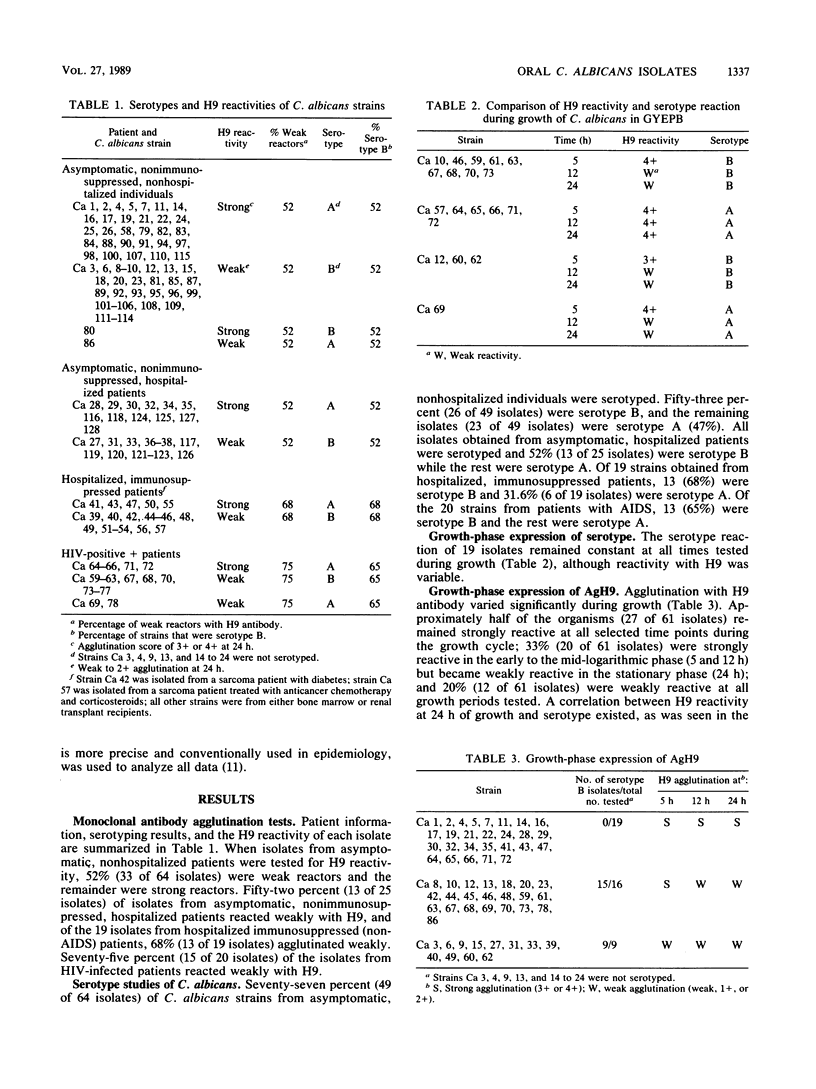
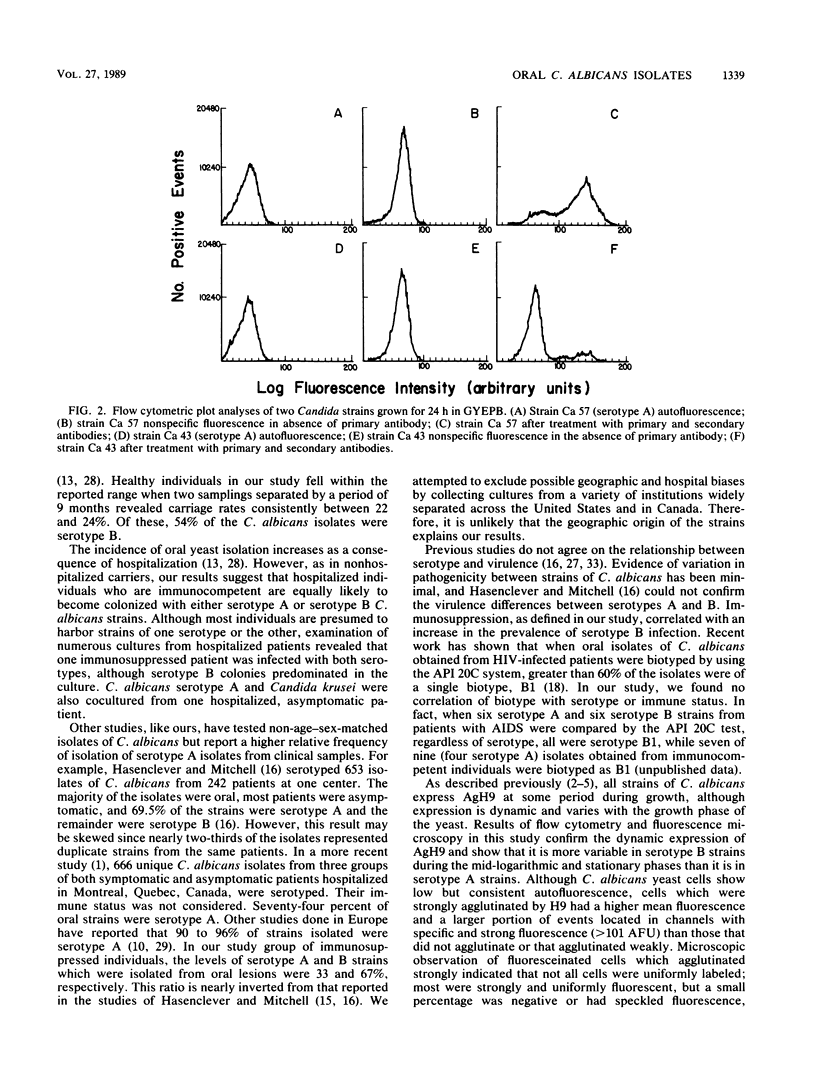
Abstract
A total of 128 human oral isolates of Candida albicans were collected from asymptomatic healthy carriers (64 isolates); asymptomatic, nonimmunosuppressed, hospitalized patients (25 isolates); immunosuppressed transplant patients (19 isolates); and human immunodeficiency virus-infected patients with symptoms of acquired immunodeficiency syndrome and oral candidiasis (20 isolates). Isolates were serotyped as A or B and tested for reactivity with an agglutinating immunoglobulin M monoclonal antibody (H9). Immunocompetent individuals colonized by oral C. albicans were almost equally likely to carry serotype A as serotype B cells, while immunocompromised individuals were at least twice as likely to be infected by serotype B than serotype A strains. The reactivity of isolates with H9 antibody followed a similar but more distinctive pattern. Approximately half of the strains from immunocompetent individuals reacted strongly with H9, and the remainder reacted weakly. However, up to 75% of the isolates from immunocompromised patients reacted weakly with H9, while the remainder reacted strongly. A correlation between H9 reactivity and the serotypes of these isolates existed (P = 0.16). The correlation between H9 reactivity and immune status was even stronger (P = 0.025). The monoclonal antibody activities described above were determined by agglutination tests during defined phases of C. albicans growth. Expression of antigen at various times during growth of several isolates was confirmed at the cellular level by analysis using fluorescence-activated cell sorting. Despite the correlation between serotype A and H9 reactivity, H9 antigen was not identical to the serotype A antigen because four serotype A strains reacted only weakly with H9 antibody, and one strain reacted strongly with H9 but was serotype B. These data indicate that oral strains of C. albicans from immunocompetent individuals differ as a group from C. albicans isolated from those who are immunosuppressed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auger P., Dumas C., Joly J. A study of 666 strains of Candida albicans: correlation between serotype and susceptibility to 5-fluorocytosine. J Infect Dis. 1979 May;139(5):590–594. doi: 10.1093/infdis/139.5.590. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Cell surface and intracellular expression of two Candida albicans antigens during in vitro and in vivo growth. Microb Pathog. 1987 Apr;2(4):249–257. doi: 10.1016/0882-4010(87)90123-9. [DOI] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Ultrastructural and biochemical studies of two dynamically expressed cell surface determinants on Candida albicans. Infect Immun. 1986 Jan;51(1):327–336. doi: 10.1128/iai.51.1.327-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of a cell surface determinant on Candida albicans as evidenced by an agglutinating monoclonal antibody. Infect Immun. 1984 Mar;43(3):966–972. doi: 10.1128/iai.43.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawner D. L., Cutler J. E. Variability in expression of cell surface antigens of Candida albicans during morphogenesis. Infect Immun. 1986 Jan;51(1):337–343. doi: 10.1128/iai.51.1.337-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Y., Webster J. H. Oral monilia study on patients with head and neck cancer during radiotherapy. Cancer. 1974 Aug;34(2):246–249. doi: 10.1002/1097-0142(197408)34:2<246::aid-cncr2820340203>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Critchley I. A., Douglas L. J. Isolation and partial characterization of an adhesin from Candida albicans. J Gen Microbiol. 1987 Mar;133(3):629–636. doi: 10.1099/00221287-133-3-629. [DOI] [PubMed] [Google Scholar]
- DeGregorio M. W., Lee W. M., Linker C. A., Jacobs R. A., Ries C. A. Fungal infections in patients with acute leukemia. Am J Med. 1982 Oct;73(4):543–548. doi: 10.1016/0002-9343(82)90334-5. [DOI] [PubMed] [Google Scholar]
- Drouhet E., Mercier-Soucy L., Montplaisir S. Sensibilité et résistance des levures pathogènes aux 5-fluoropyrimidines. I.--Relation entre les phénotypes de résistance a la 5-fluorocytosine, le sérotype de Candida albicans et l'écologie de différentes espèces de Candida d'origine humaine. Ann Microbiol (Paris) 1975 Jul-Aug;126B(1):25–39. [PubMed] [Google Scholar]
- Folb P. I., Trounce J. R. Immunological aspects of candida infection complicating steroid and immunosuppressive drug therapy. Lancet. 1970 Nov 28;2(7683):1112–1114. doi: 10.1016/s0140-6736(70)92299-3. [DOI] [PubMed] [Google Scholar]
- Fung J. C., Donta S. T., Tilton R. C. Candida detection system (CAND-TEC) to differentiate between Candida albicans colonization and disease. J Clin Microbiol. 1986 Oct;24(4):542–547. doi: 10.1128/jcm.24.4.542-547.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUHN J. G., SANSON J. Mycotic infections in leukemic patients at autopsy. Cancer. 1963 Jan;16:61–73. doi: 10.1002/1097-0142(196301)16:1<61::aid-cncr2820160109>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Antigenic studies of Candida. I. Observation of two antigenic groups in Candida albicans. J Bacteriol. 1961 Oct;82:570–573. doi: 10.1128/jb.82.4.570-573.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Antigenic studies of Candida. III. Comparative pathogenicity of Candida albicans group A, group B, and Candida stellatoidea. J Bacteriol. 1961 Oct;82:578–581. doi: 10.1128/jb.82.4.578-581.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight L., Fletcher J. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteroids, and diabetes mellitus. J Infect Dis. 1971 Apr;123(4):371–377. doi: 10.1093/infdis/123.4.371. [DOI] [PubMed] [Google Scholar]
- Korting H. C., Ollert M., Georgii A., Fröschl M. In vitro susceptibilities and biotypes of Candida albicans isolates from the oral cavities of patients infected with human immunodeficiency virus. J Clin Microbiol. 1988 Dec;26(12):2626–2631. doi: 10.1128/jcm.26.12.2626-2631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Lehrer N., Segal E., Barr-Nea L. In vitro and in vivo adherence of Candida albicans to mucosal surfaces. Ann Microbiol (Paris) 1983 Sep-Oct;134B(2):293–306. doi: 10.1016/s0769-2609(83)80042-8. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte candidacidal activity and resistance to systemic candidiasis in patients with cancer. Cancer. 1971 May;27(5):1211–1217. doi: 10.1002/1097-0142(197105)27:5<1211::aid-cncr2820270528>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I. Functional aspects of a second mechanism of candidacidal activity by human neutrophils. J Clin Invest. 1972 Oct;51(10):2566–2572. doi: 10.1172/JCI107073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOURAD S., FRIEDMAN L. Pathogenicity of Candida. J Bacteriol. 1961 Apr;81:550–556. doi: 10.1128/jb.81.4.550-556.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur H., Michelis M. A., Wormser G. P., Lewin S., Gold J., Tapper M. L., Giron J., Lerner C. W., Armstrong D., Setia U. Opportunistic infection in previously healthy women. Initial manifestations of a community-acquired cellular immunodeficiency. Ann Intern Med. 1982 Oct;97(4):533–539. doi: 10.7326/0003-4819-97-4-533. [DOI] [PubMed] [Google Scholar]
- Matthews R., Burnie J., Smith D., Clark I., Midgley J., Conolly M., Gazzard B. Candida and AIDS: evidence for protective antibody. Lancet. 1988 Jul 30;2(8605):263–266. doi: 10.1016/s0140-6736(88)92547-0. [DOI] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition, adherence, and virulence of Candida albicans. Infect Immun. 1984 Jul;45(1):6–12. doi: 10.1128/iai.45.1.6-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A. J. The acquired immune deficiency syndrome. Clin Exp Immunol. 1984 Apr;56(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Reiss E., de Repentigny L., Kuykendall R. J., Carter A. W., Galindo R., Auger P., Bragg S. L., Kaufman L. Monoclonal antibodies against Candida tropicalis mannan: antigen detection by enzyme immunoassay and immunofluorescence. J Clin Microbiol. 1986 Nov;24(5):796–802. doi: 10.1128/jcm.24.5.796-802.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDULA J., KOCKOVA KRATOCHVILOVA A., ZAMECNIKOVA A. GENUS CANDIDA BERKHOUT. II. PATHOGENICITY OF THE SPECIES CANDIDA ALBICANS (ROBIN) BERKHOUT. Folia Microbiol (Praha) 1963 Sep;41:313–317. doi: 10.1007/BF02868777. [DOI] [PubMed] [Google Scholar]
- Silverman S., Jr, Luangjarmekorn L., Greenspan D. Occurrence of oral Candida in irradiated head and neck cancer patients. J Oral Med. 1984 Oct-Dec;39(4):194–196. [PubMed] [Google Scholar]
- Silverman S., Jr, Migliorati C. A., Lozada-Nur F., Greenspan D., Conant M. A. Oral findings in people with or at high risk for AIDS: a study of 375 homosexual males. J Am Dent Assoc. 1986 Feb;112(2):187–192. doi: 10.14219/jada.archive.1986.0321. [DOI] [PubMed] [Google Scholar]
- Small C. B., Klein R. S., Friedland G. H., Moll B., Emeson E. E., Spigland I. Community-acquired opportunistic infections and defective cellular immunity in heterosexual drug abusers and homosexual men. Am J Med. 1983 Mar;74(3):433–441. doi: 10.1016/0002-9343(83)90970-1. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Langtimm C. J., McDowell J., Hicks J., Galask R. High-frequency switching in Candida strains isolated from vaginitis patients. J Clin Microbiol. 1987 Sep;25(9):1611–1622. doi: 10.1128/jcm.25.9.1611-1622.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Poulain D., Vernes A. Cytochemical and ultrastructural studies of Candida albicans. III. Evidence for modifications of the cell wall coat during adherence to human buccal epithelial cells. Arch Microbiol. 1984 Oct;139(2-3):221–224. doi: 10.1007/BF00402004. [DOI] [PubMed] [Google Scholar]
- Yap B. S., Bodey G. P. Oropharyngeal candidiasis treated with a troche form of clotrimazole. Arch Intern Med. 1979 Jun;139(6):656–657. [PubMed] [Google Scholar]



