INTRODUCTION
Gait variability, defined as the fluctuation in gait characteristics between steps, is low during walking [1]. However, increased or decreased variability is commonly reported in populations with gait abnormalities, such as elderly fallers [2, 3], older frail adults [4] and individuals with neuro-degenerative diseases (e.g, Parkinson’s disease) [5, 6]. This suggests that gait variability is associated with gait impairments. Increased gait variability has been related to balance impairments leading to falls [7]. Similarly, central nervous system impairments (such as cognitive functioning and motor performance) have been related to increased stance time variability [8], while decreased step width variability has been related to sensory impairments and balance deficits during walking [3, 8, 9]. Gait variability is also suggested to predict motor disability [8]. Therefore, current evidence suggests that gait variability is related to walking impairments and can be used as a quantifiable biomechanical marker to evaluate impaired motor performance.
Measures of gait variability may provide a sensitive assessment of the neuromotor performance reflective of additional aspects of impaired walking, beyond those commonly characterized using average gait data [10]. However, it is not known if variability in paretic gait differs from non-paretic gait. Furthermore, it is not known if gait variability relates to the severity of hemiparesis or to other measures of impaired hemiparetic performance (like low dynamic index scores and asymmetrical stepping).
Therefore, the purpose of this study was to 1) evaluate whether gait variability differs in individuals with post-stroke hemiparesis compared to healthy individuals of similar age and 2) determine if gait variability is indicative of impaired walking performance by investigating the association between gait variability and clinical measures of impaired hemiparetic performance.
METHODS
Participants
Ninety-four participants (Age = 61.4 ± 11.4 years, 69 men, 51 left-side hemiparesis, Walking speed = 0.63 ± 0.32 m/s) with chronic hemiparesis and twenty-two similarly aged healthy subjects (Age = 66.2 ± 10.0 years, 6 men, Walking speed (1.29 ± 0.21 m/s) participated in this study. Seventy participants with hemiparesis and the healthy control subjects were part of an ongoing study at the Rehabilitation Research & Development Center at VA Palo Alto Medical Center. Twenty-four participants with hemiparesis had participated in a larger gait study at the BRRC at Malcom Randall VA Medical Center and unreported data from these participants were retrospectively used for analyses. All participants provided informed consent and the study protocol was approved by the appropriate Institutional Review Boards.
Participants were at least 6 months post-stroke, had unilateral weakness, could walk 10 m in 50 seconds or less without assistance by another person and had no severe perceptual, cognitive or cardiovascular impairment that could affect walking. Subjects were excluded if they had other neurological conditions in addition to stroke, had suffered more than one cerebrovascular accident, or were unable to provide informed consent.
Experimental protocol
Participants walked at their self-selected speeds over an instrumented walkway (GAITRite) to record spatiotemporal step characteristics. Participants began walking 2m in front of the GAITRite, continued walking 2m after the mat (overall distance ~ 10m) to reach constant speed, and used their assistive devices (if any) during walking. All participants completed at least two walking trials (average = 3.4, range = 2 – 5 trials). The number of trials varied across some participants because a) many participants were unable to walk more than 2 trials due to fatigue and low functional level, and b) some participants walked fast and completed a larger number of trials (4 – 5).
Hemiparetic performance was evaluated using step length asymmetry index and clinical assessments. The asymmetry in step length during hemiparetic walking was evaluated by the Paretic Step Ratio (PSR), which is calculated as Paretic step length/ (Paretic + Non-paretic step length) and expressed as a percentage. Asymmetry in the participants with hemiparesis was characterized based on symmetry ranges calculated from the control and asymmetric groups and defined as follows: “Longer” paretic steps than non-paretic (PSR > 0.525), “Shorter” paretic steps than non-paretic (PSR < 0.475) and “Symmetric” step lengths (0.475 ≤ PSR ≤ 0.525).
Clinical assessment in sub-sets of the population was available for analysis. Eighty-one study participants underwent Lower-extremity Fugl-Meyer (LE-FM) evaluations, which is a valid [12] and reliable [13] scale to evaluate hemiparetic severity. Only synergy items (22-point) of LE-FM were utilized to grade hemiparetic severity, as in earlier studies [14] (severe=0 – 14, moderate=15 – 18 and mild=19 – 22). The dynamic gait index (DGI) evaluated dynamic balance in thirty-nine study participants. DGI rates performance of 8 walking-related tasks on an ordinal scale (0-3) and is valid and reliable to evaluate dynamic balance in ambulatory individuals with chronic stroke [15]. Balance performance on DGI was graded as: ≤ 19 (poor), > 19 (good) [16]. Only subsets were available for LE-FM and DGI assessments since a) only participants in the BRRC facility underwent DGI assessments and b) since participants in the BRRC study were part of a larger gait study, some participants were unable to complete these clinical assessments due to insufficient time.
Data analyses
All collected footfalls from all trials were analyzed. The average number of footfalls collected and analyzed per subject was 25 steps (range = 12 – 42 steps) for the hemiparetic population and 13 steps (range = 9 - 20 steps) for the control population. For the same average number of trials, three participants with hemiparesis took more steps than other study participants (48, 50 and 65 steps). The results did not alter when these participants were excluded from the analysis. Therefore, their data were included in the study. The number of steps collected and analyzed in this study was similar to that reported in earlier series [3, 8, 9, 20-23] assessing step variability and the relationship of the spatiotemporal variability to falls risk, CNS impairments, gait speed and motor disability [3, 8, 22, 24].
Swing time, pre-swing (terminal double-support) time, stride time, step length and stride width were selected for analysis based on the literature, which supports their importance in evaluating walking impairments post-stroke [25, 26]. Furthermore, there is evidence that the chosen variables can reveal meaningful conclusions on walking impairments when used in gait variability studies of clinically relevant populations [7, 17, 27]. Stance and step time were not calculated since the aim was also to select independent variables for analyses. Variability was quantified using the standard deviation in spatiotemporal characteristics across steps.
Statistical analyses
The Kolomogorov-Smirnov tests revealed deviations from normality in the spatiotemporal characteristics of participants with hemiparesis. To allow for the use of parametric statistical processing, the data were log10 transformed to achieve normality. Dependent t-tests revealed that there was no difference in variability between legs in the control subjects when assessing step length, swing and pre-swing time (p > .01). Therefore, a one-way ANOVA tested differences in step length, swing and pre-swing time variability between paretic, non-paretic and healthy (left) legs. For stride time and stride width, independent t-tests were conducted to detect differences between population groups (hemiparetic and controls).
To test differences in variability across severity and PSR groups a 3 (group) × 2 (leg) Mixed ANOVA (repeated on leg factor) was conducted for step length, swing time, pre-swing time variability and a one-way ANOVA for stride time and width variability. To test differences across DGI groups a 2 (group) × 2 (leg), Mixed ANOVA (repeated on the leg factor) was performed for step length, swing time, pre-swing time variability and an independent t-test for stride time and stride width variability. When significant effects were detected, post-hoc pairwise comparisons were performed using Bonferroni-adjusted t-tests. All statistical analyses were conducted in SPSS (version 13.0).
RESULTS
Differences in step variability between healthy and hemiparetic gait
Variability in step length, swing, pre-swing and stride time was increased in hemiparetic compared to control subjects, while stride width variability (p = .153) was not changed in hemiparetic walking (Figures 1, 2). However, when only the slower walkers (speed < 0.4m/s) were compared to controls, width variability was significantly reduced (p = .038).
Figure 1. Differences in Temporal variability between healthy (n = 22) and participants with hemiparesis (n = 94) at Self-selected (SS) walking speeds.
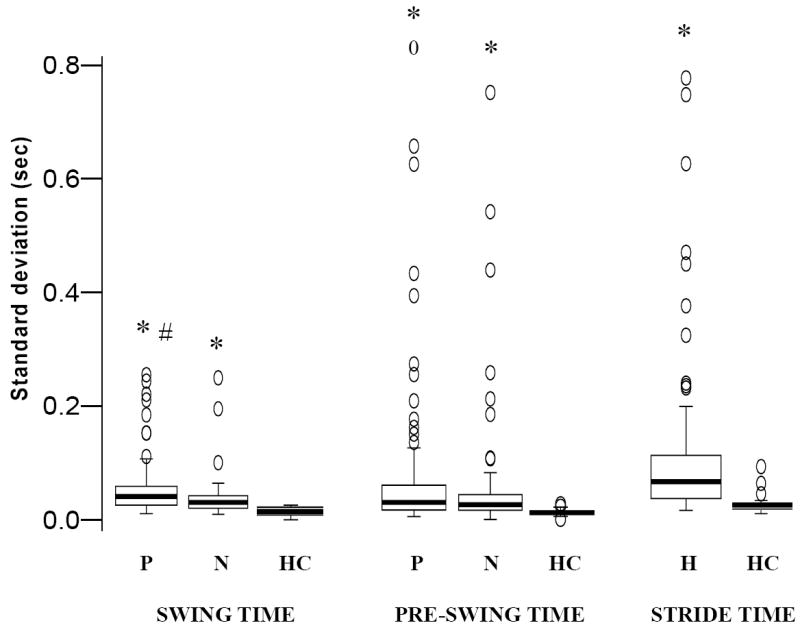
The box plots indicate the range in the data. The central horizontal line is the median of the sample. The length of the box indicates the inter-quartile range with the upper and lower boundaries of the box indicating the upper and lower quartile, respectively. Circles represent sample values that statistically indicate outlier or extreme values (by SPSS software). In impaired populations, these outlier values are true indicators of behavior and represent those persons showing excessive variability. * indicates statistically significant differences from healthy leg at p < .0001, # indicates statistically significant difference from non-paretic leg at p < .0001. Note that the statistical significance is based on the mean of the log-transformed values of the respective temporal characteristics. Variability in all temporal characteristics was increased during hemiparetic walking. Abbreviations: P – Paretic leg, N – Non-paretic leg, HC – Healthy control leg, H – Hemiparetic walking (value includes steps from both legs).
Figure 2. Differences in Spatial variability between healthy (n = 22) and participants with hemiparesis (n = 94) at Self-selected (SS) walking speeds.
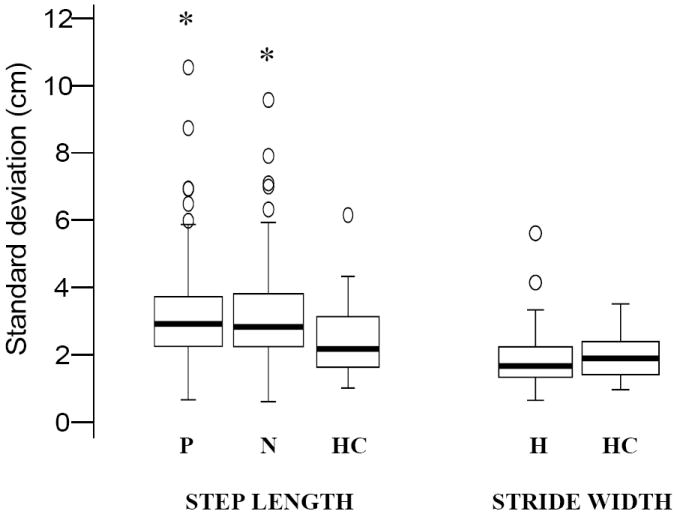
The box plots indicate the range in the data similar to Figure1. * indicates statistically significant differences from healthy leg at p < .0001 and the statistical significance is based on the mean of the log transformed values of the respective temporal characteristics. Variability in step length characteristics was increased during hemiparetic walking and that in stride width showed a trend to decrease during hemiparetic walking. Abbreviations: P – Paretic leg, N – Non-paretic leg, HC –Healthy control leg, H – Hemiparetic walking (value includes steps from both legs).
For between-leg comparisons, swing time variability was greater in paretic steps and there was a trend for paretic pre-swing (PPS) time to show greater variability compared to non-paretic pre-swing (NPS) time (p = .065, Figure 1). There was no difference in the variability between paretic and non-paretic step lengths (Figure 2).
Association between step variability, clinical assessments and asymmetry index
Differences in variability for each spatiotemporal characteristic across the three groups are presented below. Figures 3 and 4 show the differing patterns of spatiotemporal variability within the severity, asymmetrical and DGI groups and Table 1 shows the mean variability across-groups.
Figure 3. Differences in Temporal variability in hemiparetic participants based on their performance on clinical assessments.
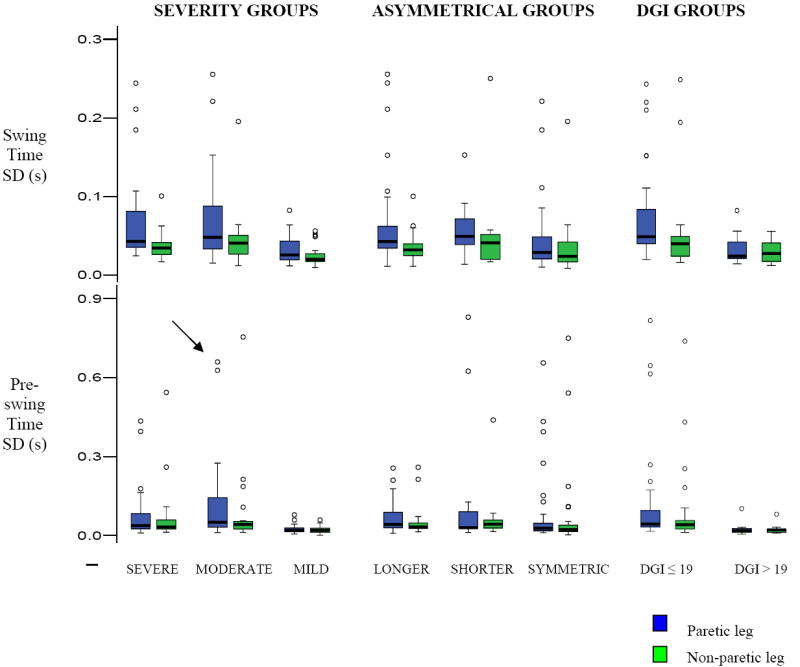
The box plots indicate the range in the data similar to Figures 1. Blue represents paretic leg and Green represents non-paretic leg for swing time and pre-swing time variability. In general, poor performance is indicated by more severe hemiparesis (lower LE-FM scores), asymmetrical gait (longer or shorter paretic steps) and poorer balance performance (lower DGI scores)]. Note the between-leg differences in swing and pre-swing time in persons with moderate and severe hemiparesis, those walking asymmetrically and in persons showing poor balance performance. Note that while stride time variability differences were observed in sub-groups of the hemiparetic population, these have not been represented in the Figure.
Figure 4. Differences in Spatial variability in hemiparetic participants based on their performance on clinical assessments.
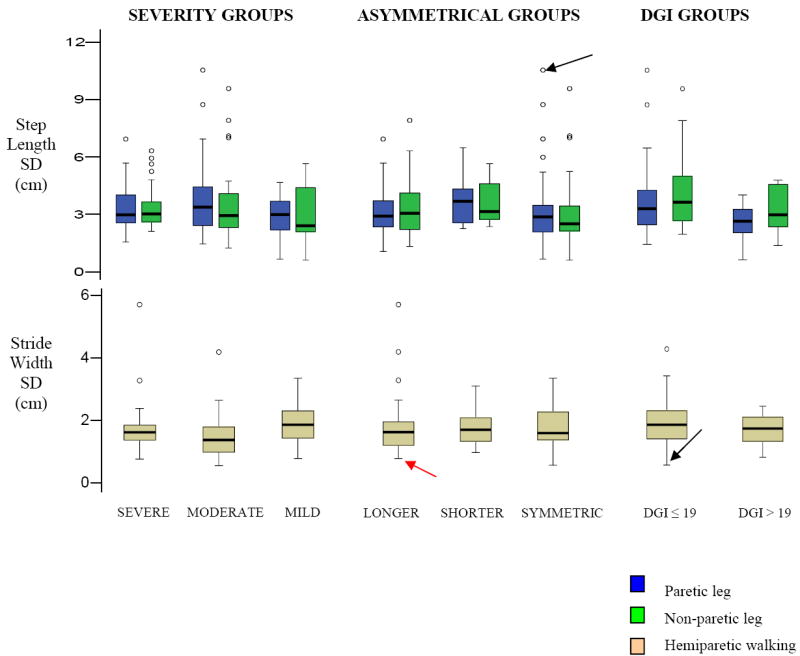
The box plots indicate the range in the data similar to Figures 1. Blue represents paretic leg and Green represents non-paretic leg for step length variability. Note that stride width variability does not show a consistent trend to differ across the different sub-groups of the hemiparetic population.
Table 1.
Step variability (expressed as standard deviation) within the hemiparetic population sub-divided based on their performance measures.
| Severity groups (n=81) | PSR groups (n=94) | DGI groups (n=39) | ||||||
|---|---|---|---|---|---|---|---|---|
| Severe | Moderate | Mild | Longer | Shorter | Symmetric | ≤19 | >19 | |
| Avg. walking speed | (n=24) (0.5m/s) | (n=23) (0.5m/s) | (n=34) (0.8m/s) | (n=35) (0.7m/s) | (n=11) (0.5m/s) | (n=48) (0.6m/s) | (n=30) (0.5m/s) | (n=9) (0.7m/s) |
| Step length variability (cm) | ||||||||
| Paretic | 3.37 | 4.01 | 2.89 | 3.13 | 3.76 | 3.14 | 3.78 | 2.51 |
| Non-paretic | 3.44 | 3.72 | 2.96 | 3.47 | 3.69 | 3.02 | 4.11 | 3.18 |
| Swing time variability (s) | ||||||||
| Paretic | 0.07 | 0.07 | 0.03 | 0.07 | 0.06 | 0.04 | 0.07 | 0.03 |
| Non-paretic | 0.04 | 0.04 | 0.02 | 0.03 | 0.05 | 0.03 | 0.05 | 0.03 |
| Pre-swing variability (s) | ||||||||
| Paretic | 0.08 | 0.13 | 0.02 | 0.06 | 0.16 | 0.07 | 0.13 | 0.03 |
| Non-paretic | 0.07 | 0.08 | 0.02 | 0.04 | 0.08 | 0.05 | 0.09 | 0.03 |
| Stride time variability (s) | 0.13 | 0.16 | 0.05 | 0.12 | 0.19 | 0.08 | 0.16 | 0.06 |
| Stride width variability (cm) | 1.83 | 1.55 | 1.90 | 1.78 | 1.77 | 1.77 | 1.87 | 1.68 |
Standard deviation in the spatiotemporal characteristics is rounded to the second decimal place. Abbreviations: PSR – Paretic step ratio, DGI – Dynamic gait index.
Swing time variability, Main effects (ME) of Group and Leg were significant across severity (p<.01), asymmetrical (p<.01) and DGI groups (p≤.02). Paretic steps showed greater variability than non-paretic ones across all groups, with greatest between-leg differences in individuals with severe and moderate hemiparesis and those taking longer paretic steps and showing poor balance performance (DG1 ≤ 19).
Pre-swing time variability, ME of both Group and Leg were significant across severity (p<.01) groups, ME of leg (p<.001) was significant across asymmetrical groups and ME of group was significant across DGI. PPS showed greater variability than NPS in the severe and moderate groups and across asymmetrical groups. Both PPS and NPS showed greater variability in individuals showing poor balance performance compared to those with good balance.
Stride time variability differed across severity groups (p < .0001). Severe and moderate groups showed greater stride time variability than the mild group (p < .003) but did not differ from each other (p > .05, Table 1). Stride time variability differed across asymmetrical groups (p = .023). Post-hoc tests demonstrated that asymmetrical groups showed only a trend (of greater variability) to differ from the symmetrical group (p ≤.109). Stride time variability (p = .007) was greater in individuals with poor balance performance (DGI ≤ 19), (Table 1, Figure 3).
Step length variability showed a trend to differ across severity groups (p = .069) and asymmetrical groups (p = .094). However, ME of group and leg were significant for step length variability (group: p = .026, leg: p = .03) across DGI groups (p≤.03), with the non-paretic leg showing greater variability than the paretic leg.
Stride width variability showed a trend to differ across severity groups (p = .045) but did not differ across the asymmetrical or DGI groups (p > .95).
DISCUSSION
Similar to other populations with gait deficits [9, 18, 28], individuals with post-stroke hemiparesis had increased variability in all spatiotemporal characteristics (except stride width) compared to healthy controls (Figure 1, 2). While increased variability has been related to gait deficits in impaired populations [5, 9, 18, 28], our study is the first to report between-leg differences in step variability. We found between-leg differences in swing and pre-swing time variability suggesting a direct association between underlying paretic leg impairment and step variability. Swing time variability on the paretic side was greater than in the non-paretic and this difference was greatest for the most affected individuals (severe and moderate hemiparesis, asymmetrical steps and those at risk for falling as predicted by lower DGI scores) (Figure 3, 4). Increased paretic leg step variability after stroke may relate to neuromuscular impairments, such as altered neural inputs to the paretic spinal half-centers, altered effects of afferent feedback to the paretic leg and impaired inter-limb coordination during walking. Furthermore, PPS variability showed a trend to be greater than NPS. Prolonged time in PPS has been related to impaired progression during hemiparetic walking [25]. The increased variability in PPS relative to NPS suggests that these neuromotor deficits may limit hemiparetic walking performance. Step length variability did not differ between-legs but was greater during hemiparetic walking compared to controls. It is likely that spatial variables such as step length (which determines the base of support during gait) are inherently more tightly coordinated between-legs. Temporal variables, however, such as step-to-step variation in one leg, can be counter-balanced by variation in the other leg to maintain steady-state walking. Stride time variability also increased during hemiparetic walking compared to controls. Increased stride time variability is reported to be strongly related to falls risk [2, 7]. This suggests that increased variability post-stroke may relate to poor dynamic balance.
In order to test the use of gait variability measures as markers of impaired performance, we investigated the relationship between step variability and hemiparetic performance. For example, the ability to produce independent voluntary movements of the paretic leg is related to motor recovery of the paretic leg and is graded using the LE –FM (higher score - greater recovery). The inverse relation between hemiparetic severity and step variability suggests that variability may decrease as motor recovery progresses. Similarly, greater step length asymmetry (both Longer paretic and Shorter paretic groups) has been related to motor control impairments [26]. In support of this hypothesis, our results revealed that both asymmetrical groups presented with greater swing time variability compared to the symmetrical group but did not differ from each other. Moreover, the inverse relation between DGI scores and step variability suggests that individuals showing greater step variability may have poor dynamic balance. Specifically, DGI scores ≤ 19 are reported to identify individuals at risk of falling [16]. In our study, hemiparetic subjects with scores ≤ 19 exhibited greater step variability.
While the overall increased step variability was related to impaired performance (lower LE – FM score, greater asymmetry and lower DGI score), the pattern across the sub-groups was not consistent for all spatiotemporal parameters (e.g. between-leg differences in pre-swing time variability was observed across severity groups but not DGI groups). Since the stroke population is immensely heterogeneous, we expected that the spatiotemporal step variability patterns would differ across sub-groups, suggesting that specific spatiotemporal measures are more strongly associated with particular aspects of hemiparetic performance. For instance, between-leg differences in swing time variability were significant across all sub-groups of participants (severity, asymmetry and DGI groups) suggesting that between-leg differences in swing time variability were most strongly related to impaired hemiparetic performance (as measured by severity, asymmetry and DGI). Stride time variability differed across all sub-groups. In comparison, , there were no differences between-legs in step length variability when considering the hemiparetic population as a group. However, in individuals at risk for falling, paretic variability was increased compared to non-paretic. This suggests that step length differences between-legs may be unmasked in the most impaired individuals. Nonetheless, the relatively small sample size of the DGI subgroups could have potentially influenced these results.
While step variability in different spatiotemporal parameters varied across participant sub-groups in combination, variability in the spatiotemporal parameters strongly predicted impaired hemiparetic performance. For instance, a participant having markedly increased PPS variability (Figure 3, black-bold arrow), increased paretic step length and reduced width variability (Figure 4, black-bold arrow) shows impaired hemiparetic walking performance. The inferences from other measures, however, were inconsistent [poor balance performance (DGI = 8), moderate hemiparetic severity, symmetric steps]. Similarly, another participant had good balance performance (DGI = 22) but walked asymmetrically with much longer paretic steps, again indicating the contrasting inferences on performance as predicted by these measures. This subject had markedly increased stride time variability and reduced width variability (Figure 4, red arrow). Overall, these examples exhibit a markedly increased variability in one or other spatiotemporal characteristic and reduced variability in stride width. This suggests that although poor hemiparetic performance was not consistently evident across all clinical assessments, gait variability strongly identified impaired walking performance.
Unlike other spatiotemporal characteristics, stride width variability was reduced in the slower walkers when compared to controls. Stride width is calculated in the frontal plane unlike other spatiotemporal characteristics that are sagittal plane measures. This suggests an inherent difference in control of this parameter. Furthermore, in population groups susceptible to falls (such as elderly individuals, patients with Parkinson’s disease and community dwelling elderly), width variability is reported to be reduced and this reduction in variability is shown to predict falls [3, 6, 17]. In our study, we also observed that participants showing markedly reduced width variability walked with wide strides. A wider step provides a wider base of support for side-to-side motion of the center of mass and may be accompanied by a reduction in variability of medio-lateral foot placement to ensure steps are consistently wide. Therefore, it is likely that the observed reduction in width variability is compensatory to maintain stability. Decreased width variability in comparison to increased variability in other spatiotemporal characteristics implies that altered variability may be specific to step characteristics and should not be generalized.
There were some study limitations. Data were collected from a limited number of steps that could have influenced the accuracy of our results [29]. However, a major strength of our methodology was the ability to measure spatial variables as well. Methods that capture hundreds of steps are based on recording temporal characteristics only [3]. Despite the fewer number of steps collected, our observed patterns of gait variability were consistent with those observed in other impaired populations [5, 7, 9]. Further, use of the GAITRite based protocol is clinically relevant and therefore, our study results have the potential to rapidly translate to clinical settings.
Subjects with stroke walked slower and, therefore, contributed more steps to the variability analysis than healthy controls. This difference in available steps for analysis may have exaggerated the difference in step variability between subject groups and affected the relationship between severity of impairment and gait variability within the stroke group. Further, we did not analyze step time variability that may have limited its comparison with step length variability and confined the subsequent discussion on asymmetry across distance and time measures.
We also evaluated gait variability using coefficient-of-variation (CV = standard deviation/mean) in step characteristics. Using CV to evaluate variability may result in questionable conclusions of increased variability, specifically when the mean value is of low magnitude. It is also likely that the differences in stride variability could be due to differences in strategies (i.e., length-frequency combinations) employed to achieve a certain speed [30]. Nonetheless, several studies have shown that stride variability, could be independent of stride length and frequency [17, 21]. Furthermore, it is likely that the trial-to-trial variability in speed resulted in the observed changes in variability. However, the speed variability did not differ (p = 0.268) between participants with hemiparesis and healthy participants, suggesting that the trial-to-trial speed variation did not contribute to the observed patterns of gait variability.
In conclusion, this study suggests that step variability is altered in individuals with stroke compared to healthy controls and relates to hemiparetic walking performance. Specifically, between-leg differences in swing time and pre-swing variability, increased step length and stride time variability and reduced width variability can be indicative of underlying sensorimotor impairments post-stroke. This suggests that these parameters are quantifiable measures of impaired hemiparetic performance. Future studies should investigate the underlying causes of altered variability and the effect of therapeutic interventions on gait variability to further validate its use to assess hemiparetic performance.
Acknowledgments
This work was funded by Merit Review grant # B2748R (Influence of Post-Stroke Gait on Bone Density) and Center of Excellence grant # F2182C (Brain Rehabilitation Research Center) from the Rehabilitation Research and Development Service of the Department of Veterans Affairs and NIH R01 HD46820 (Intramuscular Coordination of Hemiparetic Walking). This material is the result of work supported in part by the Office of Research and Development, Rehabilitation R & D Service, Department of Veterans Affairs, and the Malcom Randall VA Medical Center, Gainesville, FL. The authors would like to thank Lise Worthen and Maria Kim for their assistance in data collection at the Palo Alto facility and Mark Bowden, Sandy Davis and Erin Carr for collecting the data in the Gainesville center. The authors would also like to thank Felix Zajac for his insightful suggestions on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gabell A, Nayak US. The effect of age on variability in gait. J Gerontol. 1984;39(6):662–666. doi: 10.1093/geronj/39.6.662. [DOI] [PubMed] [Google Scholar]
- 2.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78(3):278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 3.Brach JS, Berlin JE, VanSwearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehabil. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman T, Giladi N, Gurevich T, Hausdorff JM. Gait instability and fractal dynamics of older adults with a “cautious” gait: why do certain older adults walk fearfully? Gait Posture. 2005;21(2):178–185. doi: 10.1016/j.gaitpost.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord. 1998;13(3):428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- 6.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212(12):47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 8.Brach JS, Studenski S, Perera S, Vanswearingen JM, Newman AB. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2007 doi: 10.1016/j.gaitpos.2007.1005.1016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster KE, Merory JR, Wittwer JE. Gait variability in community dwelling adults with Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(1):37–40. doi: 10.1097/01.wad.0000201849.75578.de. [DOI] [PubMed] [Google Scholar]
- 10.Lamontagne A, Stephenson JL, Fung J. Physiological evaluation of gait disturbances post stroke. Clin Neurophysiol. 2007;118(4):717–729. doi: 10.1016/j.clinph.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17(1):68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 12.Dettmann MA, Linder MT, Sepic SB. Relationships among walking performance, postural stability, and functional assessments of the hemiplegic patient. Am J Phys Med. 1987;66(2):77–90. [PubMed] [Google Scholar]
- 13.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63(10):1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 14.Kautz SA, Brown DA. Relationships between timing of muscle excitation and impaired motor performance during cyclical lower extremity movement in post-stroke hemiplegia. Brain. 1998;121(Pt 3):515–526. doi: 10.1093/brain/121.3.515. [DOI] [PubMed] [Google Scholar]
- 15.Jonsdottir J, Cattaneo D. Reliability and validity of the dynamic gait index in persons with chronic stroke. Arch Phys Med Rehabil. 2007;88(11):1410–1415. doi: 10.1016/j.apmr.2007.08.109. [DOI] [PubMed] [Google Scholar]
- 16.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77(8):812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- 17.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45(3):313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 18.Niechwiej-Szwedoa E, Innessa E, Howeb J, Jaglala S, McIlroya W, Verrier M. Changes in gait variability during different challenges to mobility in patients with traumatic brain injury. Gait Posture. 2007;25(1):70–77. doi: 10.1016/j.gaitpost.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29(34):193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekiya N, Nagasaki H, Ito H, Furuna T. Optimal walking in terms of variability in step length. J Orthop Sports Phys Ther. 1997;26(5):266–272. doi: 10.2519/jospt.1997.26.5.266. [DOI] [PubMed] [Google Scholar]
- 21.Grabiner PC, Biswas ST, Grabiner MD. Age-related changes in spatial and temporal gait variables. Arch Phys Med Rehabil. 2001;82(1):31–35. doi: 10.1053/apmr.2001.18219. [DOI] [PubMed] [Google Scholar]
- 22.Brach JS, Berthold R, Craik R, VanSwearingen JM, Newman AB. Gait variability in community-dwelling older adults. J Am Geriatr Soc. 2001;49(12):1646–1650. doi: 10.1046/j.1532-5415.2001.t01-1-49274.x. [DOI] [PubMed] [Google Scholar]
- 23.Katz-Leurer M, Rotem H, Lewitus H, Keren O, Meyer S. Relationship between balance abilities and gait characteristics in children with post-traumatic brain injury. Brain Inj. 2008;22(2):153–159. doi: 10.1080/02699050801895399. [DOI] [PubMed] [Google Scholar]
- 24.Brach JS, Studenski SA, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2007;62(9):983–988. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Quervain IA, Simon SR, Leurgans S, Pease WS, McAllister D. Gait pattern in the early recovery period after stroke. J Bone Joint Surg Am. 1996;78(10):1506–1514. doi: 10.2106/00004623-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Balasubramanian CK, Bowden MG, Neptune RR, Kautz SA. Relationship between step length asymmetry and walking performance in subjects with chronic hemiparesis. Arch Phys Med Rehabil. 2007;88(1):43–49. doi: 10.1016/j.apmr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. J Neuroeng Rehabil. 2005;2:23. doi: 10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crenshaw SJ, Royer TD, Richards JG, Hudson DJ. Gait variability in people with multiple sclerosis. Mult Scler. 2006;12(5):613–619. doi: 10.1177/1352458505070609. [DOI] [PubMed] [Google Scholar]
- 29.Owings TM, Grabiner MD. Variability of step kinematics in young and older adults. Gait Posture. 2004;20(1):26–29. doi: 10.1016/S0966-6362(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 30.Danion F, Varraine E, Bonnard M, Pailhous J. Stride variability in human gait: the effect of stride frequency and stride length. Gait Posture. 2003;18(1):69–77. doi: 10.1016/s0966-6362(03)00030-4. [DOI] [PubMed] [Google Scholar]


