Abstract
Effective semantic processing requires both stored conceptual knowledge and the ability to relate this information to our environment. In the current study we examined how neural processing of a concept's features was modulated by the semantic context in which they were presented using two types of nouns: complex nouns, in which all features contribute in a variable manner to an object's meaning (apples are usually red, but not always), and nominal kinds, for which a single feature plays a diagnostic role (an uncle must be the brother of a parent). We used fMRI to monitor neural activity while participants viewed a list of features and decided whether the list accurately described a target concept. We focused on the effect of semantic context on processing of features critical to a concept's representation. Task demands were manipulated by giving participants instructions that encouraged rule-based or similarity-based judgments. Activation patterns for feature processing were found to depend on the type of noun being evaluated and whether or not critical features were consistent with surrounding information: When processing critical features that contradicted other information, complex nouns resulted in additional recruitment compared to nominal kinds in frontal and temporal cortex. We observed modest effects of instruction condition, with rule-based instructions resulting in increased frontal processing and similarity-based instructions recruiting more temporal and parietal regions. Together, these results support the hypothesis that various classes of nouns are represented differently in semantic memory, and emphasize the dynamic interaction of process and content in semantic memory.
Keywords: human, semantic memory, concept representation, DLPFC
1. Introduction
Our ability to understand and interact with the world rests both on the information that we have acquired about objects and the flexible application of this knowledge within the contextual demands of our immediate environment. Early models of semantic memory deemphasized the importance of context and focused primarily on the storage of features that compose objects (Tulving, 1972). Recent work has demonstrated that semantic knowledge involves a dynamic interaction between storage of conceptual information (content) and the active manipulation of this knowledge in service of a task (process) (Koenig & Grossman, 2007; Martin & Chao, 2001). In the current study we focus on the interaction of process and content in semantic memory with specific attention to how different types of semantic content can engender qualitatively different processing strategies.
The notion that disparate types of semantic content are stored differently in the brain is well established. For example, it has been repeatedly demonstrated that the noun categories “animals” and “tools” rely on at least partially dissociable regions of cortex in the ventral visual pathway (Caramazza & Shelton, 1998; Martin, 2007). However, differences in content may also arise from the semantic structure of a concept—that is, how individual features contribute to a concept's representation (Crutch & Warrington, 2005; Keil, 1989). We suggest that such differences in content necessitate differences in process because divergent types of information must be evaluated. In the current study we examined the effects of context on semantic processing differences on multiple levels. First, we investigated semantic context effects within each type of noun in order to assess the degree to which implicit processing of feature knowledge depends on prior semantic context. Second, we examined processing differences resulting from dissimilarities in the semantic structure of two classes of nouns. Finally, we used two sets of experimental instructions designed to encourage distinct processing strategies for this task to see if implicit processing differences can be further altered using explicit task demands. This approach enabled us to examine processing requirements that differ based on intrinsic concept properties and those that differ based on externally imposed criteria.
One dominant framework used to approach the study of semantic memory, which we adopt for the current study, involves characterizing the meanings of concrete nouns in terms of distributions of features: An APPLE has a stem, is red, contains seeds, and grows on a tree (Hampton, 1995; McClelland & Rogers, 2003; McRae, de Sa, & Seidenberg, 1997; Smith, Shoben, & Rips, 1974). (We use capital letters to refer to a concept, and italics to its component features.) Each of these features contributes in a probabilistic manner to the representation of APPLE, but no single feature by itself can determine whether an object is an APPLE. For example, most people consider the red color of an apple to be a particularly salient feature, but do not have difficulty recognizing a green apple because it possesses a sufficient number of other positively contributing features. Although most nouns can be characterized in this fashion, we refer to these as “complex nouns” to emphasize the potentially large number of features that can contribute to a concept's meaning. Through empirical testing, it is possible to determine features of a complex noun that contribute particularly strongly to its meaning (critical features, such as the color of an apple) as well as features that are less strongly associated with an object (auxiliary features, such as whether an apple has a stem or not). Within this feature-based approach, determining the identity of an object is accomplished by assessing the number of features that contribute to its meaning, while simultaneously taking into account the relative importance of these features.
In general it may be assumed that the importance of a critical feature to a concept is tied to the importance of surrounding auxiliary features, because all of these features contribute in some degree to the representation of a concept. Thus, a critical feature may be more important in evaluating a concept when there is a mismatch between the information provided by the critical feature and the other features present. However, there are concepts for which this is not the case. These include nouns in which a single critical feature plays a diagnostic role, known as nominal kinds (Keil, 1989). A common example of a nominal kind is the word grandfather. The meaning of GRANDFATHER is constrained such that this person must be a parent's father. There exist other auxiliary features that people tend associate with GRANDFATHER, such as kindly demeanor, attends family gatherings, brings presents, and visits often. However, a person who was unkind, never present at family gatherings, didn't bring presents, and never visited—in other words, had a large number of anti-characteristic features—could still be a GRANDFATHER if he were the father of a parent. Object identity for nominal kinds therefore relies predominantly on a single critical feature. Unlike complex nouns, auxiliary features should never be able to overwhelm the contribution of the diagnostic critical feature. Accordingly, we expect processing of nominal kinds to rely less upon auxiliary attributes and more upon critical features.
The degree to which the cognitive processes underlying nominal kind concepts differ from those supporting complex nouns has been a matter of some debate. One view holds that nominal kinds rely on rule-based processes to determine category membership because of the diagnostic role played by a single, or small number, of “defining” features (Keil, 1989; Keil & Batterman, 1984; Rips, 1989). Thus, such nouns are processed in a qualitatively different way from other nouns. An alternative view suggests that in fact all concepts are processed using the same summed weighting of features, but that differences arise due to the weightings assigned to individual features (Hampton, 1997; Rosch & Mervis, 1975). For example, for nominal kinds, a feature that is particularly strongly weighted may appear to play a special diagnostic role, when in fact the categorization process is no different than any other noun (Hampton, 1995). For the current study our focus is only on the fact that the two types of nouns elicit differences in cortical processing, regardless of the nature of these differences.
Empirical evidence supporting the difference between neural representations of nominal kinds and complex nouns has been reported in one fMRI study to date (Grossman, Troiani, Koenig, Work, & Moore, 2007). In this prior report, participants evaluated whether a list of sequentially presented features accurately described a target concept. The authors examined neural activation while participants read descriptions that contributed positively to an object's meaning. When examining characteristic features, activity was stronger in parietal regions for nominal kinds relative to complex nouns. By contrast, examining features that contributed positively to the meaning of complex nouns resulted in more activity in lateral temporal and frontal regions relative to nominal kinds. Additionally, critical features were found to result in greater activation than auxiliary features. These results are consistent with the theory that complex nouns and nominal kinds are represented differently from complex nouns in semantic memory, as well as the notion that individual features differ in the degree to which they influence a concept's representation.
In the current study we re-analyze data from Grossman et al. (2007) in order to more closely examine the processing of critical features. We hypothesized that the context provided by auxiliary features would modulate the processing of critical features. In general, we predict critical features should require more activity when they contradict auxiliary features compared to when they are consistent, related to the evaluation of the semantic information presented. That is, the critical feature is the same, but is processed differently due to its importance in evaluating the target concept. We expected this effect to be less prominent in nominal kinds, because for these nouns the critical feature always plays a relatively greater role in concept representation, and thus should be affected to a lesser degree by the nature of the auxiliary features.
Above we have suggested that the neural processing of semantic features can be modulated by the nature of the conceptual content (complex noun or nominal kind) and the relative importance of these features to a concept, given their semantic context. In addition to these considerations, participants' goals during a task are also likely to affect the relative evaluation of semantic information. In the current experiment we explicitly manipulated these demands by randomly assigning participants to one of two groups. Each group received instructions designed to promote rule-based or similarity-based approaches to concept evaluation (Smith, Patalano, & Jonides, 1998). Rule-based instructions were intended to bias participants towards identifying a specific feature that plays a prominent role in the meaning of a concept. By contrast, similarity-based instructions were intended to encourage a more equally distributed evaluation of all factors. Based on previous patient and neuroimaging studies we expected rule-based instructions to subtly bias participants in this condition towards increased use of frontal brain regions associated with executive resources, and similarity-based instructions to shift activity towards temporal and parietal association cortices (Koenig et al., 2005; Koenig, Smith, & Grossman, 2006). However, in the previous report on the current data, there were no differences found between the two instruction conditions (Grossman et al., 2007). Thus, although we expected instruction effects to be apparent regardless of the type of concept being tested, we presumed any effects would be subtle.
2. Method
The current study is a reanalysis of data collected for Grossman et al. (2007).
2.1 Participants
Participants were 25 healthy adult volunteers, 14 females and 11 males, ranging in age from 18–33 (M = 23.9, SD = 3.6). All were native speakers of English, right-handed, and in good health with no history of neurological difficulty. Informed consent was obtained from all participants according to a protocol approved by the University of Pennsylvania Institutional Review Board.
2.2 Materials
Based on previous studies of semantic memory we identified four exemplars from the noun categories of animals (CAMEL, COW, RATTLESNAKE, WHALE) and tools (AXE, HAMMER, PLIERS, SCISSORS). We also identified four exemplars each from two categories that contain nominal kinds: kinship terms (BROTHER, COUSIN, GRANDSON, NIECE) and moral acts (LIE, STEAL, TEASE, CHEAT)1.
For each target noun a critical feature positively associated with the concept was identified (C+). These features were developed using pilot testing and determined to have a large impact on subjects' decisions regarding concept identity. For each target concept, we identified five auxiliary features that were consistent with the concept (A+). These features were chosen so that their absence would not prevent an exemplar from being a member of the associated category.
We then developed salient anti-characteristic critical features (C-) that prohibited category membership for the nominal kinds (i.e., were anti-diagnostic). For example, if someone is the brother of Sarah's mother, by definition they cannot be Sarah's GRANDFATHER. For complex nouns we developed critical features that were not associated with a target word, such as an APPLE that grows on a vine. Although these features are never directly associated with the target words in everyday experience, empirical testing indicated that by themselves they did not prevent category membership for the complex nouns.
Finally, we established five anti-characteristic auxiliary features (A-) for each target concept. Each anti-characteristic auxiliary feature was selected such that its presence would not automatically prevent category membership, such as a GRANDFATHER who is unfriendly or an APPLE that is purple in color. These anti-characteristic stimuli were also tested to ensure their presence did not necessarily result in the associated concept being rejected.
Example stimuli are listed in Table 1, and the full list of stimuli are provided in Supplemental Material. These stimuli allowed us to examine, for each noun type, processing associated with critical features, and whether this processing was modulated by the agreement of the surrounding auxiliary features with the target concept.
Table 1.
Example features used in experiment
| Agreement of auxiliary features | |||
|---|---|---|---|
| Noun type | Critical feature supports concept (C+) | Consistent with critical feature | Inconsistent with critical feature |
| Complex Noun (COW) | Yes | Evan sees this animal on a farm. It wears a bell. * It has an udder. It eats grass. It is white. It has black spots. |
This animal runs very fast. Scott sees it on the beach. It eats lettuce. It wears a saddle. It has long hair. * It has an udder. |
| No | This animal runs very fast. It can live on the beach. Tony sees it eating seeds. * It has antlers. It wears a saddle. It has a short tail. |
Rich sees this animal on a farm. * It has antlers. It wears a bell. It eats grass. It's black and white. It nurses its young. |
|
| Nominal Kind (GRANDSON) | Yes | Alex visits Jerry often. He sits on Jerry's lap. He is sweet and little. Jerry takes him to ballgames. Jerry reads him stories. * Jerry's son is Alex's father. |
Ralph has gray hair. * Joe's son is Ralph's father. Ralph has dentures. He sits in a rocking chair. He smokes a pipe. He listens to the radio. |
| No | Randy walks slowly. He owns a home. * Keith's brother is Randy's father. Randy has dentures. He sits in a rocking chair. He smokes a pipe. |
Billy visits Mark often. He sits in Mark's lap. Mark reads him stories. * Mark's brother is Billy's father. Billy is sweet and little. Mark takes him to ballgames. |
|
indicates critical feature.
In addition to obtaining ratings of stimuli, we examined critical features of nominal kinds and complex nouns included in the analysis along several psycholinguistic measures using data from the English Lexicon Project (Balota et al., 2007). For each critical statement we obtained the following attributes for each content word: two measures of word frequency (Kucera Francis and HAL), the number of orthographic neighbors, and the number of phonological neighbors. In addition, we obtained concreteness ratings from 27 adults on the same words. These measures were submitted to independent samples t-tests. Using a significance level of .05, there was no significant difference in Kucera Francis Frequency (MNK = 116.93, SDNK = 91.40; MCN = 110.72, SDCN = 106.20; t(58) = 0.24); HAL Frequency (MNK = 56,836, SDNK = 59,321; MCN = 94,061, SDCN = 112,331; t(58) = 1.605), number of orthographic neighbors (MNK = 6.19, SDNK = 5.15; MCN = 9.24, SDCN = 6.68; t(58) = 1.98), number of phonological neighbors (MNK = 13.18, SDNK = 10.96; MCN = 17.51, SDCN = 10.23; t(58) = 1.58), or rated concreteness (MNK = 3.89, SDNK = 0.78; MCN = 3.93, SDCN = 0.69; t(58) = 0.24).
Participants' accuracy for target complex nouns was 85.2% (SD = 7.5), and for nominal kinds 86.6% (SD = 7.4). Only descriptions that resulted in an accurate response were included in the fMRI analysis.
2.3 Procedure
Participants were presented with a target concept, followed by a list of features. After all the features were presented participants were asked to indicate whether the object described by the features matched the target concept. For example:
COW
Evan sees this animal on a farm
It wears a bell.
It has an udder.
It eats grass.
It is white.
It has black spots.
Is this animal a COW?
Thus, participants knew the target concept being tested as they read each of the features. Participants received several practice trials prior to entering the magnet to ensure they understood the instructions and were familiar with the procedure.
In the magnet, all stimuli were presented visually to participants using a projector and mirror system. Each trial commenced with presentation of the target word at the top of the display. Six brief feature descriptions then appeared sequentially below the target at equal intervals. The intervals were 3, 6, 9, or 12 sec, and were the same throughout a trial. After all feature descriptions were presented, the target concept was presented again with a question mark, and participants pressed a button to indicate whether the presented features accurately described the target concept. The next trial commenced 12 sec after the probe appeared.
Each trial involved the presentation of six features related to the target concept. One of these was a critical feature, the rest auxiliary features. In the current analysis we only focus on activity associated with the critical features. The agreement of the critical feature with the auxiliary features was varied such that for half of the trials it was consistent and half of the trials it was inconsistent. The position of the critical feature was randomized. Because our interest was in the effect of critical features when they contradicted previous information we analyzed only those critical features in which at least one preceding auxiliary feature was presented. We compared processing for critical features when they were inconsistent with auxiliary features relative to when they were consistent with these features. Thus, because the critical features themselves were the same across condition, differences can be attributed to the context in which these critical features were presented. The conditions analyzed in the current study were a 2 × 2 factorial design that crossed noun type (nominal kind or complex noun) with the consistency of the critical feature with its context (consistent or inconsistent). We conducted analyses separately for C+ and C- features to assess qualitative similarity for these conditions.
In addition to these within-subject manipulations, participants were randomly assigned to one of two instruction conditions. In the rule-based condition, participants were instructed to identify the correct response. Following each list of features, they were asked whether it was accurate (e.g., “Is this animal a COW?”). In the similarity-based condition, participants were told that descriptions more or less described the target concept, and were asked to decide whether a description reasonably described the target concept (e.g., “Could this animal be a COW?”).
2.4 Image acquisition and analysis
Scans were acquired on a Siemens Trio scanner at 3T. Each session began with acquisition of a T1-weighted structural volume using an MPRAGE protocol (TR = 1620 msec, TE = 3 msec, flip angle = 15°, 1 mm slice thickness, 192 × 256 matrix, resolution = .9766 × .9766 × 1 mm). A total of 1597 BOLD fMRI images were acquired in 8 separate scanning runs of approximately equal length. Each image was acquired with fat saturation, 3 mm isotropic voxels, flip angle of 15°, TR = 3 sec, TEeff = 30 msec, and a 64 × 64 matrix.
Image preprocessing and statistical analyses were performed using SPM5 (Wellcome Trust Centre for Functional Neuroimaging, London, UK). Analysis of imaging data was restricted to descriptions that resulted in a correct response by the participant. Data were initially analyzed separately for each participant. Low-frequency drifts were removed with high-pass filtering with a cutoff period of 128 seconds and autocorrelations modeled using a first-order autoregressive model. Images for each participant were realigned to the first image in the series (Friston et al., 1995) and coregistered with the structural image (Ashburner & Friston, 1997). The transformation required to bring a participant's images into standard MNI152 space were calculated using tissue probability maps (Ashburner & Friston, 2005), and these warping parameters were then applied to all functional images for that participant. During spatial normalization functional data were interpolated to isotropic 2 mm voxels. The data were spatially smoothed with an 8 mm FWHM isotropic Gaussian kernel.
Given that subjects were scanned for a relatively long period of time, we took several measures to reduce the amount of head movement during the experiment. First, we ensured subjects' heads were firmly situated within the head coil, and that they were in a comfortable position. Subjects were instructed to lie as still as possible throughout the session. The average maximum translation for all subjects was 2.06 mm, and the average maximum rotation .05 radians. Finally, to assess any effects of the length of the scanning session on participant movement, we compared the average translation (in any direction) from the first 300 scans (M = -.023 mm, SD = .045 mm) to that seen during the last 300 scans (M = .047 mm, SD = .190 mm). There was no difference between these two measurements, t(24) = -1.97, n.s.. Similarly we compared the average rotations for the first 300 scans (M = .0004 radians, SD = .0015 radians) to those for the last 300 scans (M = -.0009 radians, SD = .0035 radians). Again, there was no significant difference, t(24) = 1.67, n.s.. Thus we conclude that subjects did not show increased movement as the scanning session progressed.
For each stimulus category, hemodynamic response was estimated by convolving the onset times with a canonical hemodynamic response function. Motion parameters obtained from the realignment procedure were included as covariates. A general linear model approach was used to calculate parameter estimates for each variable for each subject (Friston et al., 1995), and linear contrasts for comparisons of interest. These estimates were then entered in second-level random effects analyses to allow us to make inferences across participants.
3. Results
3.1 Overall effect of condition on processing diagnostic features
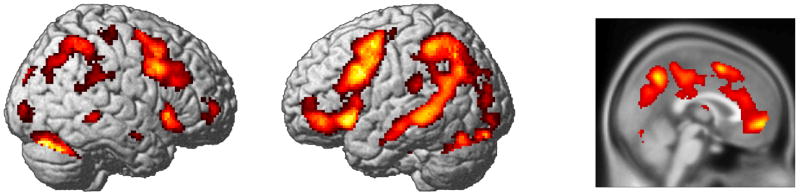
To identify regions showing a reliable effect of condition, we first conducted a second-level F test for all conditions of interest; that is, for all modeled critical features, regardless of the noun type (nominal kind or complex noun), surrounding auxiliary features (consistent or inconsistent), or instruction condition. For this contrast we controlled for false positives using a false discovery rate (FDR) threshold of p < .005 (Benjamini & Hochberg, 1995; Genovese, Lazar, & Nichols, 2002) and only accepted clusters containing a minimum of 20 contiguous voxels. This enabled us to identify regions involved in feature processing for subsequent detailed analyses. Regions identified by this procedure are shown in Figure 1, and the peaks of activation clusters listed in Table 2. (In all tables, cluster extent is indicated by the number of voxels in the cluster, using the cluster size from the normalized functional images.)
Figure 1.
Main effect of stimulus type. Results of second-level F test on all conditions of interest rendered on a standard brain template.
Table 2.
Brain regions showing a main effect of stimulus type
| Region | Peak Coordinates | Z score | # voxels |
|---|---|---|---|
| R cerebellum | 14, -76, -26 | Inf | 16956 |
| L superior parietal | -32, -60, 44 | 7.53 | |
| L middle frontal gyrus | -50, 14, 28 | 7.05 | 4287 |
| L middle frontal gyrus | -40, 0, 54 | 6.90 | |
| L inferior frontal gyrus | -48, 40, -10 | 6.78 | |
| Anterior cingulate | 0, 50, -4 | 6.87 | 3189 |
| Medial superior frontal gyrus | -2, 8, 54 | 6.81 | |
| R middle frontal gyrus | 54, 14, 34 | 6.22 | 1900 |
| R middle frontal gyrus | 44, 6, 32 | 5.24 | |
| R thalamus | 14, -4, 12 | 6.04 | 1653 |
| L thalamus | -14, -2, 14 | 5.79 | |
| R insula | 36, 24, -4 | 5.32 | 614 |
| R inferior frontal gyrus | 44, 26, -12 | 4.11 | |
| L posterior superior temporal sulcus | -62, -30, 20 | 4.76 | 130 |
| R posterior middle temporal gyrus | 60, -40, -6 | 4.63 | 102 |
| R middle frontal gyrus | 34, 56, 0 | 4.24 | 232 |
| L brainstem | -6, -32, -24 | 3.97 | 70 |
| R superior marginal gyrus | 64, -40, 26 | 3.94 | 181 |
| L medial temporal lobe | -28, -22, -6 | 3.93 | 49 |
| R medial middle temporal gyrus | 40, -32, 0 | 3.80 | 24 |
| R anterior middle temporal gyrus | 58, -4, -18 | 3.76 | 32 |
| R brainstem | 8, -32, -16 | 3.58 | 26 |
| R angular gyrus | 48, -52, 18 | 3.54 | 49 |
Effects of condition in processing critical features were seen in several regions of the brain associated with linguistic processing. This included activation in lateral and posterior inferior temporal lobes, inferior and dorsolateral frontal cortex, and parietal regions. These activations were bilateral, although they were more robust in the left hemisphere. There were also significant effects in medial regions including cingulate cortex and medial parietal cortex.
We restricted all subsequent analyses to the regions identified by this F test. Within these regions we adopted a more relaxed statistical criterion to investigate effects of stimulus type. Unless otherwise stated we report clusters in which each voxel is significant at p < .05 and the peak voxel in a cluster has a minimum Z score of 3.09 (equivalent to p < .001), with a minimum cluster extent of 20 voxels.
3.2 Effects of semantic context on characteristic critical feature processing
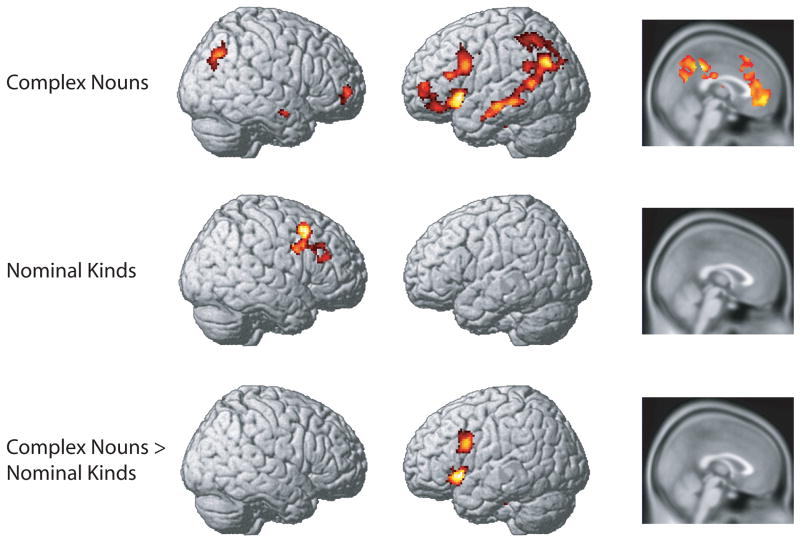
Within the region identified by our initial F test, we investigated the extra activity required to process characteristic critical features (C+) in the inconsistent relative to the consistent conditions. We initially performed this analysis for all participants, irrespective of instruction condition. Results from this comparison for complex nouns and nominal kinds are shown in Figure 2 and described in Table 3. For complex nouns, this analysis showed significant increases in left temporal and inferior parietal regions, bilateral frontal cortex, and cingulate. For nominal kinds increases in activity were restricted to right middle frontal gyrus.
Figure 2.
Increased activation associated with processing characteristic critical features (C+) that are inconsistent with surrounding auxiliary features relative to when they are consistent. Top: Inconsistent C+ > Consistent C+ for complex nouns. Middle: Inconsistent C+ > Consistent C+ for nominal kinds. Bottom: Inconsistent C+ > Consistent C+, Complex nouns > Nominal kinds. There were no regions in which nominal kinds showed a greater response than complex nouns.
Table 3.
Brain regions showing increased activation for characteristic critical features that contradicted preceding auxiliary features
| Region | Peak Coordinates | Z score | # voxels |
|---|---|---|---|
| Complex Noun: Inconsistent C+ > Consistent C+ | |||
| L superior parietal and angular gyrus | -44, -66, 30 | 4.14 | 1914 |
| L middle temporal gyrus | -50, -18, -14 | 3.27 | |
| Posterior cingulate | 0, -40, 40 | 4.08 | 493 |
| R posterior superior parietal | 44, -68, 34 | 3.71 | 242 |
| R anterior middle temporal gyrus | 60, -2, -18 | 3.68 | 32 |
| L middle frontal gyrus | -32, 58, 6 | 3.63 | 181 |
| Anterior cingulate | 0, 48, -6 | 3.57 | 2175 |
| L middle frontal gyrus | -48, 30, 18 | 3.56 | 622 |
| L ventral inferior frontal | -48, 38, -14 | 3.41 | 846 |
| R superior frontal sulcus | 30, 58, 2 | 3.40 | 122 |
| L brainstem | -10, -26, -24 | 3.38 | 69 |
| R precuneus | 4, -54, 20 | 3.32 | 1381 |
| R thalamus | 4, -16, 14 | 3.21 | 154 |
| Nominal Kind: Inconsistent C+ > Consistent C+ | |||
| R middle frontal gyrus | 30, 10, 44 | 3.34 | 596 |
| 38, 14, 48 | 3.08 | ||
| Inconsistent C+ > Consistent C+: Complex nouns > nominal kinds | |||
| L ventral inferior frontal | -32, 20, -6 | 3.50 | 546 |
| -32, 16, 8 | 3.18 | ||
| L middle frontal gyrus | -50, 12, 28 | 3.35 | 372 |
| L brainstem | -10, -26, -22 | 3.18 | 35 |
To examine differences in processing between complex nouns and nominal kinds we directly contrasted activity in the two conditions, shown in the bottom panel of Figure 2 and listed in Table 3. When critical features were inconsistent with auxiliary features, complex nouns required significantly greater activation in left inferior frontal gyrus. There were no regions associated with significantly greater activity for nominal kinds relative to complex nouns.
3.3 Effects of semantic context on non-characteristic critical feature processing
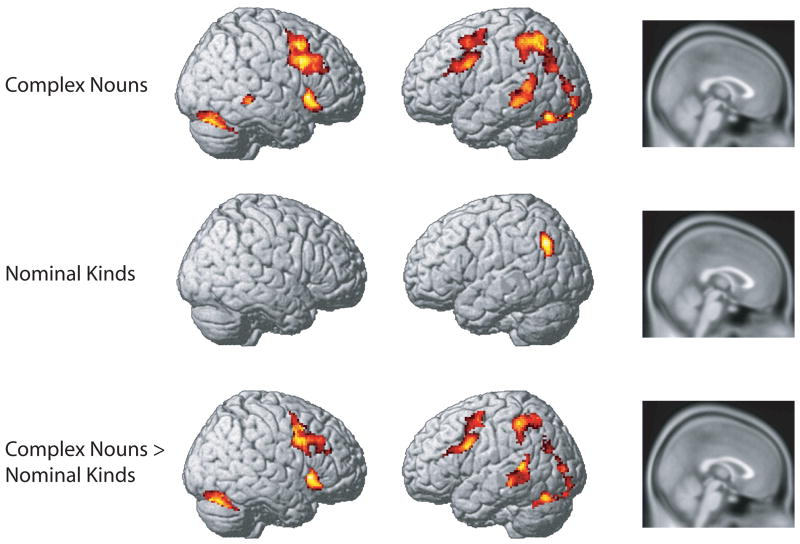
We next performed the same analyses, focusing on non-characteristic critical features (C-). These results are shown in Figure 3, with activation maxima described in Table 4. Diagnostic critical features of complex nouns that were inconsistent with previous auxiliary features resulted in increased activation in bilateral dorsolateral prefrontal cortex, as well as left middle temporal gyrus and inferior parietal lobe. For nominal kinds, increases associated with the inconsistent condition were only seen in left inferior parietal cortex.
Figure 3.
Increased activation associated with processing non-characteristic critical features (C-) that are inconsistent with surrounding auxiliary features relative to when they are consistent. Top: Inconsistent C- > Consistent C- for complex nouns. Middle: Inconsistent C- > Consistent C- for nominal kinds. Bottom: Inconsistent C- > Consistent C-, Complex nouns > Nominal kinds. There were no regions in which nominal kinds showed a greater response than complex nouns.
Table 4.
Brain regions showing increased activation for noncharacteristic critical features that contradicted preceding auxiliary features
| Region | Peak Coordinates | Z score | # voxels |
|---|---|---|---|
| Complex Noun: Inconsistent C- > Consistent C- | |||
| R globus pallidus | 14, -6, 8 | 4.38 | 616 |
| L superior parietal | -30, -58, 32 | 3.74 | 3681 |
| R cerebellum | 28, -54, -34 | 3.62 | |
| L occipital | -24, -92, -4 | 3.50 | |
| R posterior middle temporal gyrus | 62, -38, -8 | 3.64 | 82 |
| L posterior middle temporal gyrus | -50, -46, -4 | 3.64 | 707 |
| -58, -50, 16 | 2.94 | ||
| L middle frontal gyrus | -46, 6, 50 | 3.39 | 743 |
| -54, 8, 34 | 3.27 | ||
| R middle frontal gyrus | 44, 14, 22 | 3.36 | 1401 |
| 44, 4, 28 | 3.29 | ||
| L globus pallidus | -20, 0, 6 | 3.35 | 529 |
| R insula | 42, 20, -2 | 3.33 | 418 |
| 48, 22, -14 | 3.08 | ||
| Nominal Kind: Inconsistent C- > Consistent C- | |||
| L angular gyrus | -44, -64, 38 | 3.35 | 311 |
| Inconsistent C- > Consistent C-: Complex nouns > Nominal kinds | |||
| R globus pallidus | 14, -6, 8 | 4.60 | 513 |
| L middle frontal gyrus | -44, 0, 54 | 4.06 | 553 |
| -56, 6, 34 | 3.84 | ||
| L occipital | -12, -90, -18 | 3.81 | 2126 |
| L superior parietal | -38, -34, 32 | 3.79 | |
| R middle frontal gyrus | 44, 2, 28 | 3.79 | 975 |
| R insula | 44, 18, -2 | 3.58 | 398 |
| L posterior superior temporal sulcus | -62, -42, 8 | 3.55 | 536 |
| L posterior middle temporal gyrus | -46, -40, 0 | 3.27 | |
| R cerebellum | 34, -62, -20 | 3.49 | 773 |
| L globus pallidus | -10, -8, 10 | 3.12 | 340 |
To examine differences in processing between complex nouns and nominal kinds, we directly contrasted the two conditions. Results from this analysis are shown in the bottom panel of Figure 3, with activation maxima described in Table 4. Complex nouns showed significantly greater frontal activation than nominal kinds in several regions, including bilateral dorsolateral prefrontal cortex, left ventral inferior frontal gyrus, left middle temporal gyrus, and left inferior parietal. There were no regions in which nominal kinds showed more activity than complex nouns.
3.5 Effects of rule- and similarity-based instructions
As noted in the introduction, differences between rule-based and similarity-based processing are found in many behavioral and imaging studies (Allen & Brooks, 1991; Grossman et al., 2002; Koenig et al., 2006; Patalano, Smith, Jonides, & Koeppe, 2001; Smith & Sloman, 1994). To evaluate the effect of instruction type in the current study, we compared participants given rule-based instructions to those who received similarity-based instructions using a two-sample t-test. We investigated these differences for the regions revealed in our analysis of violation type (displayed in Figures 2 and 3). Because we expected these effects to be subtle, we accepted clusters in which at least 15 contiguous voxels had an uncorrected significance of p < .05, listed in Table 5. A large number of regions showed increased processing under similarity-based instructions, including left temporal and parietal regions. The only region showing significantly greater activation in the rule-based condition was the right middle frontal gyrus.
Table 5.
Brain regions showing significant differences in activation between rule- and similarity-based instructions
| Region | Peak Coordinates | Z score | # voxels |
|---|---|---|---|
| Similarity > Rule: Complex noun C+ Inconsistent > Consistent | |||
| Posterior cingulate | 2, -30, 40 | 3.20 | 208 |
| L precuneus | -4, -66, 30 | 3.14 | 642 |
| L middle temporal gyrus | -52, -16, -20 | 2.63 | 169 |
| L angular gyrus | -42, -68, 30 | 2.56 | 111 |
| Posterior cingulate | 0, -26, 30 | 2.49 | 82 |
| R angular gyrus | 40, -62, 48 | 2.41 | 122 |
| 10, -50, 10 | 2.39 | 34 | |
| L middle temporal gyrus | -48, -38, -8 | 2.26 | 30 |
| Similarity > Rule: Complex noun C- Inconsistent > Consistent | |||
| L medial parietal | -18, -56, 42 | 2.74 | 23 |
| L inferior parietal | -44, -60, 40 | 2.44 | 17 |
| Similarity > Rule: Nominal kind C- Inconsistent > Consistent | |||
| L angular gyrus | -48, -60, 42 | 2.34 | 53 |
| Rule > Similarity: Nominal kind C+ Inconsistent > Consistent | |||
| R middle frontal gyrus | 36, 14, 48 | 3.17 | 83 |
| R middle frontal gyrus | 40, 32, 34 | 2.42 | 19 |
4. Discussion
The manner in which conceptual information is stored and the various methods of accessing this knowledge can result in divergent resource demands. We broadly agree with theories positing that the meanings of most concrete nouns involve a weighted combination of characteristic features. Within such a scheme, determining object identity requires active processing to evaluate the available information and deciding how well the summed information matches a core concept. The aim of the current study was to see whether processing of individual features could be influenced by their surrounding semantic context, and whether this difference could be further modulated by the type of concept features were associated with, or by explicit task instructions. Our current results suggest that each of these manipulations impacts the cortical processing associated with noun features. Below we discuss the effects of semantic context for complex nouns and nominal kinds, and the additional impact of rule- or similarity-based processing strategies.
4.1 Effects of semantic context on processing features of nominal kinds
Because of the special place diagnostic critical features hold in the meaning of nominal kind nouns, we anticipated that they would be processed similarly regardless of how they related to preceding auxiliary features. That is, diagnostic features of nominal kinds should remain diagnostic largely irrespective of the surrounding semantic context. This hypothesis was supported by our results, which showed relatively modest increases in processing for the inconsistent condition compared to the consistent condition for nominal kinds. When the critical feature contradicting auxiliary features positively supported the meaning of a concept (C+ in A- context), increased activation was seen in right middle frontal gyrus. Although the laterality of dorsolateral processing is not always consistent, dorsolateral regions of either hemisphere have been implicated in executive processes sometimes required for evaluation and decision making (Duncan & Owen, 2000), including episodic memory tasks when semantic relationships are stressed (Miotto et al., 2006; Murray & Ranganath, 2007). Patients with neurodegenerative disease affecting dorsolateral prefrontal regions have difficulty acquiring novel semantic concepts using rule-based criteria (Koenig et al., 2006). Indeed, these nonaphasic patients with frontotemporal dementia display a variety of language problems which seem attributable to executive resource decline (Peelle & Grossman, 2008). These studies indicate that dorsolateral prefrontal regions play an important role in the executive mediation of some semantic tasks.
When the critical feature violation prohibited concept identity (C- in A+ context), activation for nominal kinds was seen in left inferior parietal lobe. Although this region is not typically reported in functional imaging studies of word processing, gray matter density in this region of left inferior parietal cortex was recently found to correlate with vocabulary growth in adolescents, and has anatomical connections to both angular gyrus and anterior supramarginal gyrus (Lee et al., 2007). These findings are consistent with a role for the left inferior parietal lobes in semantic processing. In the context of our current study, this region may reflect an accumulation of feature information regarding these nouns.
4.2 Effects of semantic context on processing features of complex nouns
Because concept representation for complex nouns is more heavily dependent on a probabilistic evaluation of feature knowledge than nominal kinds, we expected significantly greater activation when critical features were inconsistent with auxiliary features relative to when they were consistent. Indeed, activity in several regions was seen when critical features were inconsistent with surrounding context, regardless of whether the critical feature was characteristic of the target concept (C+) or not (C-).
Large regions of the temporal and parietal lobes were observed for inconsistent critical feature processing in complex nouns. Left temporal areas are associated with accessing semantic information in a variety of tasks (Martin, 2007). This includes findings that making semantic judgments about single words increases left temporal processing relative to phonological judgments about the same words (Devlin, Matthews, & Rushworth, 2003; Price, Moore, Humphreys, & Wise, 1997). The angular gyrus is often associated with semantic representation (Price, 2000) and greater activation in angular gyri is often observed for concrete relative to abstract words (Binder, Westbury, McKiernan, Possing, & Medler, 2005; Sabsevitz, Medler, Seidenberg, & Binder, 2005). This underscores the role of these regions in concrete feature knowledge. The activation of these temporal-parietal semantic regions is consistent with participants' incorporating critical features into a coherent representation.
When critical features contradicted auxiliary features, processing these features of complex nouns also resulted in significant increases in dorsolateral and anterior prefrontal activity. As discussed previously, the middle frontal gyri have been implicated in a wide variety of executive tasks, and in the context of the current paradigm we believe this activity reflects participants' evaluation of conflicting semantic feature information. In addition to the middle frontal gyrus, we also observed increased activity in left ventral inferior frontal cortex for the C+ stimuli. This is consistent with its involvement in the selection of competing semantic alternatives (Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997) and importance in making semantic decisions (Devlin et al., 2003).
Finally, C+ feature processing in the inconsistent condition resulted in significant increases in anterior and posterior cingulate cortex. Anterior cingulate in particular has been implicated in attentional processes and outcome monitoring (Botvinick, Cohen, & Carter, 2004), processes which fit well within the context of evaluating semantic features that violate a previously-established context. Posterior cingulate and surrounding activity associated with allocation of attention (Gilbert, Simons, Frith, & Burgess, 2006), but it is also often associated with episodic memory retrieval (Cabeza et al., 2003; Nestor, Fryer, Smielewski, & Hodges, 2003). In the current study, episodic memory may be important for the retrieval of specific stimulus properties of the target concept.
We have reported significantly more activation in critical conditions for complex nouns compared to nominal kinds during the processing of critical statements. The critical statements are matched across several psycholinguistic variables between nominal kinds and complex nouns, However, we cannot rule out the possibility that there are other psycholinguistic properties that systematically differ between these two types of nouns. This includes the possibility that due to a sparser category structure, characteristic features may demonstrate less conflict with auxiliary features for nominal kinds than for complex nouns, which in turn might require less mediation by frontal cortex. Consideration of multiple aspects of the representation of these nouns in future studies will prove important in elucidating the specific bases for the observed differences.
It is notable that we observed increased activity in temporal and parietal regions—typically characterized as being associated with semantic content—during semantic context manipulations that induced a change in process. (In this context, we consider “process” to refer to either the selective activation of different parts of an encoded concept, or different manipulation on whatever information is retrieved.) The close relationship between activity in these regions and the type of semantic processing required is certainly not novel. However, we feel it valuable to state what is often implicit in studies of semantic memory: namely, that although “process” and “content” are dissociable cognitive constructs, they do not map neatly onto anatomically discrete regions of cortex. To the contrary, activity in temporal-parietal semantic regions appears to be specifically upregulated in response to very specific task demands (see also Gold et al., 2006).
4.3 Effect of rule- and similarity-based instructions
In the initial investigation of these stimuli there were no differences between participants who received rule- and similarity-based instructions (Grossman et al., 2007), suggesting any differences due to instruction condition would be subtle. Given the modest effects we observed, any conclusions we reach regarding these differences must be viewed as tentative. We found that participants who were given rule-based instructions appeared to rely more on dorsolateral prefrontal regions. These regions may reflect working memory resources needed for applying rules to the decision-making processes and stimuli of the experiment (Bechara, Damasio, Tranel, & Anderson, 1998; D'Esposito, Postle, & Rypma, 2000; Strange, Henson, Friston, & Dolan, 2001). By contrast, participants who received similarity-based instructions relied more on temporal and parietal regions. Although parietal activation is ubiquitous in numerous cognitive tasks (Culham & Kanwisher, 2001), parietal cortex does appear to have a special role in perceptual similarity judgments (Wilkinson, Halligan, Henson, & Dolan, 2002). The conjunction with activity in middle temporal gyrus suggests involvement of association cortices relating to an accumulation of perceptual features.
Although direct neuroimaging evidence regarding categorization strategies is relatively rare, the current results are largely consistent with previous investigations. Several studies support increased involvement of frontal regions during rule-based processing (Grossman et al., 2002; Koenig et al., 2005), although the selectivity of frontal involvement in rule-based processing is not universal (Smith et al., 1998). Increases in posterior temporal and parietal regions have been associated with similarity-based instructions (Koenig et al., 2005). In the context of previous literature, the current results demonstrate that the approach participants bring to a semantic task can influence the regions involved in its completion. This influence appears to operate relatively independently of the specific semantic information being evaluated.
4.4 Conclusions
Our findings lead to three conclusions, all of which support a strongly interactive account of process and content in semantic memory. First, the organization of semantic categories affects the neural processing of semantic features. We specifically show differences between complex nouns and nominal kinds that arise from the nature of the relative importance of individual features to a concept. Second, task demands had a significant effect on feature processing. This was evident most strongly in implicit task demands (evaluating a feature in different semantic contexts), but also in explicit rule- and similarity-based instruction conditions. Finally, we note that semantic context modulated processing in regions typically associated with semantic storage, including left posterior middle temporal gyrus and angular gyrus, suggesting that “process” and “content” in semantic memory do not lend themselves to easy neuroanatomical dissociations.
Acknowledgments
This work was supported in part by NIH grants AG15116, AG17586, and NS54575. We are grateful to Michael Bonner and Jamie Reilly for helpful comments on this manuscript.
Footnotes
Stimuli from an additional nominal kind category of meals were also included, but behavioral data indicated that participants were not treating the intended diagnostic feature as intended, and so they were not included in any analyses reported here.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen SW, Brooks LR. Specializing in the operation of an explicit rule. Journal of Experimental Psychology: General. 1991;120(1):3–19. doi: 10.1037//0096-3445.120.3.278. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Multimodal image coregistration and partitioning - A unified framework. NeuroImage. 1997;6(3):209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, et al. The English Lexicon Project. Behavior Research Methods. 2007;39:445–459. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience. 1998;18(1):428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57(1):289–300. [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience. 2005;17(6):905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Prince SE, Rice HJ, Weissman DH, Nyberg L. Attention-related activity during episodic memory retrieval: a cross-function fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Shelton JR. Domain-specific knowledge systems in the brain: The animate-inanimate distinction. Journal of Cognitive Neuroscience. 1998;10(1):1–34. doi: 10.1162/089892998563752. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. Abstract and concrete concepts have structurally different representational frameworks. Brain. 2005;128:615–627. doi: 10.1093/brain/awh349. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive funcitons in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Experimental Brain Research. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2003;15(1):71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (Area 10) during low-demand tasks. Journal of Experimental Psychology: Human Perception and Performance. 2006;32(1):45–58. doi: 10.1037/0096-1523.32.1.45. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: Functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. Journal of Neuroscience. 2006;26(24):6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Smith EE, Koenig P, Glosser G, DeVita C, Moore P, et al. The neural basis for categorization in semantic memory. NeuroImage. 2002;17:1549–1561. doi: 10.1006/nimg.2002.1273. [DOI] [PubMed] [Google Scholar]
- Grossman M, Troiani V, Koenig P, Work M, Moore P. How necessary are the stripes of a tiger? Diagnostic and characteristic features in an fMRI study of word meaning. Neuropsychologia. 2007;45:1055–1064. doi: 10.1016/j.neuropsychologia.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton JA. Testing the prototype theory of concepts. Journal of Memory and Language. 1995;34:686–708. [Google Scholar]
- Hampton JA. Psychological representation of concepts. In: Conway MA, editor. Cognitive models of memory. Hove: Psychology Press; 1997. [Google Scholar]
- Keil FC. Concepts, kinds, and cognitive development. 1st. Cambridge: MIT Press; 1989. [Google Scholar]
- Keil FC, Batterman N. A characteristic-to-defining shift in the development of word meaning. Journal of Verbal Learning and Verbal Behavior. 1984;23:221–236. [Google Scholar]
- Koenig P, Grossman M. Process and content in semantic memory. In: Hart J Jr, Kraut MA, editors. Neural basis of semantic memory. Cambridge: Cambridge University Press; 2007. pp. 247–264. [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, et al. The neural basis for novel semantic categorization. NeuroImage. 2005;24:369–383. doi: 10.1016/j.neuroimage.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Grossman M. Semantic categorisation of novel objects in frontotemporal dementia. Cognitive Neuropsychology. 2006;23(4):541–562. doi: 10.1080/02643290542000094. [DOI] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, et al. Anatomical traces of vocabulary acquisition in the adolescent brain. Journal of Neuroscience. 2007;27(5):1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Reviews of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Current Opinion in Neurobiology. 2001;11(2):194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nature Reviews Neuroscience. 2003;4:310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- McRae K, de Sa VR, Seidenberg MS. On the nature and scope of featural representations of word meaning. Journal of Experimental Psychology: General. 1997;126(2):99–130. doi: 10.1037//0096-3445.126.2.99. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, Wilson BA, Margins MGM, Iaki S, et al. Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Human Brain Mapping. 2006;27:288–295. doi: 10.1002/hbm.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LJ, Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. Journal of Neuroscience. 2007;27(20):5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer's disease and mild cognitive impairment. Annals of Neurology. 2003;54:343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Patalano AL, Smith EE, Jonides J, Koeppe RA. PET evidence for multiple strategies of categorization. Cognitive, Affective, and Behavioral Neuroscience. 2001;1(4):360–370. doi: 10.3758/cabn.1.4.360. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Grossman M. Language processing in frontotemporal dementia: A brief review. Language and Linguistics Compass. 2008;2(1):18–35. [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197(3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS. Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience. 1997;9(6):727–733. doi: 10.1162/jocn.1997.9.6.727. [DOI] [PubMed] [Google Scholar]
- Rips LJ. Similarity, typicality, and organization. In: Vosniadou S, Ortony A, editors. Similarity and analogical reasoning. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Rosch ER, Mervis CB. Family resemblence: Studies in the internal structure of categories. Cognitive Psychology. 1975;7:573–605. [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. NeuroImage. 2005;27(1):188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Smith EE, Patalano AL, Jonides J. Alternative strategies of categorization. Cognition. 1998;65:167–196. doi: 10.1016/s0010-0277(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Smith EE, Shoben EJ, Rips LJ. Structure and process in semantic memory: A featural model for semantic decisions. Psychological Review. 1974;81(3):214–241. [Google Scholar]
- Smith EE, Sloman SA. Similarity- versus rule-based categorization. Memory and Cognition. 1994;22:377–386. doi: 10.3758/bf03200864. [DOI] [PubMed] [Google Scholar]
- Strange BA, Henson RNA, Friston KJ, Dolan RJ. Anterior prefrontal cortex mediates rule learning in humans. Cerebral Cortex. 2001;11:1040–1046. doi: 10.1093/cercor/11.11.1040. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Wilkinson DT, Halligan PW, Henson RNA, Dolan RJ. The effects of interdistracter similarity on search processes in superior parietal cortex. NeuroImage. 2002;15:611–619. doi: 10.1006/nimg.2001.0993. [DOI] [PubMed] [Google Scholar]