Abstract
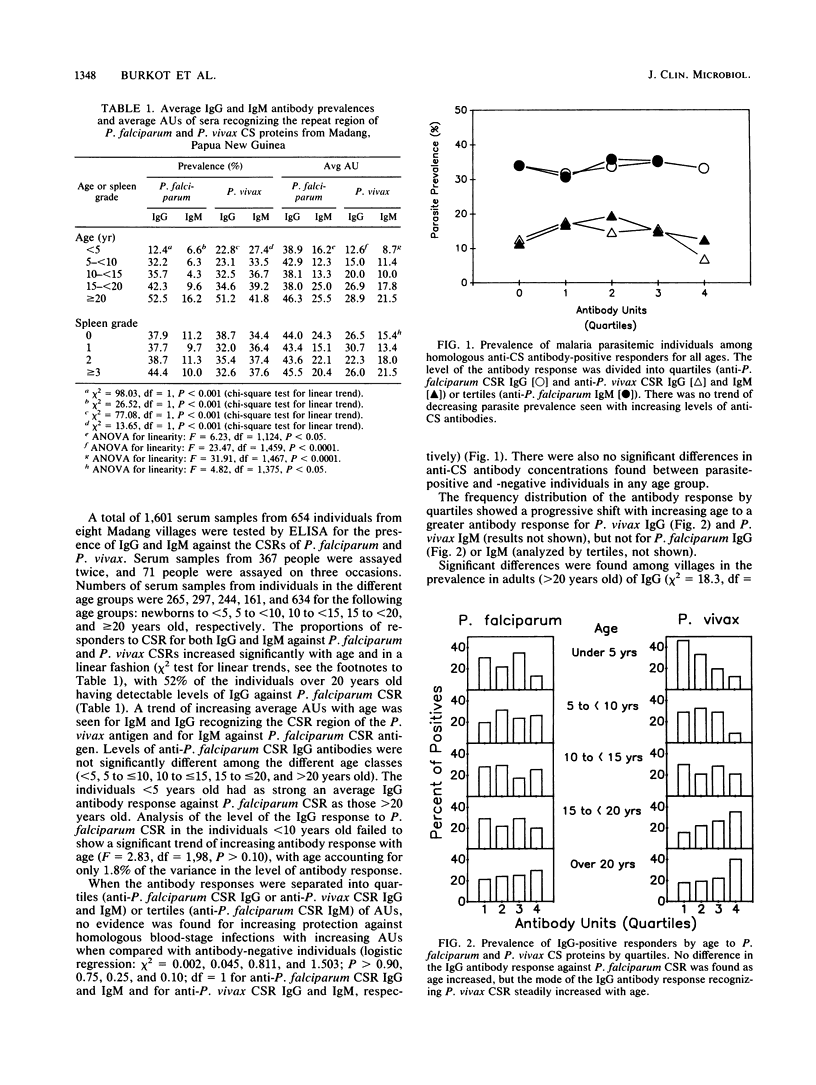
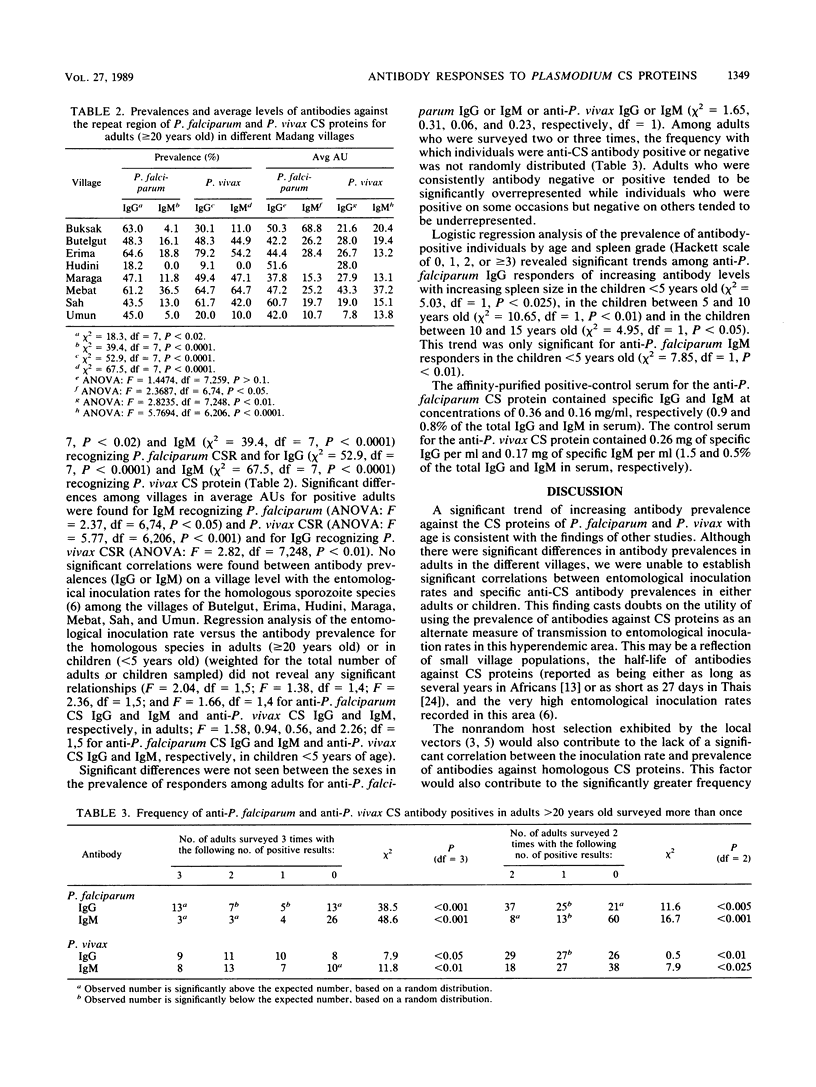
Papua New Guineans exposed to hyperendemic malaria in the Madang area showed different antibody responses to Plasmodium falciparum and Plasmodium vivax sporozoites despite comparable entomological inoculation rates. Although there was a significant trend of increasing prevalence of anti-P. falciparum circumsporozoite (CS) protein immunoglobulin G (IgG) with age, there was no significant increase in the antibody units of IgG recognizing P. falciparum CS proteins. Antibodies recognizing P. vivax CS proteins steadily increased in prevalence and antibody units with age. Significant trends of increasing prevalence of antibody responders (both IgG and IgM) with increasing splenic enlargement were found in the younger age groups for P. falciparum CS proteins but not for P. vivax CS proteins. When antibody responders were analyzed by quartiles, there was a trend of increasing antibody response with age against P. vivax CS peptide, but not for P. falciparum CS protein. There was no evidence for increasing protection against blood-stage infections with increasing antibody levels for either P. falciparum or P. vivax. Neither were any significant relationships found between entomological inoculation rates and either CS antibody prevalence or concentration among the villages studied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Burkot T. R., Graves P. M., Cattan J. A., Wirtz R. A., Gibson F. D. The efficiency of sporozoite transmission in the human malarias, Plasmodium falciparum and P. vivax. Bull World Health Organ. 1987;65(3):375–380. [PMC free article] [PubMed] [Google Scholar]
- Burkot T. R., Graves P. M., Paru R., Lagog M. Mixed blood feeding by the malaria vectors in the Anopheles punctulatus complex (Diptera: Culicidae). J Med Entomol. 1988 Jul;25(4):205–213. doi: 10.1093/jmedent/25.4.205. [DOI] [PubMed] [Google Scholar]
- Burkot T. R., Graves P. M., Paru R., Wirtz R. A., Heywood P. F. Human malaria transmission studies in the Anopheles punctulatus complex in Papua New Guinea: sporozoite rates, inoculation rates, and sporozoite densities. Am J Trop Med Hyg. 1988 Aug;39(2):135–144. doi: 10.4269/ajtmh.1988.39.135. [DOI] [PubMed] [Google Scholar]
- Burkot T. R. Non-random host selection by anopheline mosquitoes. Parasitol Today. 1988 Jun;4(6):156–162. doi: 10.1016/0169-4758(88)90151-2. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Brandling-Bennett A. D., Roberts J. M., Collins F. H., Kaseje D. C., Barber A. M., Turner A. Detection of antibodies in human sera to the repeating epitope of the circumsporozoite protein of Plasmodium falciparum using the synthetic peptide (NANP)3 in an enzyme-linked immunosorbent assay (ELISA). Am J Trop Med Hyg. 1987 Jul;37(1):17–21. doi: 10.4269/ajtmh.1987.37.17. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Collins F. H., Brandling-Bennett A. D., Schwartz I. K., Roberts J. M. Age-specific prevalence of antibody to a synthetic peptide of the circumsporozoite protein of Plasmodium falciparum in children from three villages in Kenya. Am J Trop Med Hyg. 1987 Sep;37(2):220–224. doi: 10.4269/ajtmh.1987.37.220. [DOI] [PubMed] [Google Scholar]
- Cattani J. A., Tulloch J. L., Vrbova H., Jolley D., Gibson F. D., Moir J. S., Heywood P. F., Alpers M. P., Stevenson A., Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am J Trop Med Hyg. 1986 Jan;35(1):3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Favaloro J. M., Crewther P. E., Burkot T. R., Bianco A. E., Stahl H. D., Kemp D. J., Anders R. F., Brown G. V. A blood stage antigen of Plasmodium falciparum shares determinants with the sporozoite coat protein. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5121–5125. doi: 10.1073/pnas.82.15.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Del Giudice G., Engers H. D., Tougne C., Biro S. S., Weiss N., Verdini A. S., Pessi A., Degremont A. A., Freyvogel T. A., Lambert P. H. Antibodies to the repetitive epitope of Plasmodium falciparum circumsporozoite protein in a rural Tanzanian community: a longitudinal study of 132 children. Am J Trop Med Hyg. 1987 Mar;36(2):203–212. doi: 10.4269/ajtmh.1987.36.203. [DOI] [PubMed] [Google Scholar]
- Druilhe P., Pradier O., Marc J. P., Miltgen F., Mazier D., Parent G. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfection. Infect Immun. 1986 Aug;53(2):393–397. doi: 10.1128/iai.53.2.393-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F., Lombardi S., Modiano D., Zavala F., Reeme J., Lamizana L., Coluzzi M., Nussenzweig R. S. Prevalence and levels of antibodies to the circumsporozoite protein of Plasmodium falciparum in an endemic area and their relationship to resistance against malaria infection. Trans R Soc Trop Med Hyg. 1988;82(6):827–832. doi: 10.1016/0035-9203(88)90007-7. [DOI] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S. L., Oster C. N., Plowe C. V., Woollett G. R., Beier J. C., Chulay J. D., Wirtz R. A., Hollingdale M. R., Mugambi M. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987 Aug 7;237(4815):639–642. doi: 10.1126/science.3299709. [DOI] [PubMed] [Google Scholar]
- Hoffman S. L., Wistar R., Jr, Ballou W. R., Hollingdale M. R., Wirtz R. A., Schneider I., Marwoto H. A., Hockmeyer W. T. Immunity to malaria and naturally acquired antibodies to the circumsporozoite protein of Plasmodium falciparum. N Engl J Med. 1986 Sep 4;315(10):601–606. doi: 10.1056/NEJM198609043151001. [DOI] [PubMed] [Google Scholar]
- Marsh K., Hayes R. H., Carson D. C., Otoo L., Shenton F., Byass P., Zavala F., Greenwood B. M. Anti-sporozoite antibodies and immunity to malaria in a rural Gambian population. Trans R Soc Trop Med Hyg. 1988;82(4):532–537. doi: 10.1016/0035-9203(88)90495-6. [DOI] [PubMed] [Google Scholar]
- Mellouk S., Mazier D., Druilhe P., Berbiguier N., Danis M. In vitro and in vivo results suggesting that anti-sporozoite antibodies do not totally block Plasmodium falciparum sporozoite infectivity. N Engl J Med. 1986 Sep 4;315(10):648–648. doi: 10.1056/NEJM198609043151016. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig R. S., McGregor I. A., Bryan J. H. Antibodies to sporozoites: their frequent occurrence in individuals living in an area of hyperendemic malaria. Science. 1979 Nov 2;206(4418):597–599. doi: 10.1126/science.386511. [DOI] [PubMed] [Google Scholar]
- Pang L. W., Limsomwong N., Karwacki J., Webster H. K. Circumsporozoite antibodies and falciparum malaria incidence in children living in a malaria endemic area. Bull World Health Organ. 1988;66(3):359–363. [PMC free article] [PubMed] [Google Scholar]
- Tapchaisri P., Asavanich A., Limsuwan S., Tharavanij S., Harinasuta K. T. Antibodies against malaria sporozoites in patients with acute uncomplicated malaria and patients with cerebral malaria. Am J Trop Med Hyg. 1985 Sep;34(5):831–836. doi: 10.4269/ajtmh.1985.34.831. [DOI] [PubMed] [Google Scholar]
- Tapchaisri P., Chomcharn Y., Poonthong C., Asavanich A., Limsuwan S., Maleevan O., Tharavanij S., Harinasuta T. Anti-sporozoite antibodies induced by natural infection. Am J Trop Med Hyg. 1983 Nov;32(6):1203–1208. doi: 10.4269/ajtmh.1983.32.1203. [DOI] [PubMed] [Google Scholar]
- Webster H. K., Boudreau E. F., Pang L. W., Permpanich B., Sookto P., Wirtz R. A. Development of immunity in natural Plasmodium falciparum malaria: antibodies to the falciparum sporozoite vaccine 1 antigen (R32tet32). J Clin Microbiol. 1987 Jun;25(6):1002–1008. doi: 10.1128/jcm.25.6.1002-1008.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]


