Abstract
Background
Periods of ischemia-reperfusion (I/R) during cardiac surgery are associated with transient left ventricular (LV) dysfunction and an inflammatory response. The goal of this study was to examine potential dose-dependent effects of aprotinin (APRO) on LV contractility and cytokine release in the setting of I/R.
Methods
An index of LV contractility, LV maximal elastance (Emax), was measured at baseline, 30 min of ischemia, and 60 min of reperfusion by microtransducer volumetry. Mice were randomized as follows: a) APRO 20,000 kallikrein-inhibiting units (KIU)/kg (n=11); b) APRO 4×104 KIU/kg (n=10); c) APRO 8 ×104 KIU/kg (n=10) and d) Vehicle (saline; n=10). APRO doses were calculated to reflect half, full, and twice the clinical Hammersmith dosing schedule. Following I/R, plasma was collected for cytokine measurements.
Results
Following I/R, Emax decreased from the baseline value by over 40% in the Vehicle group as well as in the APRO 4 ×104 KIU/kg and APRO 8 ×104 KIU/kg groups (p<0.05). However, Emax returned to near baseline values in the APRO 2 ×104 KIU/kg group. TNF increased ten-fold following I/R, but it was reduced with higher APRO doses.
Conclusions
This study demonstrated that a low dose of APRO provided protective effects on LV contractility, whereas higher doses suppressed TNF release. These unique findings suggest that there are distinct and independent mechanisms of action of APRO in the context of I/R.
Keywords: aprotinin, ischemia-reperfusion, LV systolic elastance, cytokines, contractility
Introduction
Cardiac surgery with cardiopulmonary bypass, often attendant with periods of myocardial ischemia-reperfusion (I/R), can induce a coagulopathy, a systemic and local inflammatory response, and transient myocardial dysfunction. The serine protease inhibitor aprotinin (APRO) was used in this clinical context to minimize blood loss. (1,2) While APRO was considered a mainstay for improving hemostasis in the context of I/R and cardiac surgery, recent retrospective analysis has identified significant concerns regarding APRO. (3) Moreover, a prospective study utilizing the clinical full Hammersmith protocol was terminated prematurely. (4) Thus, while APRO favorably affects hemostasis, which is an important determinant of morbidity and mortality in I/R and cardiac surgery, APRO likely influences other biological processes which impart deleterious effects. Studies have shown a strong association between the use of APRO and the reduction of various inflammatory markers, particularly tumor necrosis factor-α (TNF), and interleukin-6 (IL-6). (5,6) Accordingly, the overall goal of the present study was to examine the potential relationship between LV contractility and cytokine release with varying doses of APRO in the context of I/R. It has been shown that TNF levels are elevated in the setting of myocardial I/R, leading to a reduction of LV function through several pathways. (7,8) Interleukin-10 (IL-10) was demonstrated to have anti-inflammatory effects which can induce TNF. (9) Some studies have shown that cytokine release can be attenuated by APRO in the setting of myocardial I/R. (10,11) However, little is known about the overall mechanisms by which APRO attenuates the response to I/R, whether these effects of APRO are dose-dependent, and whether they lead to a preservation LV function after I/R. Accordingly, this study examined whether and to what degree APRO imparts a dose dependent effect on LV contractility and on the release of specific cytokines (TNF, IL-6, IL-10).
Methods
Experimental Design
Instrumentation and Animal Model
Adult mice (FVB strain, 10–16 wk, 24–30 gm) were anesthetized, intubated with a twenty gauge Jelco needle (Medex Medical Ltd., Rossendale, UK), and maintained under isoflurane anesthesia (2%) using a MiniVent Type 845 ventilator (Hugo Sachs Elektronik) with tidal volumes of 250µL, at a rate of 250 strokes/min, and a FiO2 of 27%. Temperature was monitored via a rectal probe during the length of the procedure, and maintained by a feedback loop to a heating pad within the operating table, as well as a heating lamp. It was previously determined by conducting baseline studies that the ventilator settings gave a pH of 7.35 ±0.01, pCO2 of 29 ±2, pO2 of 453 ±34, and an O2 saturation of 100%. The right carotid was exposed and a pre-calibrated 4-electrode-pressure sensor catheter (1.4 F, SPR-839, Millar Instruments, Houston, TX) was placed in the left ventricle (LV) with pressure tracing confirmation.
A left thoracotomy was then performed, the posterolateral aspect of the LV free wall visualized, and a purse-string placed around the left anterior descending artery just distal to the bifurcation of the left main coronary artery using 6.0 Prolene and an atraumatic needle (Ethicon, Somerville, NJ). The suture was exteriorized and the wound was closed in layers. The ligature was tightened to induce ischemia (30 minutes) and then released for reperfusion (60 minutes). In a preliminary set of studies (n=6), fluorescent microspheres (F-8838, Molecular Probes, 15µm diameter, 7.5μ104) of different emission spectra were injected at baseline, at 30 minutes of ischemia, and at 60 minutes of reperfusion by LV injection methods described previously. (12) LV regional myocardial blood flow fell to approximately 50% of baseline values with peak ischemia and returned to within baseline values with reperfusion. Thus, this murine model provided a transient period of low myocardial blood flow followed by a restoration of blood flow, and therefore allowed for the study of LV function in the context of ischemia-reperfusion (I/R). Furthermore, it was documented using this coronary occlusion model that the area at risk for the LV was 50%. At the end of the reperfusion period, the pressure sensor catheter was removed, the mouse was injected with 0.2mL of heparin, euthanized, and blood was collected from the right carotid artery for cytokine and aprotinin (APRO) analysis. Total procedure time was 120 minutes. The mice received a one time intraperitoneal normal saline bolus of 0.5mL. All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC, 1996). This protocol was reviewed and approved by the MUSC Institutional Animal Care & Use Committee (AR# 2345).
Aprotinin Protocol
APRO doses of 2mL/kg, 4mL/kg, and 8mL/kg were modeled to achieve plasma concentrations corresponding to half-Hammersmith, Hammersmith, and double Hammersmith doses, respectively, while using the standard concentration aprotinin of 10,000 Kallikrein Inhibiting Units (KIU)/mL. (13,14) Royston et al reported a model in which an APRO 4mL/kg intravenous bolus reached plasma concentrations (>250 KIU/mL) comparable to the Hammersmith, or high-dose, protocol often used clinically. (15) Our approach was to utilize this clinical dosing regimen using the weight-based initial bolus. Furthermore, this is similar to weight-based dosing regimens used in previous animal models.(16,17) For clarity and uniformity, APRO doses are reported as 2×104 KIU/kg for the half-Hammersmith, 4×104 KIU/kg for the Hammersmith, and 8×104 KIU/kg for the double-Hammersmith doses.
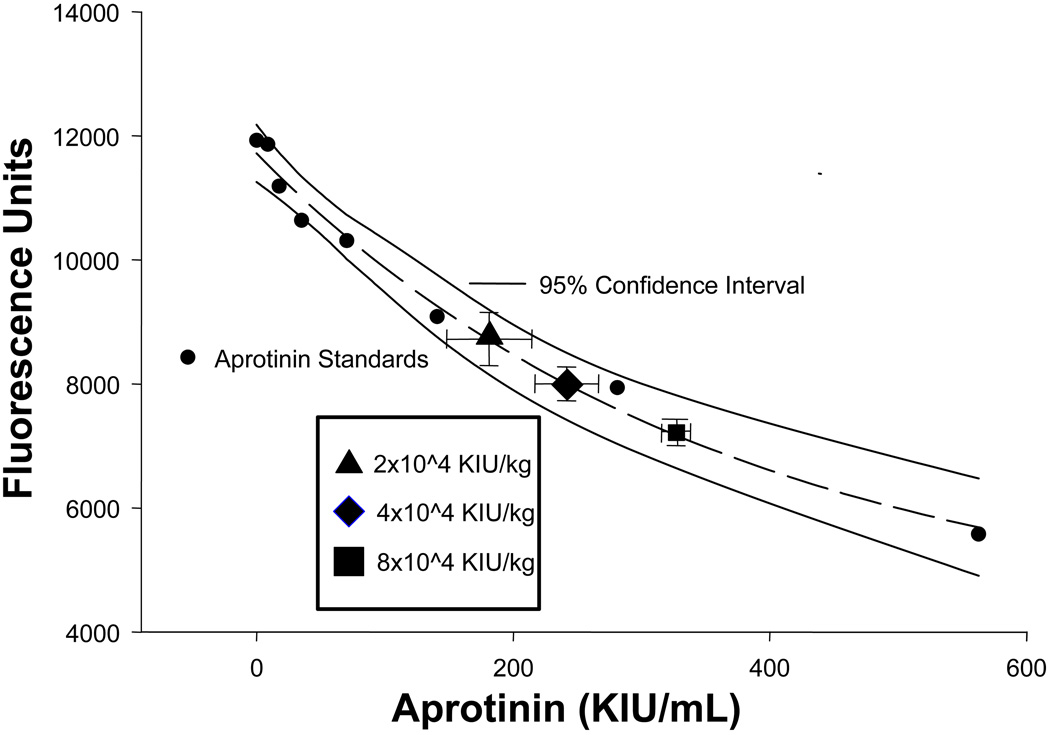
To measure relative plasma levels of APRO, a fluorogenic substrate cleaved by the serine protease plasmin was utilized in an ex-vivo assay system. Specifically, the peptide sequence D-ala-leu-lys-7-amido-4-methylcomarin (Sigma, A8171) at a fixed concentration of 10 nM, was mixed in a reaction buffer containing a 1:33 dilution of normal mouse plasma and incubated at 37degC for 15 min in the presence and absence of 7 µg/mL of plasmin (Sigma, P1876, 3 U/mg). The fluorescence of this reaction was detected in continuous fashion (Fluostar Galaxy, BMG Labtech, NC) at an excitation/emission wavelength of 365/440 nm. The plasmin substrate, plasmin concentrations, and the incubation conditions were determined from preliminary dilution studies in order to yield peak performance as defined as that which yielded a consistent and stable fluorescence signal. This reaction solution was then incubated in the presence and absence of increasing concentrations of APRO (range 0–560 KIU/mL) in order to generate a standardized enzyme activity-inhibition curve. The standard APRO inhibition curve, which was generated in triplicate, along with the 95% confidence interval for this standard curve is shown in Figure 1. This inhibition curve demonstrated the classical exponential decay, and was subjected to regression analysis yielding a significant relationship between the reduction in fluorescence to APRO concentrations (r=0.99, p<0.001). The intra-assay coefficient of variation was 5% and an inter-assay coefficient was 9%. This APRO enzyme inhibition assay was utilized to extrapolate relative APRO plasma concentrations. Specifically, at the completion of the studies described in the subsequent paragraphs, plasma was prepared and incubated in the substrate/plasmin substrate, the relative fluorescence obtained, input into the standardized APRO inhibition curve, and an APRO concentration computed.
Figure 1.
Relative plasma concentrations of aprotinin (APRO). An excellent regression fit was obtained as shown by the dashed lines, and the 95% confidence interval for the APRO assay is shown by the solid lines. The relative fluorescence and computed plasma APRO concentrations for the Hammersmith doses have been superimposed on the standard curve (error bars are the standard deviation from the estimate) and absolute values are reported in the Results. The 2×104 KIU/kg, 4×104 KIU/kg, and 8×104 KIU/kg dose nomenclature correlates to the half-Hammersmith, full-Hammersmith, and double-Hammersmith protocols presented in the Methods. Please see text for details.
(y = 4071+7643e−0.0028x; r2 = 0.98, p<0.0001)
Fifty mice were randomized to one of four treatment groups: Vehicle (0.9% saline), APRO 2×104 KIU/kg, APRO 4×104 KIU/kg, or APRO 8×104 KIU/kg. Randomization was accomplished by drawing group assignments from an envelope. After baseline hemodynamic measurements were taken, the assigned group dose of saline or aprotinin was given by intra-peritoneal injection using an equivalent final volume (0.5 mL). Ischemia was initiated 5 to 10 minutes after the injection.
LV Contractility Measurements
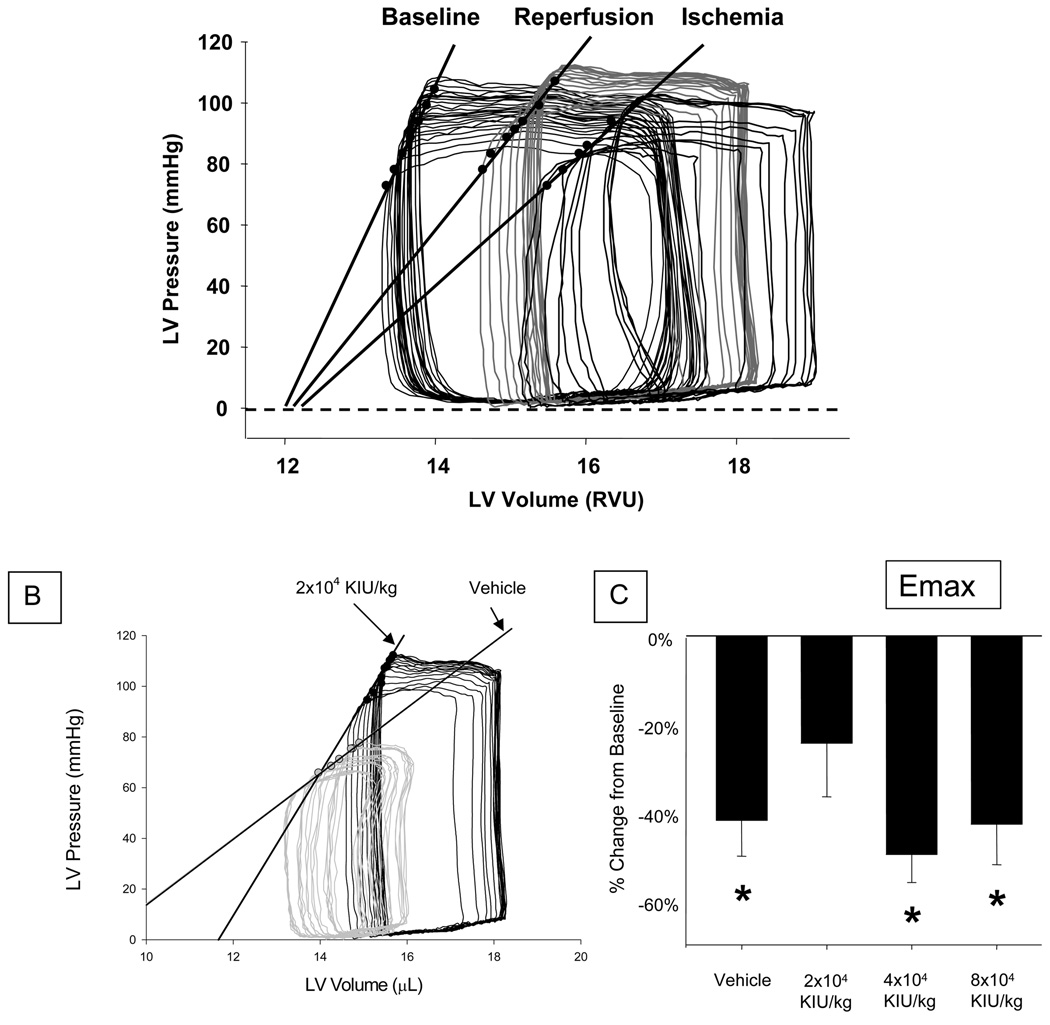
Following stabilization from the instrumentation, LV pressures (peak, diastolic) were measured along with relative volume units which were converted to true volumes using a calibration curve. For this purpose, the pressure sensor catheter was interfaced to a pressure-conductance unit (ARIA, MPCU-200, Millar) in which electrical excitation was performed under digital control (DAQ, PV Analysis Software, Millar). The continuous digital pressure and conductance signals were integrated with an ECG signal (PowerLab, AD instruments, NSW Australia) and displayed in real time using dual-display flat screen monitors (Sony Electronics Inc). This allowed for the optimal placement of the LV catheter with respect to the LV conductance signal. The LV conductance signal was converted to a LV volume using an algorithm described previously. (18) This included correction for parallel conductance and continuous in vitro calibration. Steady-state LV pressures and conductance volumetry were determined by digitizing a minimum of 12 consecutive cardiac cycles. LV peak pressure, end-diastolic pressure, and maximal rate of LV peak pressure (dP/dt) were recorded. The ventilator was then suspended during continuous recording of the LV pressure-conductance volume signal to alter venous return. This maneuver resulted in a family of LV pressure-volume loops in which definable points of the LV end-systolic pressure-volume relationship could be determined. Representative LV pressure-volume loops at baseline, peak ischemia, and reperfusion are shown in Figure 2-a, and demonstrate the linearity of the end-systolic pressure volume relationship (ESPVR). Isochronal points from the recorded LV pressure-volume loops were used to compute Emax, an index of LV contractility defined as maximal LV elastance, and ESPVR. (19,20,21) These indices were calculated for each mouse at each time point from pressure volume loops, adjusted for parallel conductance, generated from the readings obtained. Emax was indexed to LV mass (Emax/mg), which was obtained gravimetrically at autopsy and had a mean value of 81.1 ±9.8 mg.
Figure 2.
(a.) Representative left ventricular (LV) pressure-volume (P-V) loops under baseline conditions, following peak ischemia, and at 60 minutes of reperfusion (90 minutes of I/R). The solid black line represents LV Emax, a marker of LV contractility defined as maximal LV elastance. These families of P-V loops represent a marked decrease in LV contractility (LV Emax) with ischemia, followed by a recovery of contractility toward baseline values with reperfusion. (b.) Representative LV P-V loops following 60 minutes of reperfusion. The gray loops and slope are indicative of the end-systolic pressure volume relationship (ESPVR) in the Vehicle treatment group. The solid black loops and slope represent the improved ESPVR in the APRO 2×104 KIU/kg treatment group as compared to the Vehicle group. Absolute values for ESPVR are shown in Table 1. (c.) A differential effect of varying doses of APRO on myocardial contractility (Emax) and plasma cytokine release was observed with ischemia followed by 60 minutes of reperfusion. In the Vehicle group, LV contractility was decreased by 42% from baseline, and plasma cytokine levels were significantly increased. At a dose of APRO 2×104 KIU/kg, corresponding to the half-Hammersmith or a low-dose APRO regimen, LV contractility returned to within baseline values after 60 minutes of reperfusion.
(Data shown as mean ± SEM; * = p<0.05 vs Referent Control/Baseline; + = p<0.05 vs Vehicle).
Cytokine Measurements
Diluted mouse plasma samples taken after 60 minutes of reperfusion were diluted appropriately to bring the analyte into a measurable range and assayed according to manufacturer's instructions (Cat. # LUM410, LUM406, LUM417, R &D Systems, Minneapolis, MN) for TNF, IL-6, and IL-10. The relative fluorescence detected was compared to a 5 parameter logistic calibration curve generated independently for each analyte tested (Bio-Plex Manager 4.1.1). From the calibration curve, the concentration of each analyte was determined and the plasma concentration was calculated. The sensitivity for each of the measured plasma cytokines was 0.42 pg/mL for TNF, 0.71 pg/mL for IL-6, and 0.59 pg/mL for IL-10.
Control Studies
In order to examine whether and to what degree anesthesia and/or instrumentation affected LV contractility in the absence of I/R, Emax was assessed at baseline and at 90 minutes, which was equivalent to the endpoint of the I/R study. Specifically, saline was administered intraperitoneally in mice (n=9) and Emax was measured. Furthermore, in a second control study, Emax was measured in a similar fashion in mice (n=8) which received intraperitoneal APRO (8×104 KIU/kg) without I/R. In order to further address whether APRO affected plasma cytokine levels, plasma cytokine concentrations were measured at the 90 minute time point in subsets of mice injected with normal saline (n=6), 2×104 KIU/kg of APRO (n=6), 4×104 KIU/kg of APRO 9 (n=6), and 8×104 KIU/kg of APRO (n=5), and were used as referent controls.
Data Analysis
Investigators performing LV function and biochemical analyses were blinded to group assignments. All values were entered in a collective database and coded based on treatment group to maintain blinding. LV contractility, plasma cytokine levels, and the effects of APRO dosing on these parameters were first compared between the groups using analysis of variance (ANOVA). If the ANOVA revealed significant differences, post-hoc mean separation was performed using Tukey-adjusted mean square differences (Module prcomp, STATA Intercooled, v8, College Station, TX). Following this multiway ANOVA approach, data transformation was also performed in which changes with I/R on the indices of LV contractility from baseline were computed and expressed as a percentage. In addition, plasma cytokine levels determined in the reference control mice for each cytokine and percent changes from these reference values were computed following I/R. These transformed computations were then examined using an adjusted t-score (STATA). Results are presented as mean ± standard deviation (SD) unless otherwise indicated. Values of p<0.05 were considered to be statistically significant.
Results
A total of 50 mice were used in the ischemia-reperfusion (I/R) protocol, with 9 mice dying prior to the final set of measurements. These mice died of arrhythmias during reperfusion, and were equally distributed among the 3 treatment groups (Vehicle: n=3, 2×104 Kallikrein Inhibiting Units (KIU)/kg aprotinin (APRO): n=2, 4×104 KIU/kg APRO: n=2, 8×104 KIU/kg APRO: n=2). Thus, the final sample sizes were: Vehicle n=10, 2×104 KIU/kg APRO: n=11, 4×104 KIU/kg APRO: n=10, APRO 8×104 KIU/kg: n=10). Using the APRO plasma assay and standard curve shown in Figure 1, the computed APRO plasma concentrations for the 2×104 KIU/kg APRO group was 180±33, for the 4×104 APRO group APRO levels were significantly higher 242±25 KIU/mL (p<0.05), and increased again in the 8×104 APRO group (315±7 KIU/mL, p<0.05). However, it must be recognized that at high APRO doses, the assay became non-linear, which may have underestimated the higher APRO concentrations.
Steady-State Hemodynamics and LV Contractility
Baseline left ventricular (LV) function and hemodynamic measurements prior to randomization and Vehicle/APRO infusions are summarized in Table 1. Baseline values were identical across groups following randomization. LV function values at peak ischemia, and during reperfusion are shown in Table 1. At 30 minutes of ischemia, LV peak systolic pressure fell from baseline in all groups except in the APRO 2×104 KIU/kg group. At 60 minutes of reperfusion, LV peak systolic pressure recovered to within baseline values in the Vehicle, APRO 2×104 KIU/kg, and APRO 4×104 KIU/kg groups, but remained reduced in the APRO 8×104 KIU/kg group. LV maximal rate of peak pressure (dP/dt) was decreased versus baseline was decreased in all the treatment groups at peak ischemia except for the APRO 2×104 KIU/kg group. LV dP/dt returned within baseline values after 60 minutes of reperfusion for both the APRO 2×104 KIU/kg and the APRO 4×104 KIU/kg treatment groups.
Table 1.
Hemodynamics and LV Contractility with 30 Minutes of Ischemia Followed by Reperfusion: Dose-related Effects of Aprotinin
| Baseline | Ischemia | 30 Min Reperfusion | 60 Min Reperfusion | |
|---|---|---|---|---|
| Heart Rate (bpm) | ||||
| Vehicle | 516 ±75 | 518 ±28 | 501 ±28 + | 494 ±22 + |
| 2×104 KIU/kg | 516 ±43 | 515 ±60 | 520 ±73 | 513 ±53 |
| 4×104 KIU/kg | 541 ±38 | 521 ±54 | 532 ±38 | 543 ±28 |
| 8×104 KIU/kg | 556 ±28 | 543 ±35 | 536 ±47 | 502 ±54 |
| LV Peak Systolic Pressure (mmHg) | ||||
| Vehicle | 104 ±23 | 85 ±16 + | 79 ±13 + | 90 ±25 |
| 2×104 KIU/kg | 100 ±13 | 91 ±23 | 91 ±17 | 92 ±13 |
| 4×104 KIU/kg | 99 ±18 | 85 ±13 + | 89 ±13 | 89 ±13 |
| 8×104 KIU/kg | 101 ±10 | 79 ±13 + | 81 ±13 + | 79 ±9 + |
| LV End Diastolic Pressure (mmHg) | ||||
| Vehicle | 6.2 ±4.0 | 10.1 ±3.5 | 9.0 ±3.2 | 10.2 ±4.7 |
| 2×104 KIU/kg | 6.7 ±4.4 | 13.5 ±9.6 + | 9.6 ±7.3 | 6.8 ±4.6 |
| 4×104 KIU/kg | 5.2 ±5.5 | 10.4 ±3.2 | 8.7 ±2.8 | 8.9 ±1.9 |
| 8×104 KIU/kg | 5.4 ±3.2 | 10.2 ±5.4 | 8.6 ±3.2 | 8.9 ±6.0 |
| ESPVR (mmHg/µL) | ||||
| Vehicle | 21.8 ±8.7 | 12.6 ±5.7+ | 13.2 ±5.4+ | 13.3 ±7.6+ |
| 2×104 KIU/kg | 22.2 ±9.8 | 15.3 ±6.4 | 15.7 ±6.6 | 18.1 ±12.2 |
| 4×104 KIU/kg | 22.2 ±9.1 | 14.4 ±7.7 | 17.2±3.8 | 10.8 ±4.0+ |
| 8×104 KIU/kg | 22.2 ±8.0 | 10.3 ±6.9+ | 13.0 ±6.8+ | 14.7 ±6.4+ |
| LV dP/dt (mmHg/sec) | ||||
| Vehicle | 10736 ±2563 | 7525 ±1776+ | 7582 ±1407+ | 8214 ±2246+ |
| 2×104 KIU/kg | 10272 ±2612 | 8425 ±1830 | 9589 ±2182 | 9190 ±1229 |
| 4×104 KIU/kg | 10041 ±2276 | 7617 ±1577+ | 9267 ±2088 | 9786 ±1856 |
| 8×104 KIU/kg | 11159 ±2624 | 7534 ±2787+ | 8506 ±2020+ | 8021 ± 2117+ |
| Emax/mg (mmHg/µL/mg) | ||||
| Vehicle | 0.7 ±0.3 | 0.4 ±0.2 + | 0.4 ±0.2 + | 0.5 ±0.2 + |
| 2×104 KIU/kg | 0.9 ±0.3 | 0.6 ±0.3 + | 0.5 ±0.2 + | 0.6 ±0.3 |
| 4×104 KIU/kg | 0.8 ±0.4 | 0.5 ±0.2 + | 0.5 ±0.2 + | 0.4 ±0.2 + |
| 8×104 KIU/kg | 0.7 ±0.4 | 0.4 ±0.1 + | 0.4 ±0.2 + | 0.5 ±0.2 + |
p <0.05 v. baseline.
Data are reported as Mean ± SD, analyzed by PR Comp. bpm = beats per minute
LV End-systolic pressure volume relationship (ESPVR) values are summarized in Table 1. ESPVR decreased at peak ischemia in all four groups, and remained reduced at 60 minutes of reperfusion except for the APRO 4×104 KIU/kg group. Representative LV pressure-volume loops at reperfusion for a Vehicle treated mouse and an APRO 2×104 KIU/kg treated mouse at 60 minutes of reperfusion are shown in Figure 2-b. Clear changes in the slope of ESPVR depict reduced LV contractility with ischemia and some recovery of function after reperfusion. Emax decreased from baseline values at peak ischemia in all groups and remained decreased in all groups at 30 minutes of reperfusion. Emax was not reduced after 60 min of reperfusion in the APRO 2×104 KIU/kg group, but remained reduced in the Vehicle, APRO 4×104 KIU/kg, and APRO 8×104 KIU/kg groups. The absolute values for this index of contractility are summarized in Table 1. The relative changes from baseline are depicted in Figure 2-c. Emax was measured in the absence of I/R, and remained constant from a baseline value of 0.8 ±0.1mmHg/µL/mg to a 90 minute value of 0.7 ±0.1mmHg/µL/mg in both Vehicle and APRO 8×104 KIU/kg groups (p = 0.45). Thus the instrumentation alone, or APRO alone, in the absence of I/R had no effect on this index of LV contractility.
Plasma Cytokine Levels
Plasma cytokine concentrations were unaffected by APRO in the absence of I/R. For example, plasma concentrations of TNF in the Vehicle group were 3.2 ±0.33pg/mL, and were 2.8 ±0.2pg/mL in the 8×104 KIU/kg group (p = 0.40) in the absence of I/R. Accordingly, plasma cytokine concentrations for all of the non-I/R mice were pooled to construct referent control values as shown in Table 2.
Table 2.
Plasma Cytokine Levels Following Myocardial Ischemia-Reperfusion in the Mouse: Efects of Aprotinin (pg/mL)
| TNF-α | IL-6 | IL-10 | |
|---|---|---|---|
| Referent Controls | 3 ±1 | 10 ±11 | 3 ±1 |
| I/R Vehicle | 25 ±7* | 1700 ±703* | 18 ±8* |
| I/R 2×104 KIU/kg | 25 ±7* | 1250 ±755* | 19 ±11* |
| I/R 4×104 KIU/kg | 15 ±5*+ | 1733 ±1486* | 13 ±7* |
| I/R 8×104 KIU/kg | 14 ±4*+ | 1951 ±1340* | 13 ±8* |
p<0.05 vs pooled referent control
p<0.05 vs Vehicle.
Data are reported as Mean ± SD; Pooled Referent Control Samples (n=23) – see text; I/R groups – Vehicle (n=10), 2×104 KIU/kg (n=11), 4×104 KIU/kg (n=10), 8×104 KIU/kg (n=10)
Absolute values for plasma cytokine concentrations for all of the I/R groups are presented in Table 2. Plasma concentrations of TNF significantly increased in all treatment groups compared to reference controls, but were decreased in the 4×104 KIU/kg and 8×104 KIU/kg APRO groups. IL-6 and IL-10 plasma levels were significantly increased in all treatment groups versus referent controls, without any significant difference between groups.
Discussion
Using a unique murine model of I/R, the present study provided two distinct observations. First, indices of left ventricular (LV) contractility were improved by APRO at a dose that corresponds to a low, or half Hammersmith dose, but not at higher doses. Selective inhibition of tumor necrosis factor-α (TNF) release only occurred at high doses (corresponding to the Hammersmith and twice Hammersmith dosing). Taken together these findings suggest that there are potentially different mechanisms of action of APRO in the context of I/R which are dose-dependent. The present study demonstrates that a low-dose of APRO (2×104 KIU/kg), increased LV contractility while having a ‘sparing’ effect on cytokine release, whereas the high-dose APRO groups did not impart a protective effect. The current study directly measured plasma APRO levels which allowed direct correlation to the Hammersmith dosing protocols. Therefore, in terms of APRO concentrations, the findings of the present study have relevance to the clinical context. For example, the Blood conservation using antifibrinolytics (BART) trial, utilized a full-Hammersmith APRO dose in patients undergoing cardiac surgery, and reported a higher mortality rate when compared to lysine analogs.(4) In the present study, utilizing an APRO dose which was reflective of a full-Hammersmith dose, did not impart any protective effects on LV contractility and blunted IL-10 plasma concentrations, a known anti-inflammatory molecule.(9) However, extrapolating these acute findings in this acute I/R animal model to the observations regarding the long term clinical effects of a full-Hammersmith APRO dose is problematic.
Myocardial I/R is known to cause activation of an inflammatory response involving numerous cytokines. (9) TNF is the prototypical pro-inflammatory cytokine and IL-10 has been shown to be strongly anti-inflammatory. (7,8,22–24) Increased TNF levels have been documented to have time-dependent effects on LV performance, including a positive inotropic effect in the first three hours after I/R, followed by a cardiodepressant effect and an increase in the extent of reperfusion injury in subsequent hours to days. (8,23) Serine proteases play a central role in the amplification of the inflammatory response to I/R through numerous pathways, including contact activation, coagulation, cytokine release, and complement cascades, all of which are modulated by APRO. (5,6,10,11,13,14,23–26) In the present study, higher APRO doses attenuate TNF release. It may be that with a longer post-I/R period, the reduction in TNF at higher doses may eventually translate into improvements in myocardial function. (23) Another observation regarding cytokine release from the present study was that IL-6, another pro-inflammatory molecule, was unaffected by higher doses of APRO. This suggests that APRO may selectively affect cytokine release in the context of I/R.
While the present study demonstrated diverse effects on myocardial contractility and cytokine release, it must be placed in context and its limitations recognized. First, the murine I/R model does not necessarily recapitulate the transient myocardial I/R that may occur in the context of cardiac surgery. Nevertheless, the present study did cause regional ischemia accompanied by complete reperfusion, as confirmed by initial microsphere blood flow studies. Thus, the biological milieu that would be attendant with I/R injury was likely operative. Second, the APRO dosing was based upon a clinical weight based algorithm that may not be translatable to a mouse model. However, the present study directly measured plasma APRO levels at the end of the I/R period which is a unique aspect of this study, since plasma APRO measurements were directly assayed in an experimental model of I/R, rather than inferred. In a clinical study, Beath and colleagues utilized an ex-vivo approach to compute relative plasma APRO concentrations in patients undergoing cardiac surgery requiring cardiopulmonary bypass. (27) In this past study, the steady state APRO plasma concentrations obtained in patients receiving either the full or half Hammersmith dose, were very similar to those obtained in the present study. However, it must be recognized that the volume of distribution, pharmacokinetics and serine protease inhibitory profiles are likely to be different in the murine system than that of man. These limitations notwithstanding, the unique findings of the present study demonstrated differential dose dependent effects of APRO on both LV contractility and cytokine release in an intact model of I/R. Moreover, the experimental approaches defined in the present study could be utilized to examine the effects of lysine analogues with respect to LV contractility and cytokine release. In light of the recent clinical findings which have resulted in the removal of APRO from clinical application, these future mechanistic investigations would be appropriate.
ACKNOWLEDGEMENTS
This work was supported by National Heart, Lung, and Blood Institute Grants PO1-HL-48788 (FG Spinale), RO1-HL-59165 (FG Spinale), Research Service of the Department of Veterans Affairs (FG Spinale), Research Fellowships from Bayer Pharmaceuticals (MD McEvoy), and the Foundation for Anesthesia Education and Research (MD McEvoy).
List of Abbreviations
- APRO
Aprotinin
- LV
Left ventricular
- I/R
Ischemia-reperfusion
- TNF
Tumor necrosis factor-α
- IL-6
Interleukin-6
- IL-10
Interleukin-10
- KIU
Kallikrein inhibiting units
- LV dP/dt
Maximal rate of left ventricular peak pressure
- Emax
Maximal LV elastance
- ESPVR
End-systolic pressure volume relationship
- ANOVA
Analysis of variance
- BART
Blood conservation using antifibrinolytics trial
References
- 1.Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in a coronary arterty bypass graft surgery: A systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128:442–448. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, Cromheecke ME, de Jonge E, Prins MH, de Mol BJ, Briet E, Buller HR. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354:1940–1947. doi: 10.1016/S0140-6736(99)01264-7. [DOI] [PubMed] [Google Scholar]
- 3.Mangano DT, Tudor IC, Dietzel C. The Risk Associated with Aprotinin in Cardiac Surgery. NEJM. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 4.Fergusson DA, Hébert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, Teoh K, Duke PC, Arellano R, Blajchman MA, Bussières JS, Côté D, Karski J, Martineau R, Robblee JA, Rodger M, Wells G, Clinch J, Pretorius R BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008 May 29;358(22):2319–2331. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 5.Bull D, Connors R, Albanil A, Reid B, et al. Cardiopulmonary Support and Physiology. Aprotinin preserves myocardial biochemical function during cold storage through suppression of tumor necrosis factor. J Thorac Cardiovasc Surg. 2000;119:242–250. doi: 10.1016/S0022-5223(00)70179-6. [DOI] [PubMed] [Google Scholar]
- 6.Bull D, Maurer J. Aprotinin and preservation of myocardial function after ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S735–S739. doi: 10.1016/s0003-4975(02)04702-1. [DOI] [PubMed] [Google Scholar]
- 7.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274:R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 8.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95:1140–1153. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 9.Ren G, Dewald O, Frangogiannis NG. Inflammatory mechanisms in myocardial infarction. Curr Drug Targets Inflamm Allergy. 2003;2:242–256. doi: 10.2174/1568010033484098. [DOI] [PubMed] [Google Scholar]
- 10.Greilich PE, Okada K, Latham P, Kumar RR, Jessen ME. Aprotinin but not epsilon-aminocaproic acid decreases interleukin-10 after cardiac surgery with extracorporeal circulation: randomized, double-blind, placebo-controlled study in patients receiving aprotinin and epsilon-aminocaproic acid. Circulation. 2001;104:I265–I269. doi: 10.1161/hc37t1.094781. [DOI] [PubMed] [Google Scholar]
- 11.Turkoz A, Cigli A, But K, Sezgin N, Turkoz R, Gulcan O, Ersoy MO. The effects of aprotinin and steroids on generation of cytokines during coronary artery surgery. J Cardiothorac Vasc Anesth. 2001;15:603–610. doi: 10.1053/jcan.2001.26539. [DOI] [PubMed] [Google Scholar]
- 12.Krombach RS, Clair MJ, Hendrick JW, Houck WV, Zellner JL, Kribbs SB, Whitebread S, Mukherjee R, de Gasparo M, Spinale FG. Angiotensin converting enzyme inhibition, AT1 receptor inhibition, and combination therapy with pacing induced heart failure: effects on left ventricular performance and regional blood flow patterns. Cardiovasc Res. 1998;38:631–645. doi: 10.1016/s0008-6363(98)00050-9. [DOI] [PubMed] [Google Scholar]
- 13.van Oeveren W, Jansen NJ, Bidstrup BP, Royston D, Westaby S, Neuhof H, Wildevuur CR. Effects of aprotinin on hemostatic mechanisms during cardiopulmonary bypass. Ann Thorac Surg. 1987;44:640–645. doi: 10.1016/s0003-4975(10)62153-4. [DOI] [PubMed] [Google Scholar]
- 14.Royston D, Bidstrup BP, Taylor KM, Sapsford RN. Effect of aprotinin on need for blood transfusion after repeat open-heart surgery. Lancet. 1987;2:1289–1291. doi: 10.1016/s0140-6736(87)91190-1. [DOI] [PubMed] [Google Scholar]
- 15.Royston D, Cardigan R, Gippner-Steppert C, Jochum M. Is perioperative plasma aprotinin concentration more predictable and constant after a weight-related dose regimen? Anesth Analg. 2001;92:830–836. doi: 10.1097/00000539-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy RJ, Tuman K, O’Connor C, Ivankovish AD. Aprotinin pretreatment diminished postischemic myocardial contractile dysfunction in dogs. Anesth Analg. 1999;89:1096–1101. [PubMed] [Google Scholar]
- 17.Khan TA, Bianchi C, Araujo E, Voisine P, Xu SH, Feng J, Li J, Sellke FW. Aprotinin preserves cellular junctions and reduces myocardial edema after regional ischemia and cardioplegic arrest. Circulation. 2005;112:I196–I201. doi: 10.1161/CIRCULATIONAHA.104.526053. [DOI] [PubMed] [Google Scholar]
- 18.Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005 Jan;288(1):H149–H158. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 19.Suga H, Sagawa K. Mathematical interrelationship between instantaneous ventricular pressure-volume ratio and myocardial force-velocity relation. Ann Biomed Eng. 1972;1:160–181. doi: 10.1007/BF02584205. [DOI] [PubMed] [Google Scholar]
- 20.Feldman MD, Erikson JM, Mao Y, Korcarz CE, Lang RM, Freeman GL. Validation of a mouse conductance system to determine LV volume: comparison to echocardiography and crystals. Am J Physiol Heart Circ Physiol. 2000 Oct;279(4):H1698–H1707. doi: 10.1152/ajpheart.2000.279.4.H1698. [DOI] [PubMed] [Google Scholar]
- 21.Chang, et al. Effects of food restriction on systolic mechanical behavior of the ventricular pump in middle-aged and senescent rats. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):B108–B114. doi: 10.1093/gerona/56.3.b108. [DOI] [PubMed] [Google Scholar]
- 22.Giomarelli P, Scolletta S, Borrelli E, Biagioli B. Myocardial and lung injury after cardiopulmonary bypass: role of interleukin (IL)-10. Ann Thorac Surg. 2003;76:117–123. doi: 10.1016/s0003-4975(03)00194-2. [DOI] [PubMed] [Google Scholar]
- 23.Blancke F, Claeys MJ, Jorens P, Vermeiren G, Bosmans J, Wuyts FL, Vrints CJ. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm. 2005;2005:385–389. doi: 10.1155/MI.2005.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmon D, Lan W, Shorten G. The effect of aprotinin on hypoxia-reoxygenation-induced changes in neutrophil and endothelial function. Eur J Anaesthes. 2004;21:973–979. doi: 10.1017/s0265021504000365. [DOI] [PubMed] [Google Scholar]
- 25.Gott JP, Cooper WA, Schmidt FE, Brown WM, et al. Modifying risk for extracorporeal circulation: trial of four antiinflammatory strategies. Ann Thorac Surg. 1998;66:747–754. doi: 10.1016/s0003-4975(98)00695-x. [DOI] [PubMed] [Google Scholar]
- 26.Hill GE, Pohorecki R, Alonso A, Rennard S, Robbins R. Aprotinin reduces interleukin-8 production and lung neutrophil accumulation after cardiopulmonary bypass. Anesth Analg. 1996;83:696–700. doi: 10.1097/00000539-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Greilich PE, Brouse CF, Whitten CW, Chi L, Dimaio JM, Jessen ME. Antifibrinolytic therapy during cardiopulmonary bypass reduces proinflammatory cytokine levels: a randomized, double-blind, placebo-controlled study of epsilon-aminocaproic acid and aprotinin. J Thorac Cardiovasc Surg. 2003 Nov;126(5):1498–1503. doi: 10.1016/s0022-5223(03)00946-2. [DOI] [PubMed] [Google Scholar]