at least one forelimb (stage 42; ref. 51). At metamorphosis, the days since day 0 were recorded and metamorphs were held with little water until tail resorption was complete and weighed to the nearest 0.1 mg and corrected for initial mass. The experiment was terminated on 1 April 1994 (day 298); tadpoles not metamorphosed by then were recorded as dead.
Anuran larvae display large amounts of phenotypic variation in growth rate and development time and are strongly affected by food availability (52, 54). Phenotypic variation may be manifested in the timing of metamorphosis and size at metamorphosis, traits that can strongly influence fitness. High growth rates enable tadpoles to metamorphose quickly at a small size to escape drying in ephemeral ponds or predation in permanent ponds (55–57) or alternatively to maximize size at metamorphosis in ponds of greater duration (54). Larger size at metamorphosis can result in better physiological and locomotor performance in the terrestrial environment (58, 59), higher juvenile survival, earlier first reproduction, and larger size at first reproduction (60–63). Phenotypic variation in length of the larval period and size at metamorphosis are underlain by genetic variation (49, 56, 64), making these traits important for local adaptation and evolutionary change in pond-dwelling amphibians. Survival to metamorphosis, a direct component of fitness, affects juvenile recruitment and the potential for population growth (63). It is thus reasonable to assume that in these frogs the larval life-history traits we measured as surrogates of fitness are in fact highly relevant components of individual Darwinian fitness and of success of the clones.
Statistical Analyses.
Analyses were performed separately on tadpole responses at day 49 and at metamorphosis. Early growth rate was analyzed by using a four-way analysis of variance (PROC GLM with type III sums of squares; ref. 65) to determine the effects of cross type (EL, F1, LL, or RR), family nested within cross type, food level, and the interaction of food level and cross type. The main effect of block also was included in the model to eliminate subtle but nonsignificant effects resulting from vertical temperature gradients in the environmental chamber. Because developmental stage at day 49 is a noncontinuous response variable, it was analyzed by using a two-way ANOVA with weighted-least-squares estimation of parameters (PROC CATMOD with χ2 values for Wald’s statistic; ref. 65) to determine the effect of cross type, food level, and the interaction of cross type and food level. The effects of block and family could not be included in this latter model because the sample sizes in resulting treatment cells were too small. Survival to metamorphosis was analyzed by using a log-likelihood G test for the effect of cross type at both high and low food levels by pooling over all other treatments and for the effect of food level alone by pooling over cross types. Mass at metamorphosis and time to metamorphosis at the high food level were analyzed by using a one-way ANOVA (PROC GLM with type III sums of squares; ref. 65) to determine the main effect of cross type. Too few individuals survived at the low food level to include other factors in the model; preliminary analyses of block and family effects, however, were not significant for responses at metamorphosis.
Early growth rate, mass at metamorphosis, and days to metamorphosis were logarithmically transformed before analysis to increase additivity of effects and equality of the variance (66, 67). Conservative pairwise comparisons of treatment means were performed by using Scheffé’s test, which controls for experimentwise type I error rate (65).
RESULTS
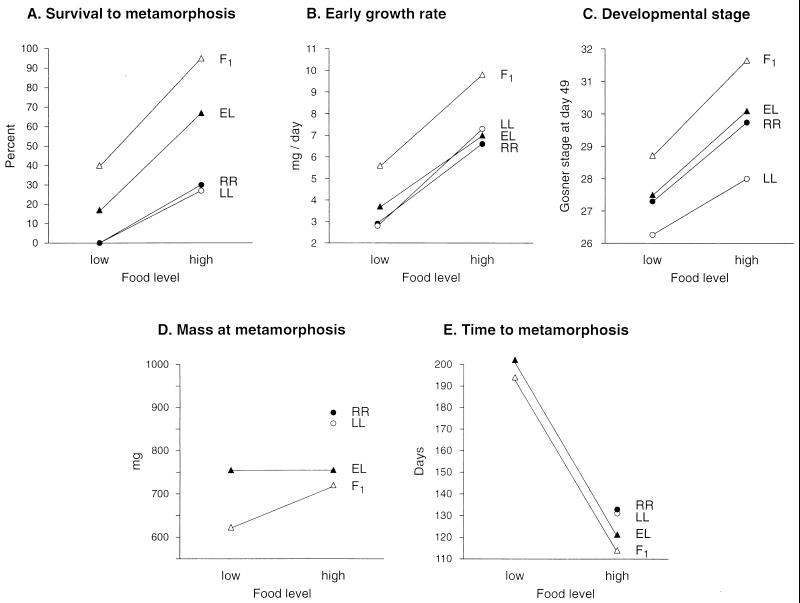
Survival among tadpoles of the four cross types was nonrandom at both the low food (G = 30.8, df = 3, P < 0.001) and high food (G = 38.8, df = 3, P < 0.001) levels. Tadpoles from the F1 crosses displayed the highest survival at both food levels, whereas tadpoles from the parental crosses (LL, RR) had the lowest survival (Fig. 1A). Tadpoles from the EL crosses were intermediate between the F1 and parental crosses (Fig. 1A). Survival was also nonrandom between food levels (G = 50.9, df = 1, P < 0.0001), with only 10.3% of all tadpoles surviving at low food whereas 26.5% of the tadpoles survived at the high food level (Fig. 1A).
Figure 1.
Interaction between cross type (F1, primary hybrids R. ridibunda × R. lessonae; EL, natural hybrid lineages R. esculenta; LL, R. lessonae; RR, R. ridibunda) and food level on larval performance in five fitness-related life-history traits. Superior performance is indicated by lower values for time to metamorphosis and by higher values for all other response variables. Values represent means of replicate individuals and families within each cross type.
Early larval growth rate was significantly affected by family nested within cross type (F10,219 = 4.54, P < 0.0001), cross type (F3,219 = 13.89, P < 0.0001), and food level (F1,219 = 122.7, P < 0.0001), but not by experimental blocks (F9,219 = 1.79, P = 0.071) or the interaction of cross type and food level (F3,219 = 2.22, P = 0.087). Scheffé’s pairwise comparisons indicate that tadpoles from the F1 crosses grew significantly faster than tadpoles from any of the other three crosses and that all tadpoles grew faster at the high food than at the low food level (Fig. 1B). The lack of a significant cross type × food level interaction indicates that the high growth rate of F1 tadpoles was independent of food level (Fig. 1B).
Developmental stage at day 49 was signicantly affected by cross type (χ2 = 56.0, df = 3, P < 0.0001) and food level (χ2 = 88.9, df = 1, P < 0.0001) but not by the interaction of cross type and food level (χ2 = 2.5, df = 3, P = 0.477). Tadpoles from the F1 crosses had the most advanced development at both food levels whereas tadpoles from one parental cross (LL) had the least advanced development (Fig. 1C). Tadpoles from the EL and RR crosses were intermediate between the F1 and LL parental crosses in developmental stage (Fig. 1C). All tadpoles were further developed at the high food than at the low food level (Fig. 1C). The lack of a significant cross type × food level interaction indicates that the greater development of F1 tadpoles was independent of food level (Fig. 1C).
Both mass at metamorphosis (F3,62 = 3.86, P = 0.0135) and days to metamorphosis (F3,62 = 5.40, P = 0.0023) were significantly affected by the cross type of tadpoles. Tadpoles from the F1 crosses were the smallest at metamorphosis but took the fewest days to metamorphose from the high food treatment (Fig. 1 D and E). Scheffé’s test indicated that the F1 tadpoles were significantly smaller than RR tadpoles but did not differ from either EL or LL tadpoles (Fig. 1D). Scheffé’s test also indicated that tadpoles from the F1 crosses had significantly shorter larval periods than tadpoles of either parental species (LL, RR) but did not differ from EL tadpoles (Fig. 1E).
DISCUSSION
Our results clearly show that F1 hybrid tadpoles R. ridibunda × R. lessonae had superior performance in four of the five life-history traits measured: compared with tadpoles of either parental species, F1 hybrids had higher survival, higher early growth rates, greater developmental stage by day 49 after becoming free-swimming, and earlier metamorphosis, but they showed lower mass at metamorphosis. In addition, F1 hybrid tadpoles displayed better performance in survival, early growth rate, and developmental stage by day 49 than tadpoles from natural lineages of R. esculenta, which for most traits had values intermediate between those of F1 offspring and those of the two parental species (Fig. 1).
The superior performance of hybrid tadpoles compared with either parental species matches abundant data on R. esculenta from natural hemiclonal lineages in various parts of their geographical range. Such R. esculenta have faster larval growth (68–70), regularly reach metamorphosis earlier (35, 69, 71; L. Berger, personal communication), are more efficient feeders (36), and have higher resistance to hypoxic conditions of hibernation after metamorphosis (37) than either parental species. Relative to their sexual host R. lessonae, R. esculenta tadpoles also produce more metamorphs in harsh conditions of drying ponds and interspecific competition (33), have higher survival and earlier metamorphosis under resource-limited conditions (49), survive better in the presence of larval dragonfly predators (72), and are less sensitive to the common agricultural fungicide triphenyltin in survival, growth rate, and time to metamorphosis (38). Moreover, R. esculenta females were found to have a higher fecundity and produce more eggs per unit body mass than either parental species, outperforming females of the sexual host R. lessonae in mean fecundity by a factor of three (73). Given hemiclonal reproduction, such enhanced hybrid traits affecting survival and fecundity are expected to contribute to enhanced fitness (“euheterosis”; ref. 74) rather than merely reflecting somatic hybrid vigor that may be coupled with sterility: the hybridogenetic gametogenesis of R. esculenta, in which sister chromatid-derived homologous chromosomes enter meiosis, circumvents severe fertility problems of hybrids resulting from incomplete synapsis that leads to aneuploid gametes (75, 76).
In all of these studies measuring fitness components of R. esculenta from natural lineages, however, the effects of hybridity and of clonal history are inextricably confounded. Clonal history includes the outcome of interclonal selection (a highly efficient process acting on whole genomes rather than the average additive effects of genes; ref. 6), which may lead either to a multitude of hemiclones, each adapted to a different, relatively narrow niche (as predicted by the Frozen Niche Variation model; refs. 19–21), or to one or a few hemiclones broadly adapted to a variety of environmental conditions (as predicted by the General-Purpose Genotype model; refs. 15–18), or to some combination (40, 77); neither of these models assumes spontaneous heterosis as a cause for ecological success of clonals. Clonal history may also include an expected accumulation of deleterious mutations on the clonally transmitted ridibunda genome through Muller’s ratchet (e.g. refs. 78–81), which may lead to a deterioration of fitness of R. esculenta lineages if some detrimental mutations are not completely recessive. In contrast to studies on natural R. esculenta lineages, however, our design using newly generated F1 hybrids permits us to test unambiguously for the effect of hybridity. The observed superior performance of F1 hybrid tadpoles compared with either parental species provides the first compelling evidence for spontaneous heterosis occurring in hybrid clonal vertebrates.
It is striking that F1 hybrid tadpoles performed not only as well, but even better than tadpoles of natural hemiclonal hybrid lineages in three of the five life-history traits measured, and performed worse, although not significantly so, only in mass at metamorphosis (Fig. 1). Time to and size at metamorphosis in frog tadpoles are usually viewed as alternative life-history traits reflecting a tradeoff between the advantages of avoiding larval death caused by aquatic predators or desiccation of ephemeral ponds and starting terrestrial life at a large size (e.g. refs. 60, 61, 82, and 83); especially under resource-limited conditions a delay of metamorphosis may be the cost for attaining a favorable size at metamorphosis (52, 84). It seems probable that the less extreme difference between these two opposing performance traits in R. esculenta from natural lineages relative to F1 hybrids (longer time to but larger mass at metamorphosis; Fig. 1) is a result of interclonal selection promoting a tradeoff optimization under conditions in natural ponds.
The superior performance of F1 hybrid tadpoles observed in this study is corroborated by data from crossing experiments with other frogs from different localities in central Poland. F1 tadpoles from primary hybridizations between R. ridibunda and R. lessonae regularly metamorphosed earlier than those of either parental species in a variety of conditions in tanks situated in the laboratory and outdoors, the superiority being greatest under unfavorable conditions of high density and interspecific competition (71; L. Berger and M. Rybacki, personal communication). These data, although not obtained in strictly controlled experiments, lend strong support to our results and their generality within the Rana system. Moreover, in another laboratory experiment, F1 tadpoles from crosses between Polish R. ridibunda and Swiss R. lessonae also had shorter time to but smaller size at metamorphosis than R. esculenta from natural lineages representing three coexisting hemiclones of one and a single hemiclone of another population (39).
The mechanistic cause underlying heterosis in F1 hybrid water frogs is not known. As a result of the permanent somatic hybridity stemming from hybridogenetic reproduction, all hemiclonal water frog lineages are characterized, just as are other hybrid clonals, by exceptionally high observed mean individual heterozygosity (H̄ values of up to 0.5 in protein-coding genes as assessed by electrophoresis; refs. 41 and 85), which reflects genetic divergence between their parental species. To us, cumulative heterozygote superiority (overdominance) contributed predominantly by a limited subset of particular protein-coding genes, such as several that are involved in central energy-metabolizing processes (cf. refs. 86–89 and refs. therein), seems an attractive and in principle testable hypothesis to explain heterosis in these frog hybrids; whether developmental disturbances caused by the combination of two disparate heterospecific genomes contribute to the accelerated growth rates observed remains to be tested.
Our results contrast distinctly with those on hybridogenetically reproducing fish hybrids of the genus Poeciliopsis. Adults of laboratory-generated hybrid hemiclones had similar broad thermal tolerances as hybrids from natural lineages, but young F1 hybrids were less tolerant than young wild hybrids and young of either parental species (13). In a test of the spontaneous heterosis hypothesis, a series of laboratory-generated hybrid hemiclones had, on average, lower survival and higher incidences of birth defects than either of the Mendelian parental species or than two hybrid hemiclones from natural lineages, although a subset of the newly generated hemiclones exhibited values within the range of Mendelian ancestors and of natural hybrid lineages (23). Among other possibilities, the contrasting results on the Poeciliopsis and Rana systems may reflect different genetic bases to hemiclonal reproduction. The differences may or may not be related to differences in the difficulty of establishing hemiclonal lineages through primary hybridizations, which apparently is far greater in Poeciliopsis than in Rana (11, 28, 76, 90).
Absence of evidence for spontaneous heterosis in the well studied Poeciliopsis hemiclonal system demonstrates that our findings do not apply globally to all hybrid clonals; but our results clearly show that heterosis stemming from hybridity must be included among the potentially important components of the observed ecological success of clonally reproducing vertebrates. Heterosis and interclonal selection are not mutually exclusive explanators of the success of hybrid clonals. We suggest that they have acted together to shape the superior performance observed in natural R. esculenta lineages: spontaneous heterosis may lead to a series of F1 hemiclones that on average perform better than either parental species in some fitness-related traits, and interclonal selection acting on the ridibunda genome will subsequently eliminate hemiclones with inferior performance from this set. Interclonal selection will also promote broadly adapted hemiclones with low fitness variance over time (General-Purpose Genotype model) or maximize ecological differences among surviving hemiclones and thus reduce competition (Frozen Niche Variation model). In this frog system, we have found support for the latter but not for the former model (40); the two are not, however, mutually exclusive (22, 40, 77). To test whether heterosis and interclonal selection coexplain fitness of R. esculenta lineages requires comparison among individual F1 hemiclones; such comparison is feasible by crossing individual F1s with the host species R. lessonae, which will lead to multiple copies of single ridibunda-genome haplotypes. The intermediate performance of R. esculenta tadpoles from natural lineages between the opposite extremes observed in F1 offspring for time to and size at metamorphosis suggests, however, that our water frog data already provide evidence for both heterosis and interclonal selection. Moreover, our results also point to the importance of uncoupling the effects of clonality and of hybridity when using hybrid clonals as models to test ideas about the evolutionary significance of maintaining sexuality.
Acknowledgments
We thank Leszek Berger (Polish Academy of Sciences) for providing frogs from Poland and Thomas Uzzell (Academy of Natural Sciences of Philadelphia) and Robert C. Vrijenhoek (Rutgers University) for constructive comments on the manuscript. This research was supported by Swiss National Fund Grants 70PK-029652 (R.D.S.), 70PP-031851 (H.H.), and 31–37579.93 (H.H., R.D.S., G.D.G.).
References
- 1.White M J D. Modes of Speciation. San Francisco: Freeman; 1978. [Google Scholar]
- 2.Bell G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality. Berkeley, CA: Univ. California Press; 1982. [Google Scholar]
- 3.Vrijenhoek R C, Dawley R M, Cole C J, Bogart J P. In: Evolution and Ecology of Unisexual Vertebrates. Dawley R M, Bogart J P, editors. Albany, NY: NY State Mus. Bull. 466; 1989. pp. 19–23. [Google Scholar]
- 4.Dawley R M, Bogart J P. Evolution and Ecology of Unisexual Vertebrates. Albany, NY: NY State Mus. Bull. 466; 1989. [Google Scholar]
- 5.Lerner I M. Genetic Homeostasis. London: Oliver and Boyd; 1954. [Google Scholar]
- 6.Wright S. Evolution and the Genetics of Populations. Vol. 3. Chicago: Univ. Chicago Press; 1977. [Google Scholar]
- 7.Mitton J B. Genetica. 1993;89:47–65. [Google Scholar]
- 8.Falconer D S, Mackay T F C. Introduction to Quantitative Genetics. 4th Ed. New York: Longman; 1996. [Google Scholar]
- 9.Hubbs C L. Syst Zool. 1955;4:1–20. [Google Scholar]
- 10.Schultz R J. Am Nat. 1969;103:605–619. [Google Scholar]
- 11.Schultz R J. In: Evolutionary Biology. Hecht M K, Steere W C, Wallace B, editors. New York: Plenum; 1977. pp. 277–331. [Google Scholar]
- 12.Cole C J. Science. 1978;201:1153–1155. doi: 10.1126/science.201.4361.1154. [DOI] [PubMed] [Google Scholar]
- 13.Bulger A J, Schultz R J. Evolution. 1979;33:848–859. doi: 10.1111/j.1558-5646.1979.tb04739.x. [DOI] [PubMed] [Google Scholar]
- 14.Darevsky I S, Kupriyanova L A, Uzzell T. In: Biology of the Reptilia. Gans C, Billett F, editors. Vol. 15. New York: John; 1985. pp. 411–526. [Google Scholar]
- 15.Baker H G. In: The Genetics of Colonizing Species. Baker H G, Stebbins G L, editors. New York: Academic; 1965. pp. 147–172. [Google Scholar]
- 16.Maslin T P. Syst Zool. 1968;17:219–231. [Google Scholar]
- 17.Parker E D, Selander R K, Hudson R O, Lester L J. Evolution. 1977;31:836–842. doi: 10.1111/j.1558-5646.1977.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 18.Lynch M. Q Rev Biol. 1984;59:257–290. [Google Scholar]
- 19.Vrijenhoek R C. Am Zool. 1979;19:787–797. [Google Scholar]
- 20.Vrijenhoek R C. In: Population Biology and Evolution. Wöhrmann K, Loeschcke V, editors. Heidelberg: Springer; 1984. pp. 217–231. [Google Scholar]
- 21.Wetherington J D, Schenck R A, Vrijenhoek R C. In: Ecology and Evolution of Livebearing Fishes (Poeciliidae) Meffe G K, Snelson F F, editors. Englewood Cliffs, New Jersey: Prentice–Hall; 1989. pp. 259–275. [Google Scholar]
- 22.Vrijenhoek R C. BioScience. 1998;48:617–628. [Google Scholar]
- 23.Wetherington J D, Kotora K E, Vrijenhoek R C. Evolution. 1987;41:721–731. doi: 10.1111/j.1558-5646.1987.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 24.Vrijenhoek R C. Annu Rev Ecol Syst. 1994;25:71–96. [Google Scholar]
- 25.Graf J-D, Polls Pelaz M. In: Evolution and Ecology of Unisexual Vertebrates. Dawley R M, Bogart J P, editors. Albany, NY: NY State Mus. Bull. 466; 1989. pp. 289–302. [Google Scholar]
- 26.Tunner H G, Heppich-Tunner S. Naturwissenschaften. 1991;78:32–34. [Google Scholar]
- 27.Uzzell T, Berger L. Proc Acad Nat Sci Philadelphia. 1975;127:13–24. [Google Scholar]
- 28.Hotz H, Mancino G, Bucci-Innocenti S, Ragghianti M, Berger L, Uzzell T. J Exp Zool. 1985;236:199–210. [Google Scholar]
- 29.Günther R. Die Wasserfrösche Europas (Anura - Froschlurche). Wittenberg Lutherstadt: Die Neue Brehm-Bücherei; 1990. [Google Scholar]
- 30.Blankenhorn H J, Heusser H, Notter P. Rev Suisse Zool. 1973;80:662–666. [Google Scholar]
- 31.Berger L. Experientia. 1983;39:127–130. [Google Scholar]
- 32.Berger L. Zool Pol. 1990;35:5–32. [Google Scholar]
- 33.Semlitsch R D, Reyer H-U. Evolution. 1992;46:665–676. doi: 10.1111/j.1558-5646.1992.tb02074.x. [DOI] [PubMed] [Google Scholar]
- 34.Semlitsch R D. Evolution. 1993;47:1805–1818. doi: 10.1111/j.1558-5646.1993.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 35.Berger L, Berger W A. Amphibia-Reptilia. 1992;13:135–146. [Google Scholar]
- 36.Rist L, Semlitsch R D, Hotz H, Reyer H-U. Funct Ecol. 1997;11:735–742. [Google Scholar]
- 37.Tunner H G, Nopp H. Naturwissenschaften. 1979;66:268–269. doi: 10.1007/BF00571614. [DOI] [PubMed] [Google Scholar]
- 38.Fioramonti E, Semlitsch R D, Reyer H-U, Fent K. Environ Toxicol Chem. 1997;16:1940–1947. [Google Scholar]
- 39.Semlitsch R D, Schmiedehausen S, Hotz H, Beerli P. Evol Ecol. 1996;10:531–543. [Google Scholar]
- 40.Semlitsch R D, Hotz H, Guex G-D. Evolution. 1997;51:1249–1261. doi: 10.1111/j.1558-5646.1997.tb03972.x. [DOI] [PubMed] [Google Scholar]
- 41.Hotz H. Ph.D. Dissertation. Universität Zürich; 1983. [Google Scholar]
- 42.Günther R, Uzzell T, Berger L. Mitt Zool Mus Berlin. 1979;55:35–57. [Google Scholar]
- 43.Günther R. Biol Zentralbl. 1977;96:457–466. [Google Scholar]
- 44.Blankenhorn H J. In: The Reproductive Biology of Amphibians. Taylor D H, Guttman S I, editors. New York: Plenum; 1977. pp. 389–410. [Google Scholar]
- 45.Abt G, Reyer H-U. Behav Ecol Sociobiol. 1993;32:221–228. [Google Scholar]
- 46.Tunner H G. Z Zool Syst Evol Forsch. 1974;12:309–314. [Google Scholar]
- 47.Berger L, Uzzell T, Hotz H. Proc Acad Nat Sci Philadelphia. 1988;140:220–239. [Google Scholar]
- 48.Hotz H, Beerli P, Spolsky C. Mol Biol Evol. 1992;9:610–620. doi: 10.1093/oxfordjournals.molbev.a040744. [DOI] [PubMed] [Google Scholar]
- 49.Semlitsch R D. Evolution. 1993;47:510–519. doi: 10.1111/j.1558-5646.1993.tb02110.x. [DOI] [PubMed] [Google Scholar]
- 50.Berger L, Rybacki M, Hotz H. Amphibia-Reptilia. 1994;15:408–413. [Google Scholar]
- 51.Gosner K L. Herpetologica. 1960;16:183–190. [Google Scholar]
- 52.Alford R A, Harris R N. Am Natural. 1988;131:91–106. [Google Scholar]
- 53.Travis J. Growth. 1980;44:167–181. [Google Scholar]
- 54.Wilbur H M, Collins J P. Science. 1973;182:1305–1314. doi: 10.1126/science.182.4119.1305. [DOI] [PubMed] [Google Scholar]
- 55.Smith D C. Ecology. 1983;64:501–510. [Google Scholar]
- 56.Newman R A. Evolution. 1988;42:763–773. doi: 10.1111/j.1558-5646.1988.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 57.Newman R A. Evolution. 1988;42:774–783. doi: 10.1111/j.1558-5646.1988.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 58.Pough F H, Kamel S. Oecologia. 1984;65:138–144. doi: 10.1007/BF00384476. [DOI] [PubMed] [Google Scholar]
- 59.Goater C P, Semlitsch R D, Bernasconi M V. Oikos. 1993;66:129–136. [Google Scholar]
- 60.Berven K A, Gill D E. Am Zool. 1983;23:85–97. [Google Scholar]
- 61.Smith D C. Ecology. 1987;68:344–350. [Google Scholar]
- 62.Semlitsch R D, Scott D E, Pechmann J H K. Ecology. 1988;69:184–192. [Google Scholar]
- 63.Berven K A. Ecology. 1990;71:1599–1608. [Google Scholar]
- 64.Travis J, Emerson S B, Blouin M. Evolution. 1987;41:145–156. doi: 10.1111/j.1558-5646.1987.tb05777.x. [DOI] [PubMed] [Google Scholar]
- 65.SAS Institute. SAS/STAT User’s Guide, Release 6.03. Cary, NC: SAS Inst.; 1988. [Google Scholar]
- 66.Snedecor G W, Cochran W G. Statistical Methods. 7th Ed. Ames, Iowa: Iowa State Univ. Press; 1980. [Google Scholar]
- 67.Sokal R R, Rohlf F J. Biometry: The Principles and Practice of Statistics in Biological Research. 3rd Ed. New York: Freeman; 1995. [Google Scholar]
- 68.Berger L. Zool Pol. 1971;21:345–393. [Google Scholar]
- 69.Heusser H, Blankenhorn H J. Rev Suisse Zool. 1973;80:543–569. [Google Scholar]
- 70.Berger L, Uzzell T. Zool Pol. 1977;26:291–317. [Google Scholar]
- 71.Berger L. Acta Zool Cracov. 1968;13:301–324. [Google Scholar]
- 72.Semlitsch R D. Oikos. 1993;67:40–46. [Google Scholar]
- 73.Berger L, Uzzell T. Folia Biol (Kraków) 1980;28:3–25. [PubMed] [Google Scholar]
- 74.Dobzhansky T. Genetics and the Origin of Species. New York: Columbia Univ. Press; 1951. [Google Scholar]
- 75.Bucci S, Ragghianti M, Mancino G, Berger L, Hotz H, Uzzell T. J Exp Zool. 1990;255:37–56. doi: 10.1002/jez.1402550107. [DOI] [PubMed] [Google Scholar]
- 76.Guerrini F, Bucci S, Ragghianti M, Mancino G, Hotz H, Uzzell T, Berger L. J Exp Zool. 1997;279:163–176. doi: 10.1002/(sici)1097-010x(19971001)279:2<163::aid-jez7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 77.Vrijenhoek R C, Pfeiler E. Evolution. 1997;51:1593–1600. doi: 10.1111/j.1558-5646.1997.tb01482.x. [DOI] [PubMed] [Google Scholar]
- 78.Muller H J. Mutat Res. 1964;1:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- 79.Nei M. Am Natural. 1970;104:311–322. [Google Scholar]
- 80.Felsenstein J. Genetics. 1974;78:737–756. doi: 10.1093/genetics/78.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lynch M, Bürger R, Butcher R, Gabriel W. J Hered. 1993;84:339–344. doi: 10.1093/oxfordjournals.jhered.a111354. [DOI] [PubMed] [Google Scholar]
- 82.Travis J. Evolution. 1983;37:496–512. doi: 10.1111/j.1558-5646.1983.tb05566.x. [DOI] [PubMed] [Google Scholar]
- 83.Newman R A. Evolution. 1994;48:1773–1785. doi: 10.1111/j.1558-5646.1994.tb02213.x. [DOI] [PubMed] [Google Scholar]
- 84.Travis J. Ecology. 1984;65:1155–1160. [Google Scholar]
- 85.Hotz H, Uzzell T, Berger L. In: II International Symposium on Ecology and Genetics of European Water Frogs, 18–25 September 1994, Wroclaw, Poland. Ogielska M, editor. Wroclaw: Zool. Pol.39; 1994. pp. 243–266. [Google Scholar]
- 86.Mitton J B. In: The Natural History of Inbreeding and Outbreeding. Thornhill N W, editor. Chicago: Univ. Chicago Press; 1993. pp. 17–41. [Google Scholar]
- 87.Powers D A, Smith M, Gonzales-Villasenor I, DiMichele L, Crawford D, Bernardi G, Lauerman T. In: Oxford Surveys in Evolutionary Biology 9. Futuyma D, Antonovics J, editors. New York: Oxford Univ. Press; 1993. pp. 43–107. [Google Scholar]
- 88.Watt W B. Genetics. 1994;136:11–16. doi: 10.1093/genetics/136.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zouros E, Pogson G H. In: Genetics and Evolution of Aquatic Organisms. Beaumont A, editor. London: Chapman & Hall; 1994. pp. 135–146. [Google Scholar]
- 90.Vrijenhoek R C. In: Evolution and Ecology of Unisexual Vertebrates. Dawley R M, Bogart J P, editors. Albany, NY: NY State Mus. Bull. 466; 1989. pp. 24–31. [Google Scholar]