Abstract
The rostral ventromedial medulla (RVM) has been established as part of a descending pain-modulatory pathway. While the RVM has been shown to modulate homosegmental nociceptive reflexes such as tail flick or hindpaw withdrawal, it is not known what role the RVM plays in modulating the magnitude of multisegmental, organized motor responses elicited by noxious stimuli. Using local blockade of glutamate receptors with the non-specific glutamate receptor antagonist kynurenate (known to selectively block nociceptive facilitatory ON-cells), we tested the hypothesis that the RVM facilitates the magnitude of multi-limb movements elicited by intense noxious stimuli. In male Sprague-Dawley rats, we determined the minimum alveolar concentration (MAC) of isoflurane necessary to block multilimb motor responses to noxious tail clamp. MAC was determined so that all animals were anesthetized at an equipotent isoflurane concentration (0.7 MAC). Supramaximal mechanical stimulation of the hindpaw or electrical stimulation of the tail elicited synchronous, repetitive movements in all four limbs that ceased upon, or shortly after (<5sec) termination of the stimulus. Kynurenate microinjection (2 nmol) into the RVM significantly attenuated, by 40- 60%, the peak and integrated limb forces elicited by noxious mechanical stimulation of the hindpaw (p< 0.001; two-way ANOVA; n= 8) or electrical stimulation of the tail (peak force: p<0.011, two-way ANOVA; n= 8), with significant recovery 40-60 min following injection. The results suggest that glutamatergic excitation of RVM neurons, presumably ON-cells, facilitates organized, multi-limb escape responses to intense noxious stimuli.
Keywords: nociception, pain; descending modulation; glutamate; motor reflex; rostral ventromedial medulla
INTRODUCTION
The RVM is part of a descending pathway that along with the midbrain periaqueductal gray mediates bidirectional pain modulation including opioid-induced analgesia as well as hyperalgesic states [2;6;35]. The nucleus raphe magnus and nucleus reticularis magnocellularis of the RVM contain two neuronal classes of bulbospinal nociceptive reflex-modulating neurons, ON cells and OFF cells, which are believed to facilitate and to suppress nociceptive reflexes, respectively, by modulating dorsal horn activity [2;10]. A noxious stimulus excites ON-cells and inhibits OFF-cells in association with a tail flick or hindpaw withdrawal reflex [9;14;21]. Advancing the OFF-cell pause will shorten the latency of a nociceptive reflex [32], as will agents that selectively increase ON-cell activity [27]. Appropriately, opiates suppress ON-cell excitatory responses and OFF-cell inhibitory responses to noxious stimuli while increasing the latency of nociceptive reflexes [9;12;18], whereas hyperalgesic states are associated with increased ON-cell activity [3;19;28].
Whether a reduction in ON-cell activity alone disfacilitates nociceptive motor responses was previously not supported. Microinjection of the non-specific glutamate receptor antagonist kynurenate into the RVM was found to selectively inhibit ON-cell firing without changing OFF-cell activity or the latency of tail-flick to noxious heat [17]. However, in another study using repeated noxious laser stimuli, it was shown that the RVM modulates nociceptive motor responses following their initiation [14]. This suggests that the RVM has an important role in modulating ongoing nociceptive behaviors in response to repeated (or perhaps sustained) noxious stimuli. Therefore the magnitude of nocifensive behavior might serve as a useful indicator of RVM modulation.
Movement occurring under anesthesia in response to supramaximal noxious stimulation consists of organized movements such as repetitive, synchronous movement of all limbs resembling a galloping behavior in rats [1]. The minimum alveolar concentration (MAC) is defined by abolition of such movement and is commonly used to determine anesthetic immobilizing potency [31]. Our prior study showed that single limb withdrawal forces and ON-cell responses to noxious thermal stimuli were reduced 90% by isoflurane concentrations slightly exceeding MAC [21]. However, it is not known if decreases in ON-cell activity result in disfacilitation of multisegmental motor activity elicited by supramaximal stimuli as used in MAC determination. The aim of the present study was to use a manipulation previously shown to selectively decrease ON-cell activity (RVM microinjection of kynurenate) [17] to test whether this attenuates the magnitude of limb movements elicited by supramaximal intensity noxious stimulation.
MATERIALS AND METHODS
Surgery and Monitoring
The UC Davis animal care and use committee approved this study. Experiments were conducted in eight male Sprague-Dawley rats (450-555 g). Rats were anesthetized with isoflurane (3-4%) in an acrylic chamber followed by mask anesthesia (2%). Rats were then intubated through a tracheostomy, and pump ventilated with isoflurane in 100% oxygen for the remainder of the experiment. We continuously monitored rectal temperature and carotid blood pressure (model PB-240, Puritan-Bennett, Hazelwood, MO). End-tidal CO2 and isoflurane concentration were monitored with a calibrated Ohmeda Rascal II analyzer (Helsinki, Finland). The jugular vein was cannulated for administration of lactated Ringer's solution. A craniotomy was made to permit placement of the injection pipette into the RVM.
MAC Determination
MAC was determined in each animal prior to data collection to ensure that all animals were maintained at an equipotent isoflurane concentration. The animal was equilibrated to an approximate population MAC concentration for at least 15 min (1.2-1.4%). A noxious tail clamp was applied to the middle of the tail for up to 60 seconds (but removed as soon as a positive movement response occurred) [21;31]. Multi-limb movements or head turning in response to the clamp was considered a positive response, whereas tonic limb or neck extensions or complete lack of movement was considered a negative response. Anesthetic concentration was increased or decreased by 0.2% followed by at least a 15 min equilibration period. When the two concentrations that just permitted and just prevented movement were determined, these two values were averaged and designated as the animal's MAC value.
Limb Force Measurement and Injection Procedures
Following MAC determination, the animal was secured to a stereotaxic frame (model 1430, David Kopf Instruments, Tujunga, CA) with earbars, an incisor clamp, and a hip clamp. Each of all four limbs was attached via 1-0 silk sutures to a force transducer (model FT03, Grass Instruments, West Warwick, RI). Limbs were in an extended position with 50 g passive tension. Limb forces were amplified, digitized, and stored on a PC (Powerlab with Chart software, AD Instruments, Sydney Australia). A glass pipette was pulled with an outer tip diameter 80- 100 μm and attached to a 10 μl hamilton microsyringe via PE-20 tubing. The injection cannula assembly was filled with either saline or kynurenate (Sigma-Aldrich, St. Louis, MO) dissolved in saline. Injection patency and volume was verified by the movement of a small air bubble over a calibrated distance within the tubing. The injection pipette was lowered into the brainstem (midline, 11.0- 12.0 mm Caudal to bregma, 8.5 to 9.5 mm ventral to the cerebellar surface). Saline was first injected and 2-4 responses noxious hindpaw stimulation and 2-4 responses to noxious tail stimulation were recorded. The injection pipette was then retracted, filled with kynurenate solution (2 nmol in 400 nl), and replaced into the same track and at the same depth as for the prior saline injection. Injections were made using a syringe pump (MD 1001, Bioanalytical Sciences, West Lafayette, IN) over a period of 3 minutes.
Experimental Design and Analyses
All data were collected under an isoflurane anesthetic concentration of 0.7 MAC. Noxious mechanical stimulation was delivered with a small A-clamp (170 g/mm2 over a 40 mm2 area) applied to the middle portion of the hindpaw for 12 seconds. Noxious electrical tail stimulation (12 seconds, 60 mA, 100 Hz; 0.2 ms pulse duration; model NS252J; Fisher and Paykel Healthcare, Auckland, New Zealand) was delivered through platinum needle electrodes placed in each side of the tail 5-7 cm from the proximal end. Baseline responses to mechanical stimulation of the hindpaw and electrical stimulation of the tail were collected every 3-4 minutes, with alternating tail and hindpaw stimulation. Two responses to hindpaw stimulation and two responses to tail stimulation were obtained within 15 min of the RVM microinjection of vehicle or kynurenate. The stimulus series was repeated 20-40 min and again 40-60 min following the microinjection of kynurenate to test for recovery. Peak force and integrated force across 15 seconds were measured for each response in each of the four limbs and these measurements were averaged across the two stimulus trials for each stimulus series. Data from all four limbs were averaged and compared using a two-way ANOVA followed by a Tukey multicomparison test (SPSS, Inc., Chicago, IL). For hindpaw stimulation, data were analyzed separately for the stimulated hindlimb and for the contralateral hindlimb. Single pairwise comparisons were made using a paired t-test. A p-value of < 0.05 was considered statistically significant.
After data collection, animals were killed with saturated KCl under anesthesia. The brain was excised and placed in formalin followed by 30% sucrose. The brain was then frozen, cut in 40 μm transverse sections, counterstained with cresyl violet, and mounted to identify injections sites.
RESULTS
Motor Responses to Intense Noxious Stimulation of the Tail or Hindpaw
Noxious electrical tail stimulation and noxious mechanical hindpaw stimulation under 0.7 MAC isoflurane elicited bouts of repetitive, synchronous limb movement in all four limbs (fig. 1) as previously described (Antognini et al., 1999). It should be noted, however, that multilimb movement responses to noxious stimuli are not to be interpreted as an indication of the animal “waking up”. Animals did not exhibit spontaneous movement nor did the movement outlast the stimulus by more than a few seconds. Usually animals displayed one or two brief withdrawal movements upon or immediately following termination of the stimulus and stopped. As mentioned, MAC was determined in each animal to ensure that all animals were anesthetized with an equipotent isoflurane concentration of 0.7 MAC, which is approximately twice the concentration necessary to ablate consciousness and memory in both rats and humans [7;8]. Furthermore, similar types of movements occur in rats following decerebration, which does not alter isoflurane MAC [33].
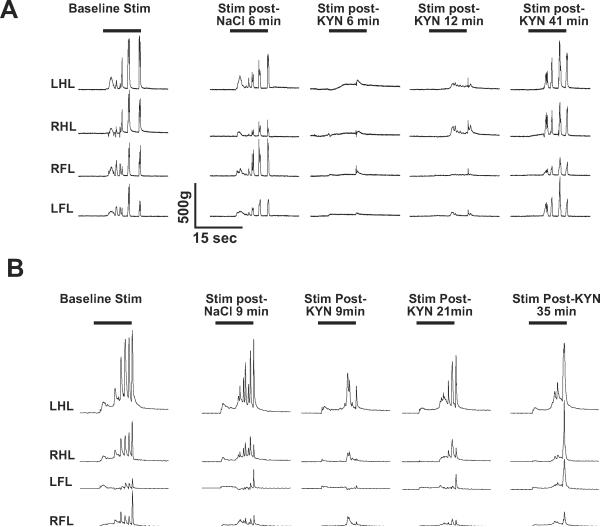
Figure 1.
Individual examples of limb movement forces elicited by noxious electrical tail stimulation (A) or noxious mechanical hindpaw stimulation (B). Kynurenate microinjection into the RVM (2 nmol) significantly reduced the magnitude of movement in all limbs, however with hindpaw stimulation the stimulated hindpaw was reduced less by kynurenate compared to the contralateral hindlimb (B, top trace). Abbreviations: left hindlimb (LHL), right hindlimb (RHL), left forelimb (LFL), right forelimb (RFL).
Effects of RVM Microinjection of Kynurenate on Limb Forces Elicted by Intense Noxious Stimuli
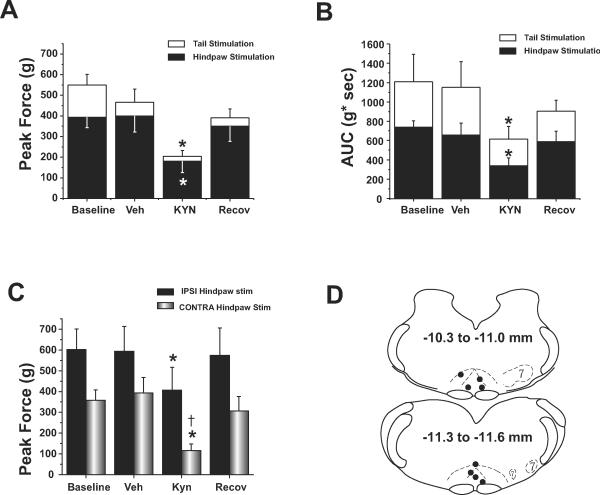
Injection of kynurenate usually caused a modest, transient reduction (10-20mmHg) in baseline blood pressure that recovered to baseline levels before testing responses to noxious stimuli. Kynurenate microinjection into the RVM decreased the force of limb movements for both noxious electrical tail stimulation and noxious mechanical hindpaw stimulation. The nadir of motor depression occurred 6- 12 min after kynurenate microinjection, followed by a gradual recovery over the ensuing 20- 60 min. For tail stimulation, kynurenate microinjection into the RVM significantly decreased peak limb forces to 44% of control, and significantly decreased integrated limb forces by 54% of control compared to saline injection alone (p< 0.001 and p< 0.023, respectively). For hindpaw stimulation, kynurenate microinjection into the RVM significantly decreased peak hindlimb forces to 45% of control and significantly decreased integrated limb forces to 52% of control, compared to saline injection alone (p< 0.001 and p< 0.007, respectively). For both tail and hindpaw stimulation, significant recovery occurred 40-60 min following kynurenate injection (tail stimulation: peak force p<0.023, integrated force: p< 0.050; hindpaw stimulation: peak force p< 0.002, integrated force p< 0.044). Mean responses among baseline, vehicle, and recovery conditions were not significantly different. Individual examples of the effect of kynurenate microinjections are shown in fig. 1, and mean data for pooled limb forces are shown in figures 2A and 2B.
Figure 2.
Effects of kynurenate on mean peak force (A) and area under the curve (B) of limb forces elicited by noxious stimulation of the tail (open bars) or hindpaw (solid bars). (C) Effect of kynurenate on stimulated (IPSI, solid bars) vs. unstimulated (CONTRA) hindpaw (shaded bars) are shown for mean peak force generated by the respective hindlimb. (D) Representative coronal section templates [30] depicting histologically-identified kynurenate injection sites. Templates are taken from 10.8 mm caudal to bregma (top) and 11.6 mm caudal to bregma (bottom) and on each template is the indicated range of injection sites included. * significantly decreased compared to vehicle control and recovery. † Significantly less than stimulated hindpaw forces. Abbreviations: 7, facial nucleus.
Effects of RVM Microinjection of Kynurenate on Stimuluated vs. Unstimulated Hindlimb Forces
For responses to hindpaw stimulation, we further analyzed effects of kynurenate microinjection on forces generated by the stimulated hindlimb compared to forces generated by the contralateral (unstimulated) hindlimb. RVM microinjections of kynurenate significantly reduced peak force of the stimulated hindlimb by 31% (from 594 g± 339 SD to 407 g± 311 SD) and significantly reduced the integrated force of the stimulated hindlimb by 60% (from 1116 g*sec± 233 SD to 451g*sec± 165 SD). In the unstimulated limb, kynurenate also significantly reduced the peak force by 70% and significantly reduced the integrated force by 53%. Although kynurenate significantly reduced movement forces in both hindlimbs, the force generated by the unstimulated hindlimb showed significantly greater depression compared to the stimulated hindlimb (p< 0.003, paired t-test). Mean data for kynurenate effects on stimulated vs. unstimulated peak hindlimb forces are shown in figure 2C.
Data were analyzed from animals in which we histologically verified injection sites located within or on the borders of the nucleus raphe magnus and the alpha part of the gigantocellular reticular nucleus (n= 8). Figure 2D displays injection sites on a sagittal template adapted from Paxinos and Watson [30]. Three animals were not included in this analysis because injection sites were clearly lateral or dorsal to the RVM (data not shown). These three animals did not show any discernable decrease in limb forces, except in one animal which showed a delayed and short-lived force reduction that lasted for only one tail and one hindpaw stimulus.
DISCUSSION
The present study demonstrates that blockade of glutamatergic transmission within the RVM, and presumably blockade of ON-cell responses, results in reduction of the magnitude of noxious stimulus-elicited multi-limb movement. Furthermore, the depression was not limited to the stimulated appendage, as limb forces in response to noxious tail stimulation were depressed as well as forces generated by the contralateral hindlimb during noxious hindpaw stimulation (fig. 2). For the most caudal microinjections, it is possible that part of the motor depression could have resulted from kynurenate spread into inferior olive, known to participate in motor coordination [25]. However, microinjections were placed throughout the rostrocaudal extent of the RVM and there were no significant differences in the effects elicited by microinjection at caudal versus rostral injection sites. Furthermore, kynurenate injection sites outside the RVM in three animals had short-lived or no discernable effect on limb forces. These data expand the role of the RVM by demonstrating that it is capable of modulating the magnitude of multisegmentally-organized “escape” movements elicited by noxious stimuli.
We observed in the present study that RVM microinjection of kynurenate decreased the magnitude, or force, of limb movements elicited by high-intensity noxious stimulation (figs. 1 and 2), while RVM kynurenate injection in a prior study failed to alter tail flick latency [17], despite producing a large reduction in ON cell responses. One explanation of this discrepancy might be that the magnitude of noxious stimulus evoked movement is affected by ON cell depression to a greater extent than is the latency (“threshold”) of a nociceptive motor response. In support of this, Foo and Mason (2003) have shown with double-pulse noxious laser stimulation that both ON and OFF neurons in the RVM play an important role in modulating nociceptive reflexes following their initiation. Thus, the magnitude of noxious stimulus-evoked movements might be a more sensitive measure of RVM-mediated modulation compared to latency measurements. We should note, however, that the stimuli used in our present study (and in Foo and Mason) were quite different from many previous studies that typically use a ramping thermal stimulus. It is thus possible that the degree of ON or OFF-cell involvement in determining threshold vs. magnitude may vary depending on the type, intensity, and duration of the noxious stimulus applied. We did not see any consistent changes in movement latencies following RVM kynurenate injection. However in our study, the instantaneous application of high-intensity noxious stimuli precluded us from making any meaningful latency (i.e., “threshold”) measurements, and as such we thought it inappropriate to present any formal latency analysis here.
The reduction in multisegmental movement by kynurenate is likely to be at least partly explained by disfacilitation of dorsal horn neuronal responses to noxious stimuli that in turn reduce nociceptive transmission to distant spinal segments or the contralateral hemisegment. However, in addition to dorsal horn projections, RVM neurons including ON cells also project to the spinal intermediate zone and ventral horn [11;20;36]. Evidence for noxious stimulus-induced heterosegmental descending facilitation ventral to the dorsal horn is supported in a prior study where noxious thermal tail stimulation was used to modulate hindlimb withdrawal responses to noxious thermal hindpaw stimulation [26]. In this prior study the noxious tail stimulus (that was not of sufficient intensity to actually elicit limb movements) facilitated single hindlimb withdrawals to noxious thermal hindpaw stimulation while inhibiting dorsal horn neurons with hindpaw receptive fields; the facilitatory effect was absent in spinalized animals. The present study, using intense noxious stimulation to initiate multi-limb movement, suggests that the RVM can facilitate movement even in segments not receiving direct noxious stimulation.
Interestingly, during noxious hindpaw stimulation, we found that peak forces generated by the stimulated hindlimb were reduced significantly less than for the unstimulated hindlimb (fig. 2C). Therefore, RVM modulation of movement in segments not receiving direct nociceptive input appears to be greater than in the segment receiving noxious stimulation, at least for hindpaw stimulation, as we did not measure tail movements. This would be expected if movement of the unstimulated limbs relied more on descending facilitation compared to the stimulated limb, in which motoneurons will receive both supraspinal input as well as local nociceptive input from dorsal horn neurons. Consistent with the present study, we previously found that acute reversible T8 spinal cold block or chronic spinal transection in rats had a greater effect in reducing multisegmental movement in response to supramaximal noxious hindpaw and tail stimulation (as used in the present study) compared to single limb withdrawals [22].
The RVM does not modulate nociception alone. In unanesthetized animals, RVM neurons respond to innocuous as well as noxious stimuli [29], and appear to play a more diverse role in homeostatic processes such as autonomic regulation, sleep-wake cycling, and micturition [24] as well as feeding behavior [13]. Furthermore, because RVM activity is also modulated by stimuli that might be considered potentially dangerous but not necessarily noxious, the RVM might be involved in engaging and integrating processes involved in complex motor behavior and associated autonomic “flight/fight” responses in addition to pain modulation. Prior studies have shown that the mesencephalic locomotor region (MLR) is located in the cuneiform nucleus and pedunculopontine tegmentum, that along with the PAG and hypothalamic areas are proposed to form a “primary defense system”[23;34]. Electrical or chemical stimulation of the MLR elicits a pressor response, increased ventilation, as well as locomotion and other escape-associated behaviors [4;16] through projections to RVM reticulospinal neurons [15]. Interestingly neurons in the cuneiform and pedunculopontine nuclei, areas belonging to the MLR, were shown to exhibit responses to noxious stimuli resembling RVM ON and OFF-cells [5]. Because kynurenate microinjection into the RVM has been shown to selectively suppress ON-cell activity [17], and we presently found that RVM kynurenate microinjection reduces the force of organized multilimb movement in response to heterotopic noxious stimuli, it is therefore possible that at least a subset of ON-cells are involved in descending locomotor drive during noxious stimulus-evoked escape responses, such as those observed in the present study. Based on present and prior results, we propose that kynurenate blockade of glutamatergic transmission in the RVM reduces ON-cell firing, resulting in disfacilitation of organized, multi-limb movement elicited by intense noxious stimulation. Further studies are needed to clarify the role of RVM neuronal classes in modulating multisegmentally-organized motor and escapeassociated behavior elicited by noxious stimuli.
ACKNOWLEDGEMENTS
Supported by NIGMS GM 78167 (to SLJ); NIDCR 13685 (to EEC); NIGMS GM 61283 and GM 47818 (to JFA). The authors thank Mirela Iodi-Carstens for the histological preparation of brain tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Antognini JF, Wang XW, Carstens E. Quantitative and qualitative effects of isoflurane on movement occurring after noxious stimulation. ANESTHESIOLOGY. 1999;91:1064–1071. doi: 10.1097/00000542-199910000-00027. [DOI] [PubMed] [Google Scholar]
- [2].Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- [3].Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is associated with increased on-cell activity in the rostral ventromedial medulla. Somatosens. Mot. Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- [4].Bedford TG, Loi PK, Crandall CC. A model of dynamic exercise: the decerebrate rat locomotor preparation. J. Appl. Physiol. 1992;72:121–127. doi: 10.1152/jappl.1992.72.1.121. [DOI] [PubMed] [Google Scholar]
- [5].Carlson JD, Selden NR, Heinricher MM. Nocifensive reflex-related on- and off-cells in the pedunculopontine tegmental nucleus, cuneiform nucleus, and lateral dorsal tegmental nucleus. Brain Res. 2005;1063:187–194. doi: 10.1016/j.brainres.2005.09.036. [DOI] [PubMed] [Google Scholar]
- [6].Dubner R. The neurobiology of persistent pain and its clinical implications. Suppl Clin. Neurophysiol. 2004;57:3–7. doi: 10.1016/s1567-424x(09)70337-x. [DOI] [PubMed] [Google Scholar]
- [7].Dutton RC, Maurer AJ, Sonner JM, Fanselow MS, Laster MJ, Eger EI. The concentration of isoflurane required to suppress learning depends on the type of learning. ANESTHESIOLOGY. 2001;94:514–519. doi: 10.1097/00000542-200103000-00024. [DOI] [PubMed] [Google Scholar]
- [8].Dwyer R, Bennett HL, Eger EI, Heilbron D. Effects of isoflurane and nitrous oxide in subanesthetic concentrations on memory and responsiveness in volunteers. ANESTHESIOLOGY. 1992;77:888–898. doi: 10.1097/00000542-199211000-00009. [DOI] [PubMed] [Google Scholar]
- [9].Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J. Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu. Rev. Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- [11].Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J. Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- [12].Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- [13].Foo H, Mason P. Sensory suppression during feeding. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16865–16869. doi: 10.1073/pnas.0506226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Foo H, Mason P. Discharge of raphe magnus ON and OFF cells is predictive of the motor facilitation evoked by repeated laser stimulation. J. Neurosci. 2003;23:1933–1940. doi: 10.1523/JNEUROSCI.23-05-01933.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 1987;411:13–20. doi: 10.1016/0006-8993(87)90676-7. [DOI] [PubMed] [Google Scholar]
- [16].Garcia-Rill E, Skinner RD, Fitzgerald JA. Chemical activation of the mesencephalic locomotor region. Brain Res. 1985;330:43–54. doi: 10.1016/0006-8993(85)90006-x. [DOI] [PubMed] [Google Scholar]
- [17].Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: implications for circuitry. Pain. 1998;75:247–255. doi: 10.1016/s0304-3959(97)00226-1. [DOI] [PubMed] [Google Scholar]
- [18].Heinricher MM, Morgan MM, Tortorici V, Fields HL. Disinhibition of off-cells and antinociception produced by an opioid action within the rostral ventromedial medulla. Neuroscience. 1994;63:279–288. doi: 10.1016/0306-4522(94)90022-1. [DOI] [PubMed] [Google Scholar]
- [19].Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J. Neurophysiol. 2004;92:1982–1989. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- [20].Holstege G, Kuypers HG. The anatomy of brain stem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog. Brain Res. 1982;57:145–175. doi: 10.1016/S0079-6123(08)64128-X. [DOI] [PubMed] [Google Scholar]
- [21].Jinks SL, Carstens E, Antognini JF. Isoflurane differentially modulates medullary ON and OFF neurons while suppressing hind-limb motor withdrawals. ANESTHESIOLOGY. 2004;100:1224–1234. doi: 10.1097/00000542-200405000-00026. [DOI] [PubMed] [Google Scholar]
- [22].Jinks SL, Dominguez CL, Antognini J. Drastic Decrease in Isoflurane Minimum Alveolar Concentration and Limb Movement Forces Following Thoracic Spinal Cooling and Chronic Spinal Transection in Rats. ANESTHESIOLOGY. 2005 doi: 10.1097/00000542-200503000-00022. IN PRESS. [DOI] [PubMed] [Google Scholar]
- [23].Jordan LM. Initiation of locomotion in mammals. Ann. N. Y. Acad. Sci. 1998;860:83–93. doi: 10.1111/j.1749-6632.1998.tb09040.x. [DOI] [PubMed] [Google Scholar]
- [24].Mason P. Ventromedial medulla: pain modulation and beyond. J. Comp Neurol. 2005;493:2–8. doi: 10.1002/cne.20751. [DOI] [PubMed] [Google Scholar]
- [25].Modianos DT, Pfaff DW. Brain stem and cerebellar lesions in female rats. I. Tests of posture and movement. Brain Res. 1976;106:31–46. doi: 10.1016/0006-8993(76)90071-8. [DOI] [PubMed] [Google Scholar]
- [26].Morgan MM, Heinricher MM, Fields HL. Inhibition and facilitation of different nocifensor reflexes by spatially remote noxious stimuli. J. Neurophysiol. 1994;72:1152–1160. doi: 10.1152/jn.1994.72.3.1152. [DOI] [PubMed] [Google Scholar]
- [27].Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- [28].Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- [29].Oliveras JL, Vos B, Martin G, Montagne J. Electrophysiological properties of ventromedial medulla neurons in response to noxious and non-noxious stimuli in the awake, freely moving rat: a single-unit study. Brain Res. 1989;486:1–14. doi: 10.1016/0006-8993(89)91271-7. [DOI] [PubMed] [Google Scholar]
- [30].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- [31].Quasha AL, Eger EI, Tinker JH. Determination and applications of MAC. ANESTHESIOLOGY. 1980;53:315–334. doi: 10.1097/00000542-198010000-00008. [DOI] [PubMed] [Google Scholar]
- [32].Ramirez F, Vanegas H. Tooth pulp stimulation advances both medullary off-cell pause and tail flick. Neurosci. Lett. 1989;100:153–156. doi: 10.1016/0304-3940(89)90676-9. [DOI] [PubMed] [Google Scholar]
- [33].Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. ANESTHESIOLOGY. 1993;78:707–712. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- [34].Sinnamon HM. Preoptic and hypothalamic neurons and the initiation of locomotion in the anesthetized rat. Prog. Neurobiol. 1993;41:323–344. doi: 10.1016/0301-0082(93)90003-b. [DOI] [PubMed] [Google Scholar]
- [35].Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- [36].Zagon A, Bacon SJ. Evidence of a Monosynaptic Pathway Between Cells of the Ventromedial Medulla and the Motoneuron Pool of the Thoracic Spinal Cord in Rat: Electron Microscopic Analysis of Synaptic Contacts. Eur. J. Neurosci. 1991;3:55–65. doi: 10.1111/j.1460-9568.1991.tb00811.x. [DOI] [PubMed] [Google Scholar]