Abstract
Background
Cardiac surgery can result in LV ischemia and reperfusion (I/R), the release of cytokines such as tumor necrosis factor, and oxidative-stress with release of myeloperoxidase. While aprotinin has been used in cardiac surgery, the likely multiple effects of this serine protease inhibitor limits clinical utility. This study tested the hypothesis that different APRO doses cause divergent effects on LV contractility, cytokine release and oxidative stress in the context of I/R.
Methods/Results
LV I/R (30 min I/60 min R) was induced in mice and LV contractility (maximal end-systolic elastance; Emax) determined. Mice were randomized to 2×104 KIU/kg aprotinin (n=11), 4 × 104 KIU/kg aprotinin (n=10), and Vehicle (saline, n=10). Based upon a fluorogenic assay, aprotinin doses of 2 and 4×104 KIU/kg resulted in plasma concentrations similar to those of the half and full Hammersmith doses, respectively. Following I/R, Emax fell by over 40% from baseline (p<0.05), and this effect was attenuated with 2 ×104 KIU/kg but not 4 ×104 KIU/kg aprotinin. Tumor necrosis factor increased by over 60% from control (p<0.05) with I/R, bit was reduced with 4 ×104 KIU/kg aprotinin. Myeloperoxidase increased with I/R, and was reduced to the greatest degree by 2 ×104 KIU/kg aprotinin.
Conclusions
Aprotinin influences LV contractility, cytokine release and oxidative stress which are dose dependent. These results provide mechanistic evidence that multiple pathways are differentially affected by aprotinin in a context relevant to cardiac surgery.
Keywords: aprotinin, cardiac surgery, inflammation, ischemia, reperfusion
Introduction
Cardiac surgery with cardiopulmonary bypass (CPB), often attendant with periods of myocardial ischemia-reperfusion (I/R), can result in a systemic and local inflammatory response, oxidative stress and transient myocardial dysfunction. Aprotinin (APRO), a naturally occurring serine protease inhibitor, has been commonly used in this setting as it has been shown to favorably affect coagulation pathways and reduce blood loss and transfusion requirements.1,2 However, due to the fact that APRO is a non-specific serine protease inhibitor, multiple biological pathways and systems can be affected. The downstream consequences of APRO on these multiple systemic pathways has raised potential concerns about mechanisms of action and ultimately clinical outcomes.3 For example, APRO has been shown to modify various markers of inflammation such as the release of cytokine tumor necrosis factor-α (TNF) and interleukin-6 (IL-6).4, 5 However, these effects are not uniformly observed and may be due to the APRO dose utilized.6 For example, in a clinical study by Engleberger et al, when APRO was added to the CPB priming solution, the degree of TNF receptor activation was unaffected in the early post-CPB period.6 Moreover, past studies have demonstrated that APRO may have myocardial protective effects in the context of I/R.6–10 However, an integrative examination to determine whether and to what degree a mechanistic relationship exists between APRO administration on LV contractile function, cytokine release and oxidative stress with I/R has not been performed. Accordingly, this study was performed in order to test the central hypothesis that using clinically relevant APRO concentrations in an intact murine model of I/R, differential effects on LV contractility, cytokine release and oxidative stress would occur.
Methods
Overview
This study performed an integrative set of measurements in an intact murine model of LV I/R in which the APRO doses were computed to reflect clinical dosing protocols (Hammersmith) and administered prior to the induction of I/R. Plasma APRO concentrations were computed in this animal model in order to confirm that these APRO doses would reflect relevant plasma concentrations encountered clinically when utilizing the half or full Hammersmith APRO dosing protocol. Response variables from this protocol included in-vivo measurements of LV contractility, plasma levels of TNF and IL-6, and in-situ quantitation of oxidative stress by a myeloperoxidase reaction.
Experimental Design
Instrumentation and Protocol
Adult FVB mice (10–16 wk, 24–30 gm) were induced, intubated, and maintained under isoflurane anesthesia (2%). The right carotid was exposed and a pre-calibrated 4-electrode-pressure sensor catheter (1.4 F, SPR-839, Millar Instruments, Houston, TX) was placed in the LV. A left thoracotomy was then performed and a purse-string placed around the left anterior descending artery just distal to the bifurcation of the left main coronary artery using 6.0 Prolene. The ligature was tightened to induce ischemia (30 minutes) and then released for reperfusion (60 minutes). At then end of the reperfusion period, the catheter was removed and blood was collected from the carotid, and decanted plasma stored for subsequent analysis. The heart was then perfused with ice-cold saline, the LV excised and a circumferential section placed in cryogenic freezing medium for histochemical analysis. Six FVB mice served as reference controls for plasma and histological measurements. All animals were treated and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, Washington, DC, 1996).
For the in-vivo study, mice were randomized to one of 3 treatment groups: Vehicle (0.9% saline), 2×104 KIU/kg APRO, or 4 ×104 KIU/kg APRO (KIU-kallikrein inhibitory units). Randomization was performed prior to instrumentation and APRO or vehicle was administered immediately following the completion of baseline measurements. An additional 6 mice were anesthetized and instrumented, but did not undergo I/R, and were used for reference control purposes for the biochemical and histochemical studies. Using this targeted coronary occlusion model, we have demonstrated previously that the relative area at risk is 35% with a coefficient of variation of 5%.11,12 In a preliminary set of studies (n=6), fluorescent microspheres (F-8838, Molecular Probes, 15μm diameter, 7.5×104) of different emission spectra were injected at baseline, at 30 minutes of ischemia, and at 60 minutes of reperfusion by LV injection. Total LV regional myocardial blood flow fell to approximately 50% of baseline values with peak ischemia and returned to within baseline values with reperfusion. Thus, this murine model of coronary artery occlusion provided a transient period of low myocardial blood flow followed by a restoration of blood flow, and therefore allowed for the study of LV function in the context of I/R.
Aprotinin Protocol and Dosing Validation
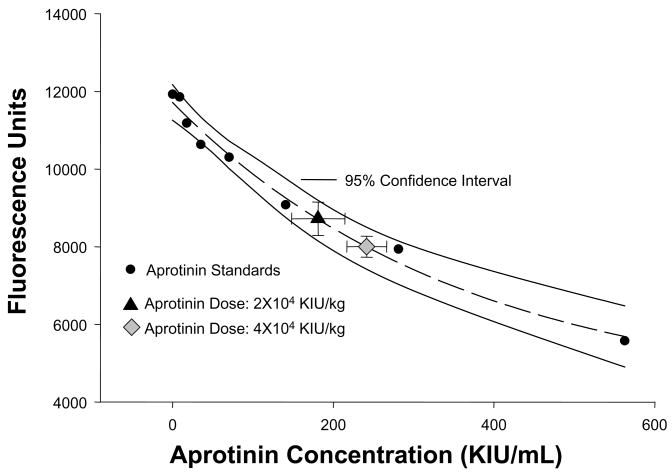
APRO (10,000; KIU/mL) was used at doses of 2 ×104 KIU/kg and 4 ×104 KIU/kg in order to approximate plasma concentrations which reflect APRO plasma concentrations encountered clinically when utilizing the half-Hammersmith and full Hammersmith, doses, respectively.9,10 It has been demonstrated previously that using APRO a 4 ×104 KIU/kg intravenous bolus reached plasma concentrations corresponding to the full Hammersmith clinical dosing protocol.9 Furthermore, this APRO dosing schedule is similar to weight-based dosing regimens used in previous animal models to achieve representative plasma APRO activity.7 Nevertheless, the present study administered APRO in a unique model of I/R and therefore a procedure was developed to measure relative plasma levels of APRO. For this approach, a fluorogenic substrate cleaved by the serine protease plasmin was utilized in an ex-vivo assay system. Specifically, the peptide sequence D-ala-leu-lys-7-amido-4-methylcomarin (Sigma, A8171) at a fixed concentration of 10 nM, was mixed in a reaction buffer containing a 1:33 dilution of normal mouse plasma and incubated at 37degC for 15 min in the presence and absence of 7 ug/mL of plasmin (Sigma, P1876, 3 U/mg). The fluorescence of this reaction was detected in continuous fashion (Fluostar Glaaxy, BMG Labtech, NC) at an excitation/emission wavelength of 365/440 nm. The plasmin substrate, plasmin concentrations, and the incubation conditions were determined from preliminary dilution studies in order to yield peak performance as defined as that which yielded a consistent and stable fluorescence signal. This reaction solution was then incubated in the presence and absence of increasing concentrations of APRO (range 0–560 KIU/mL) in order to generate a standardized enzyme activity-inhibition curve. The standard APRO inhibition curve, which was generated in triplicate, along with the 95% confidence interval for this standard curve is shown in Figure 1. This inhibition curve demonstrated the classical exponential decay, and was subjected to regression analysis yielding a significant relationship between the reduction in fluorescence to APRO concentrations (r=0.99, p<0.001). The intra-assay coefficient of variation was 5% and an inter-assay coefficient was 9%. This APRO enzyme inhibition assay was utilized to extrapolate relative APRO plasma concentrations. Specifically, at the completion of the studies described in the subsequent paragraphs, plasma was prepared and incubated in the substrate/plasmin substrate, the relative fluorescence obtained, input into the standardized APRO inhibition curve, and an APRO concentration computed.
Figure 1.
Relative plasma concentrations of APRO were computed using a fluorogenic assay in which a specific peptide, cleaved by plasmin was utilized. Using fixed concentrations of the plasmin peptide and plasmin, a standardized curve could be generated with increasing concentrations of APRO. The specific reagents and incubation conditions are detailed in the text. An exponential APRO inhibition curve was generated which was then fit to an exponential regression equation. An excellent regression fit was obtained as shown by the dashed lines (y=4071+7643e−0.003x; r2=0.98, p<0.0001), and the 95% confidence interval for the APRO assay is shown by the solid lines. The relative fluorescence and computed plasma APRO concentrations for the 2×104 KIU/kg and the 4×104 KIU/kg doses have been superimposed on the standard curve (error bars are the standard deviation from the estimate).
LV Contractility Measurements
Following initial instrumentation and a 30 min stabilization period, LV pressures and relative volumetric units were continuously recorded using a pressure-conductance unit (ARIA, MPCU-200, Millar) and integrated electrical stimulation (DAQ, PV Analysis Software, Millar). The LV pressure and volume signals were integrated with an ECG signal and digitized (PowerLab, AD instruments, NSW Australia). The placement of the LV conductance catheter, validation procedures, and algorithm have been described previously.11,12 This included correction for parallel conductance and regular in vitro calibration. With continuous recording of the LV pressure-volumetric signal, the ventilator was suspended for several seconds and then restarted which altered venous return, and thereby LV preload. An adequate reduction in venous return was defined as an approximate, transient reduction in LV systolic pressure of 30%. This resulted in a family of LV pressure-volume loops in which definable points of the LV end-systolic pressure-volume relationship could be determined. Isochronal points from the recorded LV pressure-volume loops were used to compute the slope which in turn was used to compute maximal LV elastance (Emax).13 In addition, the isochronal values for LV end-diastolic volume and stroke volume were obtained from the LV pressure-volume relationships and used to compute preload recruitable stroke work (PRSW).
Plasma Analysis
Plasma samples were assayed for TNF and IL-6 using an enzyme linked multiplex suspension array (Luminex #X500002Z15, Bio-Rad, Hercules, CA). The relative fluorescence obtained for each cytokine was converted to an absolute concentration using standards that were included in each assay. The standards for TNF and IL-6 resulted in highly linear calibration curves (r2=0.9979, r2=0.9984, respectively p<0.05), and the sensitivity range for TNF was 0–400 pg/mL and for IL-6; 0–150 pg/mL. The intra-assay coefficient of variation was approximately 15%. In addition, plasma was subjected to a mouse specific, high sensitivity cardiac troponin-I enzyme linked immunoassay (2010-1-HSP, Life Diagnostics, PA).
Myocardial Myeloperoxidase Histochemistry
In order to assess the degree of oxidative stress induced by the I/R protocol, relative content of myeloperoxidase was assessed by immunohistochemistry. LV frozen sections (5 um) were briefly fixed in an ice cold acetone solution for 5 minutes, and washed twice in PBS. The sections were then incubated in a 1:100 dilution of a rabbit polyclonal anti-myeloperoxidase (Abcam AB 535-500) for 1 hour, washed 3 times in PBS, and then incubated with a 1:250 dilution of an anti-rabbit IgG (Vector Labs, 6101). Visualization of the primary antisera binding sites was performed using the 3′,3′-diaminobenzidine-hydrogen peroxide substrate (Vector Labs). Negative controls were utilized in all staining protocols and included substitution with nonimmune anti-sera. The LV free wall was imaged (Axioskop-2, Zeiss) and 5 transmural images at a magnification of 20X (Plan-Neofluar, Zeiss) were digitized (AxioCam MRc, Zeiss). The digitized LV images were quantified using a computer assisted method which has been optimized for histomorphometric measurements.11 Briefly, following background subtraction, the digitized images were set to a 0–100% grey-scale reference, and regions corresponding to a threshold signal of 70% or greater were identified as positive myeloperoxidase staining using a standardized algorithm (SigmaScan Pro 5, SPSS). The positive stained area was divided by LV myocardial sampling area (387×103 um2/field sample) yielding a percent area of myeloperoxidase staining. Reference control LV sections were included in all staining protocols and used to normalize the myeloperoxidase values.
Data Analysis
All of the data collected in this study were coded and the code not broken until the conclusion of the study. The observers for the histological measurements were blinded to the treatment assignments throughout. LV pressure and contractility were compared using a two-way analysis of variance (ANOVA) model. Single point measurements were compared between treatment and control groups using a one-way ANOVA. Following the ANOVA, pair-wise comparisons were performed using unpaired t-tests corrected for the number of comparisons. In order to examine the magnitude of change in LV contractility, with respect to I/R and APRO, the percent change from the respective baseline value was computed. The test statistic was set to an arithmetic value of 0, and comparisons performed using a t-statistic. A similar approach was performed for TNF and MPO where the comparative changes were computed from reference control values. All statistical analyses were performed using the STATA statistical software package (Statacorp, College Station, TX). Values of p<0.05 were considered to be statistically significant.
Results
A total of 40 mice were enrolled in the I/R protocol, with 9 mice dying prior to the final set of measurements. These mice died of arrhythmias during reperfusion, and were equally distributed among the 3 treatment groups (vehicle: n=3, 2 ×104 KIU/kg APRO: n=3, 4 ×104 KIU/kg APRO: n=4; Chi Square analysis, p>;0.70). Thus, the final sample sizes were: vehicle n=10, 2 mL/kg APRO: n=11, 4 mL/kg APRO: n=10). Using the APRO plasma assay and standard curve shown in Figure 1, the computed APRO plasma concentrations for the 2 ×104 KIU/kg APRO group was 180±33 and for the 4 ×104 KIU/kg APRO group was significantly higher 242±25 KIU/mL (p<0.05). Plasma troponin-I values were not detectable in non I/R mouse plasma samples and at the completion of the I/R protocol, were 0.41±0.04 ng/mL in the vehicle group, 0.55±0.22 ng/mL in the 2 mL/kg group and 0.38±0.05 ng/mL in the 4 mL/kg group, with no differences amount groups (ANOVA, p=0.77).
Baseline hemodynamic and LV contractility indices, examined using the statistical approach previously described, were equivalent between all 3 groups at baseline (prior to randomization) and therefore were pooled for data presentation and clarity. Baseline heart rate was 531±6 bpm which is consistent for this murine model under stable, ambient conditions.11,12 Heart rate was continuously monitored during the administration of vehicle or either APRO dose, and remained unchanged from baseline values. At the completion of the I/R protocol, heart rate was slightly lower from baseline in the vehicle group (494±7 bpm, p<0.05) and was similar to baseline in both the 2 and 4 ×104 KIU/kg APRO groups (513±18 and 543±9 bpm, respectively). LV peak systolic pressure fell from baseline (98±2 mmHg) at peak ischemia in the vehicle group and in the 4 ×104 KIU/kg group (85±5 and 85±4 mmHg, p<0.05, respectively), but not in the 2 ×104 KIU/kg group (91±7 mmHg). At 60 min of reperfusion, LV peak systolic pressure returned to within baseline values in all groups.
LV Contractility with I/R: Effects of Aprotinin
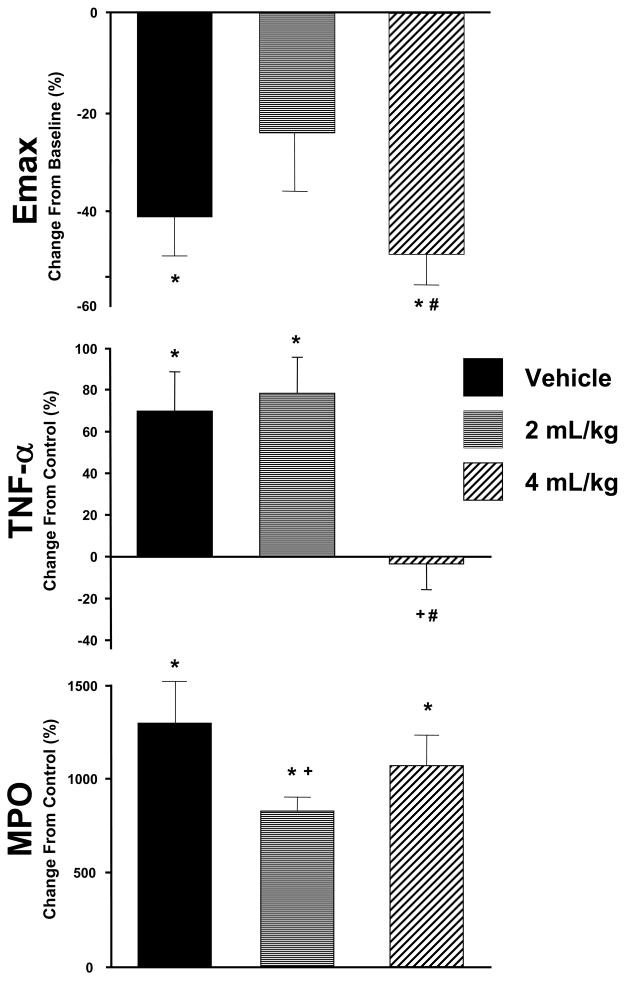
LV contractility as defined by Emax, fell from baseline values (0.78±0.06 mmHg/uL/mg) by approximately 50% at peak ischemia in all groups (p<0.05). While Emax returned to within baseline values after 60 min of reperfusion in the APRO 2 ×104 KIU/kg group (0.59±0.09 mmHg/uL/mg), Emax remained significantly reduced in the vehicle and APRO 4 ×104 KIU/kg groups (0.39±0.05 and 0.45±0.06 mmHg/uL/mg, respectively, p<0.05). The relative changes in Emax at reperfusion as a function of baseline values is shown in Figure 2 and highlights the differences in this index of LV contractility between the vehicle and APRO groups. PRSW, another index of LV contractility, followed similar trends with respect to I/R and APRO. Specifically, PRSW was significantly reduced from baseline values (0.85±0.16 mmHg/mg) following I/R in the vehicle and APRO 4 ×104 KIU/kg groups (0.39±0.07 and 0.48±0.11 mmHg/mg, respectively, p<0.05) but was similar to baseline in the APRO 2 ×104 KIU/kg group (0.78±0.16 mmHg/mg).
Figure 2.
(TOP) The relative change in Emax, an index of LV contractility, was computed as a percent of baseline values following I/R. A significant reduction in Emax occurred in the vehicle (n=10) and 4×104 KIU/kg APRO group (n=10). However, this depression in LV contractile function was ameliorated in the 2×104 KIU/kg APRO group (n=11). (MIDDLE) Plasma levels for the cytokine TNF were increased following I/R in both the vehicle and 2×104 KIU/kg APRO groups when computed as a percent change to reference control values. This I/R induced TNF release was abrogated in the 4 ×104 KIU/kg APRO group. (BOTTOM) Myeloperoxidase (MPO) content, as assessed by immunohistochemistry, was increased significantly from control values in all I/R groups when compared to reference control values. However, MPO levels were reduced from vehicle values in the 2 ×104 KIU/kg APRO group. Absolute values for these indices are provided in the Results. (*p<0.05 vs respective baseline/referent control; +p<0.05 vs vehicle; #p<0.05 vs 2 ×104 KIU/kg APRO)
Cytokine Release with I/R: Effects of Aprotinin
Reference control plasma levels for the cytokine TNF were 24.5±1.8 pg/mL and were not detectable for IL-6. Following I/R, plasma TNF levels were increased by approximately 2-fold in the vehicle and 2 ×104 KIU/kg APRO groups (41.5±4.6, 43.6±4.3 pg/mL, respectively, p<0.05), but remained within normal reference values in the 4 mL/kg group (24.4±3 pg/mL). The relative changes in TNF levels as a function of reference control value are shown in Figure 2. With respect to IL-6, a strong signal was detected in the vehicle group (471±56 pg/mL, p<0.05 vs a referent control value of 0). IL-6 values were similar to vehicle values in both the 2 and ×104 KIU/kg APRO groups following I/R (532±201, 467±96 pg/mL, respectively).
Myocardial Myeloperoxidase with I/R: Effects of Aprotinin
Representative histochemical staining for MPO is shown in Figure 3 for reference controls, following I/R in the vehicle and APRO groups. While minimal MPO staining could be detected in reference control LV sections, a significant increase in MPO staining occurred following I/R and these relative changes are summarized in Figure 2. While MPO staining was increased in both APRO groups, the relative levels were reduced significantly in the 2 ×104 KIU/kg group. Upon inspection of higher power images, differences in the relative distribution of MPO staining could be appreciated between the vehicle and the 2 ×104 KIU/kg APRO group (Figure 3). Specifically, MPO staining appeared to be associated with interstitial cells in all I/R groups, most probably inflammatory cells and macrophages. However, MPO staining was diffusely distributed in and around these interstitial cells in the vehicle group whereas MPO staining was more punctuate and localized to intracellular compartments in the 2 ×104 KIU/kg group.
Figure 3.
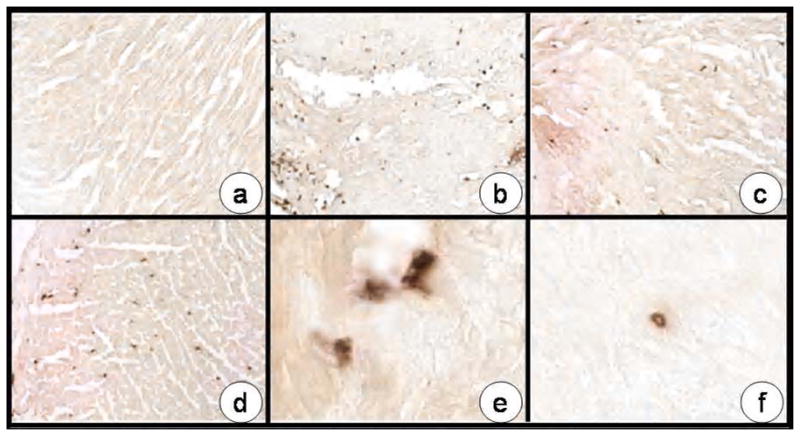
Representative photomicrographs of LV sections stained for myeloperoxidase (MPO) taken from: (a) reference control; (b) following I/R in the vehicle only group; (c) in the 2 ×104 KIU/kg APRO group; and (d) in the 4 ×104 KIU/kg APRO group. A significant and robust increase in MPO staining was observed in the vehicle group and while evident in the APRO groups, appeared reduced. Quantitative results are presented in Figure 2. Higher power images of an LV section taken from the vehicle group revealed an egress of MPO staining from interstitial cells (e) whereas in the 2 ×104 KIU/kg APRO group, MPO staining appeared to be confined to the intracellular compartment of these interstitial cells (f). Original magnification: a–d: 20X, e,f: 63X.
Discussion
Up until recently, a major clinical indication for utilizing aprotinin (APRO) in cardiac surgery was for hemostatic purposes. Past clinical and basic science reports have demonstrated that this serine protease inhibitor may have multiple effects in the context of myocardial ischemia-reperfusion (I/R) injury.4–7 However, the majority of these past studies have either focused upon a single biological event, such as cytokine release, or a functional response such as LV function. The present study addressed this issue through a set of integrative measurements in an intact murine model of I/R in which clinically relevant APRO doses were utilized. The new and unique findings of the present study were 2-fold. First, using an APRO dose that achieved plasma concentrations consistent with a half-Hammersmith clinical dosing regimen (2 ×104 KIU/kg), LV contractility was improved and a determinant of oxidative stress reduced following I/R. Second, APRO utilized at an APRO dose which achieved plasma concentrations similar to a full-Hammersmith clinical dose (4 ×104 KIU/kg) completely abrogated the release of the cytokine, tumor necrosis factor (TNF), but failed to provide protective effects on indices of LV contractile function or oxidative stress. These results further demonstrate the multiple effects of APRO in the context of I/R and that these effects are not uniform across dosing regimens.
Aprotinin and LV Function
Past studies have examined the effects of APRO in intact animal systems with respect to LV systolic performance.7,14,15 In these studies, utilizing a full Hammersmith APRO dose (4 ×104 KIU/kg), protective effects on regional and global systolic function have been demonstrated following brief periods of ischemia. However, these past studies did not serially assess LV pressure-volume relationships in order to determine LV contractility and different APRO doses were not incorporated into the study design. The present study demonstrated that despite a recovery in LV pressure development with I/R, intrinsic defects in myocardial contractility were present and could be ameliorated with a low APRO dose (2 ×104 KIU/kg), but not with a 2-fold higher dose of APRO. In order to more carefully examine the relevance of the APRO dosing paradigm used in the present study, actual plasma APRO calculations were also performed. This is a unique aspect of this study, and to our knowledge is the first time that plasma APRO measurements have been directly assayed in an experimental model of I/R, rather than inferred. In a clinical study, Beath and colleagues utilized an ex-vivo approach to compute relative plasma APRO concentrations in patients undergoing cardiac surgery requiring cardiopulmonary bypass.16 In this past study, the APRO plasma concentrations obtained in patients receiving either the full or half Hammersmith dose, at the time immediately following separation from cardiopulmonary bypass, were very similar to those obtained in the present study. However, it must be recognized that the volume of distribution, pharmacokinetics and serine protease inhibitory profiles are likely to be different in the murine system than that of man. Nevertheless, the present study demonstrated that at different plasma APRO concentrations, which are achieved in clinical applications, impart differential effects on LV contractility and cytokine release.
Aprotinin and Cytokines
It is well established that myocardial I/R is accompanied by the activation and subsequent release of a number of inflammatory mediators including TNF and the interleukins such as IL-6. While the effects of TNF and IL-6 are mediated through specific cognate receptors, the downstream consequences include the induction of a cascade of biological signaling molecules and the formation of mediators of oxidative stress. The exposure of the myocardium to TNF can result in a duality of responses with respect to contractile function and structure.17,18 Specifically, acute and robust exposure of the myocardium to TNF can elicit a positive effect on contractile function whereas prolonged exposure can result in diminished myocardial performance. Proteases, such as serine proteases contribute to the amplification and processing of cytokines, and therefore APRO has been demonstrated to attenuate cytokine release in the context of I/R.4–6 TNF is produced in a membrane bound form and requires proteolytic processing by specific proteases such as TNF convertase (TACE or ADAM-17) to yield a soluble TNF which can interact with the TNF receptor complex.17,18 Moreover, TACE in and of itself requires proteolytic processing to attain full catalytic activity.19 In light of the fact that APRO is a non-selective serine protease inhibitor, it is likely that APRO modulates the signaling cascades which culminate in TNF expression and activation. It has been well established that APRO affects the activity of proteolytic pathways and systems in a concentration dependent manner.20–22 For example, at very low concentrations (<100 KIU/mL) plasmin activity is inhibited, but at twice this concentration (>200 KIU/mL) other proteases such as kallikrein and elastase can be inhibited. Since these APRO concentrations can be easily reached and often exceeded using a full Hammersmith dosing protocol, then it is likely that a number of proteolytic pathways can be affected.
Aprotinin and Oxidative Stress
An important downstream consequence of I/R and the elaboration of mediators of inflammation is the formation of reactive oxygen species such as myeloperoxidase (MPO). With I/R an influx of inflammatory cells occur, most notably neutrophils and this infiltration occurs early with I/R and is considered to be a cellular hallmark of myocardial injury and dysfunction.20,21 Closely integrated with this cellular infiltrate is the formation and release of MPO.22,23 In the present study, increased myocardial MPO occurred following I/R consistent with the activation of inflammatory cells.20,21 The low dose of APRO (2 ×104 KIU/kg) reduced the relative content of MPO, suggesting a modification of inflammatory cell recruitment/activation with I/R. One potential mechanism for this effect is that this low dose of APRO appeared to prevent the release of MPO from preformed stores contained within interstitial inflammatory cells. The present study provides new evidence to suggest that the modification of MPO by APRO may not be entirely due to the suppression of cytokines.
Summary and Limitations
APRO has been historically recognized for improving hemostasis during and after cardiac surgery requiring cardiopulmonary bypass and up until recently formed an important pharmacological tool in the post-surgical armamentarium. However, since APRO is a serine protease inhibitor, then multiple biological pathways can be affected, some of which may be detrimental to specific physiological processes.3 The present study builds upon the current body of knowledge regarding APRO by demonstrating that myocardial specific effects such as contractile function and oxidative stress can be achieved without affecting more global processes such as systemic TNF release. These observations may hold clinical relevance with respect to more selective dosing strategies of APRO and potentially reducing undesired non-cardiac effects. Specifically, the present study utilized APRO dosing strategies which achieved plasma concentrations consistent with those obtained in clinical studies using either the half or full Hammersmith protocols, distinctly different responses were acheived. Using an APRO dose of 2 ×104 KIU/kg, which achieved plasma concentrations consistent with a half-Hammersmith dose, local myocardial effects were achieved following a period of I/R. In contradistinction, a 2-fold higher dose of APRO (4×104 KIU/kg), which achieved plasma concentrations consistent with a full Hammersmith dose, reduced systemic cytokine release, but did not provide an improvement in LV contractility. However, extending these findings to the clinical context must be done with caution and in light of inherent study limitations. First, this was an acute I/R study, and therefore the longer term effects of APRO on myocardial contractility, cytokine release and oxidative stress remain to be determined. Second, a limited number of cytokines and markers of oxidative stress were evaluated and how APRO may affect a larger portfolio of inflammatory markers and determinants of oxidative stress remain unknown. Third, while the present study measured relative APRO concentrations and utilized a clinically applicable dosing regimen, the measurements were performed in a murine model which did not simulate cardiopulmonary bypass per se, and therefore clinical extension of these plasma APRO concentrations must be done with caution. The mouse was utilized in the present investigation since we have developed a means to measure LV contractility in this model and the mouse has been demonstrated to possess a cytokine cascade similar to humans.24,25 Moreover, murine transgenic constructs exist in which the TNF pathway has been genetically altered.18,27 These TNF transgenic constructs could then be utilized in future studies in order to identifying the mechanistic underpinnings for the effects of APRO in the context of I/R. These mechanistic studies regarding APRO are either not feasible or problematic in a clinical context. The results of the present study underscore the importance of identifying the basic mechanisms of action and pathways affected by APRO in order to address the current clinical controversies and questions which surround the use of this pharmacological approach.3, 24
Acknowledgments
National Heart, Lung, and Blood Institute Grants PO1-HL-48788 (FG Spinale), RO1-HL-59165 (FG Spinale), Research Service of the Department of Veterans Affairs (FG Spinale), Research Fellowships from Bayer Pharmaceuticals (MD McEvoy), and the Foundation for Anesthesia Education and Research (MD McEvoy).
List of Abbreviations
- APRO
aprotinin
- Emax
Maximal LV elastance
- IL-6
interleukin-6
- I/R
ischemia/reperfusion
- MPO
myeloperoxidase
- PRSW
preload recruitable stroke work
- TNF
tumor necrosis factor-α
References
- 1.Sedrakyan A, Treasure T, Elefteriades JA. Effect of aprotinin on clinical outcomes in coronary artery bypass graft surgery: a systematic review and meta-analysis of randomized clinical trials. J Thorac Cardiovasc Surg. 2004;128(3):442–8. doi: 10.1016/j.jtcvs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, Cromheecke ME, de Jonge E, Prins MH, de Mol BJ, Briet E, Buller HR. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet. 1999;354(9194):1940–7. doi: 10.1016/S0140-6736(99)01264-7. [DOI] [PubMed] [Google Scholar]
- 3.Mangano DT, I, Tudor C, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354(4):353–65. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 4.Bull DA, Maurer J. Aprotinin and preservation of myocardial function after ischemia-reperfusion injury. Ann Thorac Surg. 2003;75(2):S735–9. doi: 10.1016/s0003-4975(02)04702-1. [DOI] [PubMed] [Google Scholar]
- 5.Tassani P, Augustin N, Barankay A, Braun SL, Zaccaria F, Richter JA. High-dose aprotinin modulates the balance between proinflammatory and anti-inflammatory responses during coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2000 Dec;14(6):682–6. doi: 10.1053/jcan.2000.18328. [DOI] [PubMed] [Google Scholar]
- 6.Englberger L, Kipfer B, Berdat PA, Nydegger UE, Carrel TP. Aprotinin in coronary operation with cardiopulmonary bypass: does “low-dose” aprotinin inhibit the inflammatory response? Ann Thorac Surg. 2002;73(6):1897–904. doi: 10.1016/s0003-4975(02)03535-x. [DOI] [PubMed] [Google Scholar]
- 7.Khan TA, Bianchi C, Voisine P, Feng J, Baker J, Hart M, Takahashi M, Stahl G, Sellke FW. Reduction of myocardial reperfusion injury by aprotinin after regional ischemia and cardioplegic arrest. J Thorac Cardiovasc Surg. 2004;128(4):602–8. doi: 10.1016/j.jtcvs.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 8.Lazar HL, Bao Y, Tanzillo L, O’Gara P, Reardon D, Price D, Crowley R, Cabral HJ. Aprotinin decreases ischemic damage during coronary revascularization. J Card Surg. 2005 Nov-Dec;20(6):519–23. doi: 10.1111/j.1540-8191.2005.00136.x. [DOI] [PubMed] [Google Scholar]
- 9.Royston D, Cardigan R, Gippner-Steppert C, Jochum M. Is perioperative plasma aprotinin concentration more predictable and constant after a weight-related dose regimen? Anesth Analg. 2001;92(4):830–6. doi: 10.1097/00000539-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 10.van Oeveren W, Jansen NJ, Bidstrup BP, Royston D, Westaby S, Neuhof H, Wildevuur CR. Effects of aprotinin on hemostatic mechanisms during cardiopulmonary bypass. Ann Thorac Surg. 1987;44(6):640–5. doi: 10.1016/s0003-4975(10)62153-4. [DOI] [PubMed] [Google Scholar]
- 11.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284(1):H364–71. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 12.Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol. 2005 Jan;288(1):H149–58. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 13.Suga H, Sagawa K. Mathematical interrelationship between instantaneous ventricular pressure-volume ratio and myocardial force-velocity relation. Ann Biomed Eng. 1972;1(2):160–81. doi: 10.1007/BF02584205. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy RJ, Tuman KJ, O’Connor C, Ivankovich AD. Aprotinin pretreatment diminishes postischemic myocardial contractile dysfunction in dogs. Anesth Analg. 199989(5):1096–100. [PubMed] [Google Scholar]
- 15.Khan TA, Bianchi C, Voisine P, Feng J, Baker J, Hart M, Takahashi M, Stahl G, Sellke FW. Reduction of myocardial reperfusion injury by aprotinin after regional ischemia and cardioplegic arrest. J Thorac Cardiovasc Surg. 2004;128(4):602–8. doi: 10.1016/j.jtcvs.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Beath SM, Nuttall GA, Fass DN, Oliver WC, Jr, Ereth MG, Oyen LJ. Plasma aprotinin concentrations during cardiac surgery: full- versus half-dose regimens. Anesth Analg. 2001;91:257–264. doi: 10.1097/00000539-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95(12):1140–53. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 18.Mann DL. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu Rev Physiol. 2003;65:81–101. doi: 10.1146/annurev.physiol.65.092101.142249. [DOI] [PubMed] [Google Scholar]
- 19.Srour N, Lebel A, McMahon S, Fournier I, Fugere M, Day R, Dubois CM. TACE/ADAM-17 maturation and activation of sheddase activity require proprotein convertase activity. FEBS Lett. 2003;554(3):275–83. doi: 10.1016/s0014-5793(03)01159-1. [DOI] [PubMed] [Google Scholar]
- 20.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- 21.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100(5):2700–5. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112(18):2812–20. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158(3):879–91. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Heart Association. Advisory: Bayer suspends marketing of aprotinin (Trasylol) at FDA request. November 2007 Web site: http://www.informz.net/heart/archives/archive_518051.html.
- 25.Han SN, Meydani SN. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans. J Infect Dis. 2000 Sep;182(Suppl 1):S74–80. doi: 10.1086/315915. [DOI] [PubMed] [Google Scholar]
- 26.Vallejo JG, Nemoto S, Ishiyama M, Yu B, Knuefermann P, Diwan A, Baker JS, Defreitas G, Tweardy DJ, Mann DL. Functional significance of inflammatory mediators in a murine model of resuscitated hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2005 Mar;288(3):H1272–7. doi: 10.1152/ajpheart.01003.2003. [DOI] [PubMed] [Google Scholar]
- 27.Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, Wilson EM, Spinale FG, Mann DL. Targeted overexpression of noncleavable and secreted forms of tumor necrosis factor provokes disparate cardiac phenotypes. Circulation. 2004 Jan 20;109(2):262–8. doi: 10.1161/01.CIR.0000109642.27985.FA. [DOI] [PubMed] [Google Scholar]