Abstract
The cardioprotective effects of moderate alcohol consumption have been well documented in animal models and in humans. Protection afforded against ischemia and reperfusion injury (I/R) proceeds through an ischemic preconditioning-like mechanism involving the activation of epsilon protein kinase C (εPKC) and is dependent on the time and duration of ethanol treatment. However, the substrates of εPKC and the molecular mechanisms by which the enzyme protects the heart from oxidative damage induced by I/R are not fully described. Using an open-chest model of acute myocardial infarction in vivo, we find that intraperitoneal injection of ethanol (0.5 g/kg) 60 minutes prior to (but not 15 minutes prior to) a 30-minute transient ligation of the left anterior descending coronary artery reduced I/R-mediated injury by 57% (measured as a decrease of creatine phosphokinase release into the blood). Only under cardioprotective conditions, ethanol treatment resulted in the translocation of εPKC to cardiac mitochondria, where the enzyme bound aldehyde dehydrogenase-2 (ALDH2). ALDH2 is an intra-mitochondrial enzyme involved in the detoxification of toxic aldehydes such as 4-hydroxy-2-nonenal (4-HNE) and 4-HNE mediates oxidative damage, at least in part, by covalently modifying and inactivating proteins (by forming 4-HNE adducts). In hearts subjected to I/R after ethanol treatment, the levels of 4-HNE protein adducts were lower and JNK1/2 and ERK1/2 activities were diminished relative to the hearts from rats subjected to I/R in the absence of ethanol. Together, this work provides an insight into the mitochondrial-dependent basis of ethanol-induced and εPKC-mediated protection from cardiac ischemia, in vivo.
INTRODUCTION
The beneficial effects of moderate alcohol consumption on the heart have been well documented in both men and women [1–5]. While chronic moderate consumption of ethanol prior to and following an acute myocardial infarction (AMI) can improve patient prognosis [6, 7], acute ethanol treatment may also significantly protect the heart from I/R injury [8, 9]. In addition to changes in lipid levels and hemostatic factors [10, 11], the protective effects of acute and chronic ethanol treatment proceed through direct preconditioning-like mechanisms within the cardiomyocyte [8, 9, 12, 13]. Treatment of isolated cardiomyocytes or isolated hearts with 50 mM ethanol diminishes injury associated with prolonged ischemia and improves cardiac function [8, 9, 14]. However, other studies found no protection by acute ethanol treatment [15–17]. It is now evident that the acute cardioprotective effects of ethanol are largely dependent upon the timing and dose of ethanol treatment; ethanol administration directly before prolonged ischemia does not protect the heart from injury as shown in experimental models [18–20]. Downey and collaborators made the important finding that protection afforded by ischemic preconditioning is lost if ethanol is not washed out or sufficiently metabolized before the onset of ischemia [19], an effect that has been recently corroborated in humans [21].
The protective effects of ethanol administration require εPKC activation [8, 14, 22, 23]. Additionally, we have determined through the use of ex vivo models of acute myocardial infarction that the protective effects of ethanol are due to a time-dependent mechanism involving δPKC-mediated adenosine release, resulting in εPKC activation [14, 22]. However, protection afforded by the time-dependent activation of εPKC upon acute ethanol treatment has yet to be shown, in vivo. Treatment of isolated cardiomyocytes and isolated hearts, ex vivo with as little as 10 mM ethanol induces translocation of εPKC to the cellular particulate fraction [8, 23, 24]. Following translocation, εPKC interacts with its anchoring protein, εRACK (Receptor for Activated C-Kinase) and phosphorylates nearby substrates, conferring cardioprotection [23]. However, the protein target of εPKC following acute ethanol treatment is still unknown. Interestingly, several mitochondrial targets have been implicated for εPKC in ischemic preconditioning, including the mitochondrial ATP-sensitive K+ channel (mitoKATP) [25, 26], cytochrome-c oxidase (COIV) [27], and the permeability transition pore (MPTP) [28]. However, either conflicting evidence [9, 19] or no evidence exists for a role of these targets in ethanol-mediated preconditioning and ethanol-stimulated mitochondrial localization of εPKC has not been reported.
Recently we found that εPKC activates the intra-mitochondrial enzyme ALDH2 in an ex vivo model of myocardial infarction, using the Langendorff preparation [29]. Using an in vivo model of acute myocardial infarction in rats, we show here that ethanol treatment 60 minutes prior to prolonged ischemia protected the heart from injury. This protection coincided with the translocation of εPKC to cardiac mitochondria, where it associated with the mitochondrial enzyme, aldehyde dehydrogenase-2 (ALDH2). These effects were dependent upon the timing of ethanol exposure and did not occur in the absence of I/R. Additionally, we show here that ethanol treatment prior to prolonged ischemia increased the activity of ALDH2 and decreased the formation of 4-hydroxy-2-nonenal (HNE)-protein adducts and activation of JNK1/2 and ERK1/2, all hallmarks of I/R injury. These data represent a novel protective mechanism by which ethanol increases the detoxification of cytotoxic aldehydes that accumulate during I/R in vivo by a mechanism involving the time-dependent εPKC-mediated activation of mitochondrial ALDH2.
MATERIALS and METHODS
In vivo model of left anterior descending coronary artery (LAD) ligation
Male Wistar rats (250–300g) were anesthetized by 3% isoflurane 15 or 60 minutes (indicated in text) after intraperitoneal (i.p.) injection of 0.5 g/kg ethanol. The surgical procedures used for left anterior descending coronary artery (LAD) ligation are based on a previously published protocol [30]. Briefly, animals were incubated, and ventilated with a Harvard rodent ventilator at a rate of 80 breaths per minute (5–15 mm Hg). Maintenance anesthesia was provided via 1% inhalational isoflurane and body temperature was maintained at 37°C using a rectal probe linked to a thermocoupled thermometer and an appropriate heating blanket. The heart was exposed by median sternotomy and control and ethanol-treated rats were subjected to a 10 minute period of stabilization, followed by a ligature being placed around the LAD coronary artery, close to its origin from the aortic root. The normoxia control animals (sham) were exposed to the same procedure with no ligation. The free ends of the ligature were used to form a noose around a syringe plunger which was placed flat on the myocardium. Coronary occlusion was achieved by tightening the noose around the plunger for 30 minutes. Occlusion was determined by observation of immediate pallor of the left ventricular free wall and reflow was achieved by release of the ligature for 15 min. At the end of reperfusion, hearts were excised and flushed with 0.9% saline to remove blood.
For administration of ethanol, rats were mildly anesthetized with 3% isoflurane and 0.5g/kg ethanol was administered through intraperitoneal (i.p.) injection after which the animals were returned to their cages for either 15 or 60 minutes prior to surgery. Animals in the control groups received an injection of saline for the same durations prior to LAD occlusion. For measurement of humoral creatine phosphokinase (CPK), 0.45ml of blood was withdrawn into a 0.05ml heparin-primed syringe during the 15 minute reperfusion period at 5 min intervals via an apical punch of the left ventricle. Serum was separated by centrifugation at 5,000g for 5 minutes on a tabletop centrifuge and serum CPK values were determined using a CK-SL assay kit (Diagnostic Chemicals Ltd, Oxford, Connecticut). Background levels of CPK were taken prior to LAD occlusion and were subtracted from all CPK values and statistical analyses used are listed in the figure legends. All the animal protocols were approved by the Institutional Animal Care and Use Committees of Stanford University.
Western blot analysis
Following LAD occlusion and 15 minutes of reperfusion, the left ventricular free wall (without septum) was dissected away from the right ventricle and atria and homogenized in buffer A (210 mM mannitol, 70 mM sucrose, 5 mM MOPS and 1 mM EDTA). Tissue extract was centrifuged at 700g to pellet nuclei and unbroken cellular debris, followed by centrifugation at 10,000g to collect mitochondrial-enriched fractions. For εPKC translocation, mitochondrial fractions were resuspended in buffer A, analyzed by Western blot using antibodies against εPKC (Santa Cruz Biotechnology, Santa Cruz, CA; 1:500 dilution) and normalized to total cellular εPKC levels. For detection of HNE protein adducts in mitochondrial fractions, we used a specific antibody against the reductively stabilized HNE amino acid adducts (Calbiochem, Gibbstown, NJ; 1:1000 dilution). The levels of phosphorylated and unphosphorylated JNK, and Erk1/2 were analyzed using their respective antibodies (Cell Signaling, Danvers, MA) and mitochondrial purity was assessed with antibodies against the Na+K+ ATPase (Millipore, Billerica, MA), prohibitin (Santa Cruz Biotechnology), enolase (Santa Cruz Biotechnology,), and the ER targeting sequence KDEL (StressGen Corporation, Ann Arbor MI).
Immunoprecipitation analysis
For determination of ALDH2 and εPKC interactions, 700 μg of mitochondrial lysate protein was diluted into buffer A and immunoprecipitated with ALDH2 or εPKC-specific antibodies (Santa Cruz Biotechnology) (2 μg). After 3 hours of incubation, protein A/G agarose beads were added and incubated for an additional 2 hours before spinning down. Immunocomplexes were washed 3 times with buffer A and separated on an 8% SDS gel after which they were analyzed for the presence of associated proteins by Western blot using antibodies for εPKC, ALDH2 or εPKC (Santa Cruz Biotechnology) and visualized using a One-Step IP-Western kit (GenScript corp., Piscataway, NJ).
Enzymatic measurement of aldehyde dehydrogenase-2
Enzymatic activity of ALDH2 was determined spectrophotometrically by monitoring the reductive reaction of NAD+ to NADH at A340nm. The assays were carried out at 25°C in 50 mM sodium pyrophosphate buffer, pH=9.5. To this volume, 10 mM acetaldehyde and 200 μg of mitochondrial lysate protein isolated from the left ventricle of ethanol-treated or untreated rat hearts that underwent LAD occlusion were added. To start the reaction, 2.5 mM NAD was added and the accumulation of NADH was monitored for 5 minutes with measurements being taken every 30 seconds. The reaction rates were recorded, compared to animals that were not treated with ethanol and expressed as % control.
Electron microscopic analysis of mitochondrial purity and integrity
Tissue was homogenized in buffer A as described above and fixed in 4% paraformaldehyde and 0.1% gluteraldehyde. The fixed material was sectioned by the Stanford Electron Microscopy Facility. Sections were taken between 75 and 80 nm, picked up on formvar/Carbon coated 75 mesh Ni grids and stained for 20 seconds in 1:1 saturated uracetate (~7.7%) in acetone followed by staining in 0.2% lead citrate for 3 to 4 minutes for contrast. Mitochondrial samples were observed in a JEOL 1230 transmission electron microscope at 80kV and photos were taken using a Gatan Multiscan 791 digital camera.
RESULTS
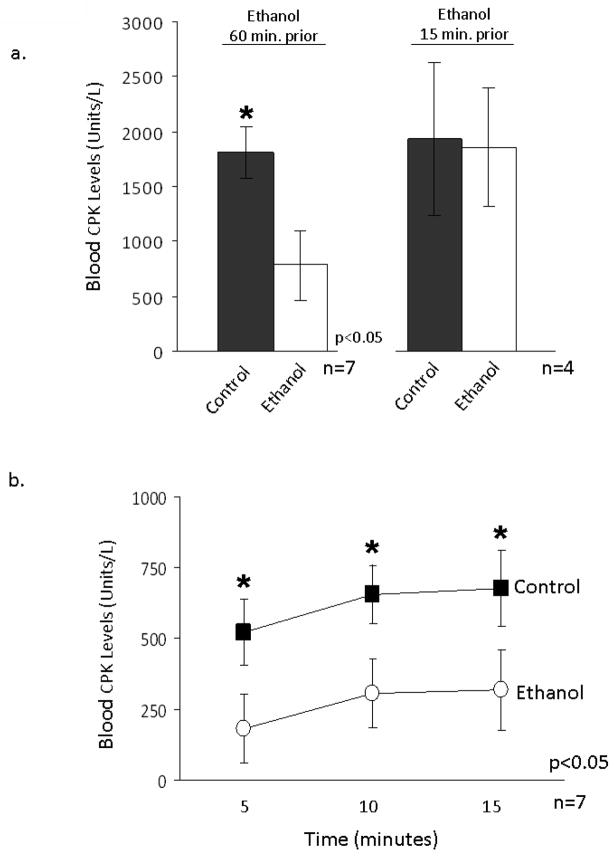
Occlusion of the left anterior descending coronary artery (LAD) results in ischemia, which subsequently leads to irreversible damage to the myocardium and tissue loss. We have previously shown in an ex vivo rat model of ischemia/reperfusion (I/R) injury that ethanol exposure prior to the ischemic event protects the heart from injury through an εPKC-dependent mechanism [8]. Very recently, we also found that activation of mitochondrial ALDH2 is required and sufficient to produce cardioprotection afforded by ethanol pretreatment, ex vivo [29]. In the current study, we first determined whether ethanol treatment in rats leads to cardioprotection from acute myocardial infarction, in vivo. As shown in Figure 1a, i.p. administration of 0.5 g/kg of ethanol 60 minutes prior to 30 minutes of LAD occlusion followed by 15 minutes of reperfusion resulted in a 57+/−9% decrease in creatine phosphokinase (CPK) release into the blood relative to rats that were injected with saline (p<0.05; n=7 animals/group). As seen in figure 1b, ethanol administration 60 minutes prior to ischemia significantly diminished the release of CPK over the 15 minutes of reperfusion as compared to controls (Fig. 1b, p<0.05; n=7). In agreement with the previous findings using ex vivo models [18, 22, 31], ethanol administration 15 minutes prior to LAD occlusion had no significant protective effects on the myocardium following reperfusion (Fig. 1a, n=4 animals/group).
Figure 1. Ethanol decreases CPK release into the blood in an in vivo model of acute myocardial infarction.
Rats were i.p. injected with 0.5 g/kg of ethanol 15 or 60 minutes prior to occlusion of the left anterior descending (LAD) coronary artery (ethanol treated) and compared to animals, which were injected with saline (control treated). Hearts were reperfused for 15 minutes by release of the ligature and humoral creatine phosphokinase (CPK) levels were monitored every five minutes in blood drawn through a cardiac apical punch. CPK released over the 15 minutes of reperfusion was summed together and expressed as % control. CPK values from sham animals that underwent surgery, but did not undergo coronary artery ligation were subtracted from the experimental groups. Comparisons between multiple groups were made using analysis of variance (ANOVA) with a Newman-Keuls multiple comparison test and individual group comparisons were made with a student’s t-test. The number of animals/group with standard error and statistical significance for all data are listed in the figures with a p-value of 0.05 being considered significant. (a) A significant difference in CPK release was seen with ethanol administration 60 minutes prior to ischemia but not in animals injected 15 minutes prior. (b) CPK release from animals injected with ethanol 60 minutes prior to occlusion were monitored every five minutes and expressed as blood CPK units/L. Ethanol significantly decreased CPK release relative to controls at each time point tested.
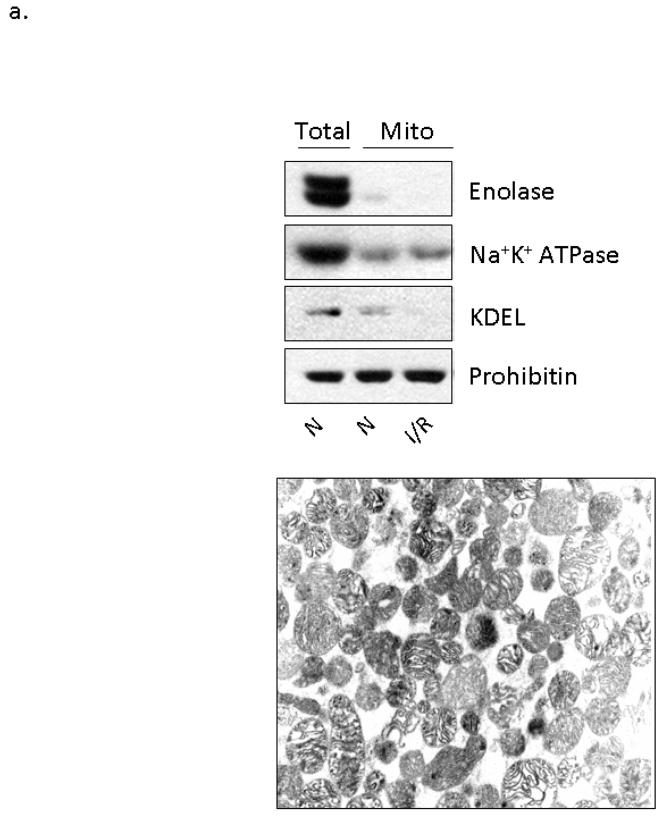
Several studies demonstrated that treatment of whole hearts and isolated cardiomyocytes with ethanol results in the activation and translocation of εPKC to the particulate fraction of the cellular homogenate [8, 14, 22, 23]. However, the subcellular localization and consequences of εPKC translocation had not been determined. Since under some conditions εPKC has been reported to be localized in the mitochondrial fraction [32, 33], we isolated mitochondria from the left ventricle of hearts treated with 0.5 g/kg of ethanol 15 and 60 minutes prior to LAD ligation and performed Western blot analysis to determine the localization of εPKC. To confirm the purity of this mitochondrial preparation, we first evaluated the presence of contaminating fractions. In order to visualize mitochondrial proteins alongside total lysate proteins, we loaded equal amounts of mitochondrial and total lysate proteins, thereby concentrating the mitochondrial fraction. As seen in the top panel of Figure 2a, the mitochondrial fraction had minimal contamination of cytosolic proteins (enolase), plasma membrane proteins (Na+K+ATPase) and ER membrane proteins (KDEL). Furthermore, as evidenced by electron microscopy (Fig 2a, bottom panel), mitochondrial membrane integrity was maintained in this fraction.
Figure 2. Ethanol-mediated protection is associated with εPKC translocation to cardiac mitochondria.
Mitochondria were isolated from the left ventricles of animals that were treated with 0.5 g/kg of ethanol 15 and 60 minutes prior to LAD occlusion (Et) and compared to animals that were not treated with ethanol (C) and to animals that were not subjected to LAD occlusion (N). Following homogenization, mitochondrial lysates and total cellular lysates were analyzed by Western blot analysis utilizing an anti-εPKC antibody and equal loading was determined by monitoring the levels of mitochondrial ALDH2. (a) Electron micrographs illustrating the membrane integrity of isolated mitochondria. Additionally, the presence of mitochondrial protein (prohibitin), and the lack of cytosolic proteins (enolase), plasma membrane proteins (Na+K+ ATPase), and ER resident proteins (KDEL) illustrates the purity of our mitochondrial preparation. (b) Data from two separate groups of animals treated with ethanol 60 minutes prior to LAD ligation are shown. Two different molecular weight forms of mitochondrial εPKC are marked with arrows. (c) Quantification of εPKC translocation to the mitochondria from 7 different experiments was done using NIH Image-J software, expressed as % control and significance was determined with a 2 way t-test (*= p<0.05). Significant changes were observed between the ethanol treated and control and normoxic hearts. (1c Insert) Hearts from wildtype and εPKC knock-out mice were isolated and homogenized for Western blot analysis. The lower 87kDa band is present in both wildtype and knock-out animals suggesting non-specific antibody recognition while the upper band represents εPKC. (d) Animals were subjected to ethanol treatment without LAD occlusion and data from two representative experiments are shown. (e) Representative data from 4 separate experiments from animals treated with ethanol 15 minutes prior to LAD ligation are shown.
We next found that cardioprotection induced by ethanol treatment 60 minutes prior to LAD occlusion correlated with translocation of εPKC to cardiac mitochondria, as compared with mitochondrial fractions isolated from rat hearts maintained under normoxic conditions or control hearts obtained from rats subjected to LAD ligation without ethanol pretreatment (Fig. 2b, c; n=7 animals/group). Furthermore, subfractionation experiments demonstrated that translocated εPKC was found to be associated with the inner mitochondrial membrane (data not shown). The total cellular levels of εPKC did not change by any of the treatments. Occlusion of the LAD alone (control hearts in Fig. 2b) did not result in translocation of εPKC, suggesting that ethanol treatment prior to the ischemic event is necessary for this εPKC translocation. In a recent publication, volatile anesthetics, such as sevoflurane, were shown to enhance ethanol-induced cardiac preconditioning through PKC activation [34]. Therefore, to determine the effect of the anesthetic used here, isoflurane, we injected control animals with 0.5 g/kg of ethanol followed 60 minutes later by an acute lethal dose of sodium pentobarbital. This study confirmed that isoflurane did not activate εPKC and that ethanol alone was not sufficient to induce translocation of εPKC (Fig. 2d; n=3 animals/group). Importantly, the lack of protection associated with ethanol treatment 15 minutes prior to LAD occlusion correlated with a lack of translocation of εPKC to the mitochondria (Fig. 2e, n=4 animals/group). These data suggest that translocation of εPKC may be dependent upon an ethanol-mediated priming event in the cytosol, which enables εPKC activation and translocation to cardiac mitochondria following I/R.
As seen in Figures 2b, d, e, two εPKC immunoreactive bands of 87 and 95 kDa are present in this fraction. To determine if these bands represent two different forms of εPKC, we subjected mitochondrial homogenate isolated from wild type and εPKC knockout mice to Western blot analysis. It was determined from these studies that the lower 87kDa band is a non-specific immunoreactive band and does not represent εPKC (insert Figure 2c).
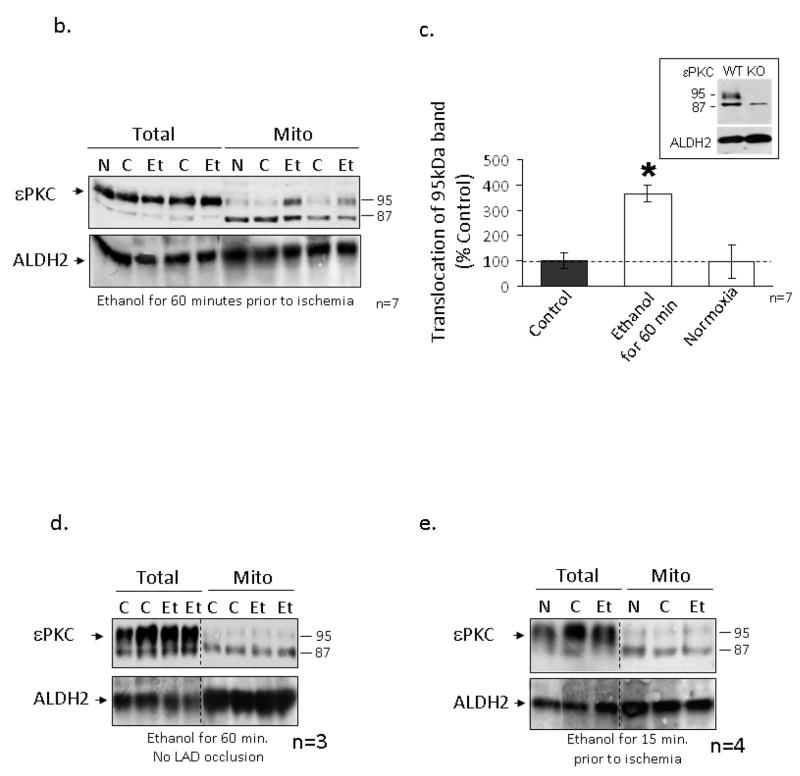
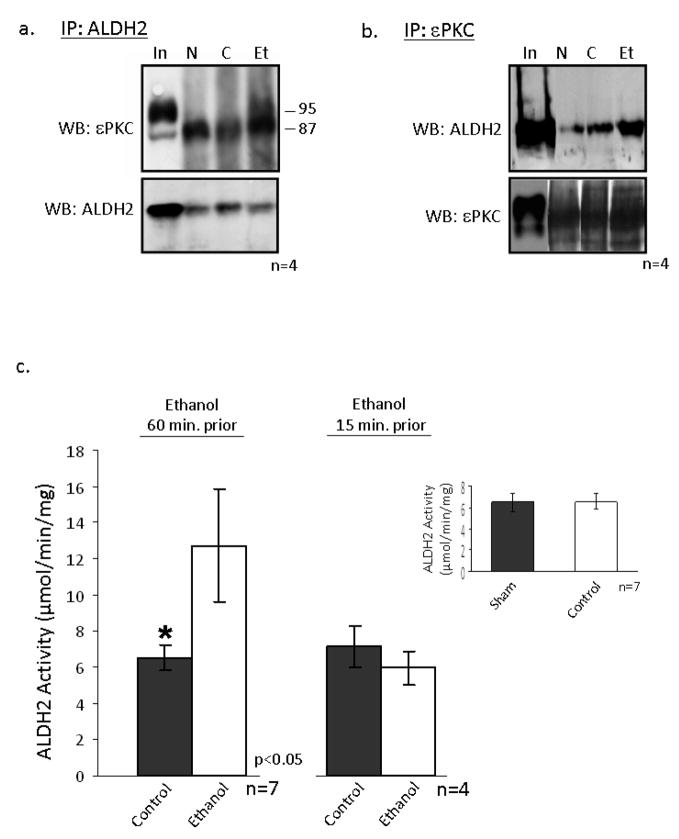
We recently found that ALDH2 activation is required and sufficient to mediate cardioprotection from ischemic injury [29]. Since ethanol treatment protects the heart from injury and, in conjunction with I/R induces εPKC translocation to the mitochondria, we hypothesized that εPKC may increase ALDH2 activity to diminish the accumulation of toxic aldehydes, which damage the heart during I/R. Co-immunoprecipitation experiments conducted on mitochondria isolated from hearts subjected to I/R with and without ethanol showed that εPKC (95kDa) associated with ALDH2 (Fig 3a; n=4 animals/group) and ethanol treatment 60 minutes before I/R resulted in a 70% increase in ALDH2 activity (Fig. 3c; n=7 animals/group, p<0.05). There was no difference in ALDH2 activity between sham-treated rats and rats subjected to LAD ligation without ethanol treatment (control; insert figure 3c). Furthermore, ethanol treatment 15 minutes prior to I/R did not result in ALDH2 activation, correlating with our earlier findings that these conditions do not induce ethanol-mediated protection (Figure 3c; n=7 animals/group).
Figure 3. εPKC associates with mitochondrial ALDH2 following ethanol treatment.
Mitochondrial protein was isolated from the left ventricles of animals that were administered 0.5 g/kg of ethanol 60 minutes prior to LAD occlusion (Et) and compared to animals that were not treated with ethanol (C) and animals which did not undergo LAD occlusion (N). (a) Following homogenization 700 μg of mitochondrial protein was immunoprecipitated with an anti-ALDH2 antibody and subjected to Western blot analysis with anti-εPKC. As a control, mitochondrial lysate was used (In). (B) Proteins were subjected to a reverse immunoprecipitation using the antibodies listed in the Figure. (C) ALDH2 activity was measured in mitochondrial fractions isolated from control and animals treated with ethanol 15 and 60 minutes prior to LAD occlusion and the results are expressed as μmole NADH produced/minute/mg of protein of either 7 or 4 independent experiments, respectively. Differences in activity (as determined by a two way t-test) were observed between the control and ethanol groups of the animals injected with ethanol 60 minutes prior to ischemia (*= p<0.05) but not in animals treated 15 minutes prior. (Insert) There was no significant difference in ALDH2 activity between sham and control treated animals.
Ethanol is metabolized to acetaldehyde by alcohol dehydrogenase (ADH) and is further converted to acetate by the mitochondrial enzyme aldehyde dehydrogenase-2 (ALDH2). However, this mechanism is unlikely responsible for ethanol-mediated cardiac protection from I/R. During cardiac I/R, accumulation of the toxic aldehyde, 4-hydroxynonenal (4-HNE), has been proposed to be an indicator of oxidative damage and reperfusion injury [35–40]. We therefore determined whether the increased interaction with and activation of ALDH2 by εPKC affects 4-HNE levels. We found that the increase in enzymatic activity of ALDH2 (Fig. 3), was associated with a decrease in the formation of select HNE-protein adduct accumulation within the mitochondria (Figure 4, n=5 animals/group). These data suggest that the protective effects of ethanol may proceed through increased detoxification of the cytotoxic and highly reactive aldehyde, 4-HNE due to ALDH2 activation.
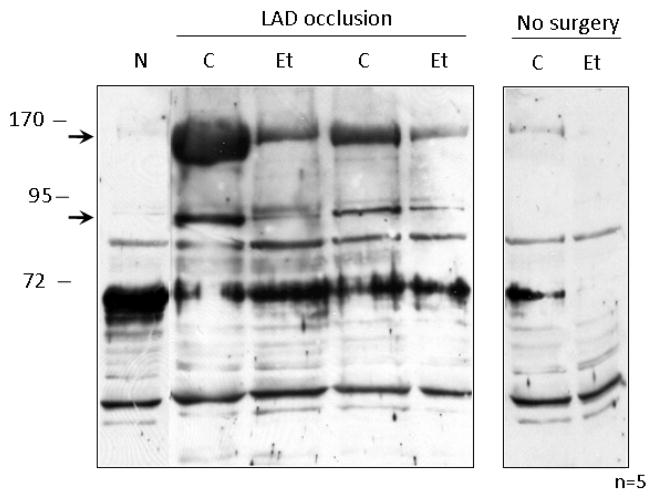
Figure 4. Ethanol treatment reduces HNE protein-adduct formation following I/R.
Mitochondrial protein was isolated from the left ventricles of animals that were administered 0.5 g/kg of ethanol 60 minutes prior to LAD occlusion (Et) and compared to animals that were not treated with ethanol (C) and animals which did not undergo LAD occlusion (N). The right panel shows basal levels of HNE formation in cardiac mitochondria that were not subjected to surgery. Following homogenization, mitochondrial lysate was analyzed by Western blot utilizing antibodies which recognize HNE protein adducts. Two protein bands which showed a reduction upon in HNE-adduct formation upon ethanol treatment are denoted by arrows.
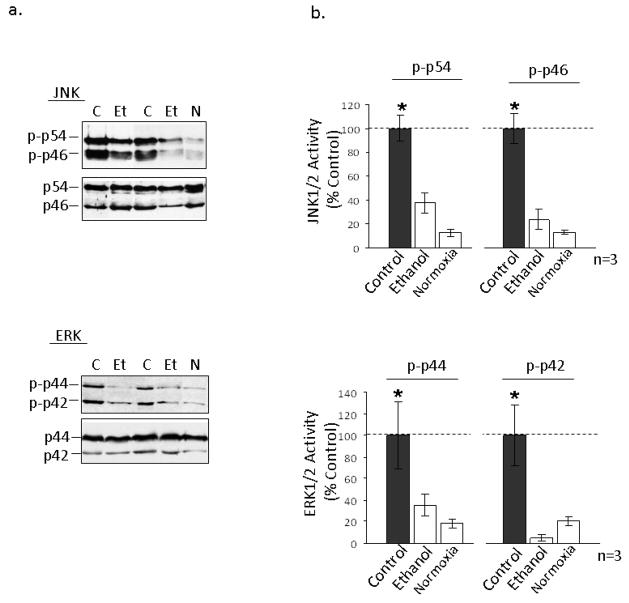
Pro-oxidants in the heart can lead to apoptosis via activation of JNK1/2 and ERK1/2 signaling pathways [41]. Additionally, 4-HNE was found to directly modify and activate JNK isoforms in hepatocytes and PC12 cells resulting in apoptosis [42, 43]. In this study, we found that both cardiac isoforms of JNK1/2 (p46 and p54) and ERK1/2 (p42 and p44) were hyper-phosphorylated after LAD occlusion, in vivo (Fig. 5a, b; n=3 animals/group). Intraperitoneal injection of 0.5g/kg of ethanol 60 minutes prior to I/R blocked this activation, suggesting that the cardioprotective effects of ethanol may proceed through εPKC-mediated activation of ALDH2 to diminish the accumulation of 4-HNE and downstream pro-death signaling.
Figure 5. Ethanol treatment decreases activation of MAPK pathway signaling molecules.
Mitochondrial protein was isolated from the left ventricles of animals that were administered 0.5 g/kg of ethanol 60 minutes prior to LAD occlusion (Et) and compared to animals that were not treated with ethanol (C) and animals which did not undergo LAD occlusion (N). (a) Following homogenization, cardiac total lysate from the left ventricle was analyzed by Western blot to determine the phosphorylation levels of JNK1/2 (p-p46 and p-p54), and Erk1/2 (p-p44 and p-p42). Data from two separate groups of experiments are shown. (B) Quantification of the data in panel a was done using NIH Image-J software, expressed as % control and significance was determined using a 2 way t-test.
DISCUSSION
In the current study, we have identified a mitochondrial target of εPKC and have elucidated a protective mechanism of ethanol-mediated preconditioning in vivo. Our results demonstrate for the first time that acute administration of ethanol 60 minutes prior to coronary occlusion results in εPKC translocation to cardiac mitochondria, ALDH2 and εPKC mitochondrial association, increased ALDH2 activity, diminished HNE-protein adduct formation and decreased pro-death signaling.
Many epidemiological studies in the past decade have demonstrated that chronic moderate consumption of ethanol decreases the risk of myocardial infarction [1–6]. We have previously found that acute ethanol exposure before ischemia is sufficient to protect the heart from damage [8]. Using an isozyme-selective inhibitor, we demonstrated that εPKC mediates the protective effects of ethanol in an adult rat heart ex vivo model of I/R and in isolated cardiac myocytes in vitro [8]. Several groups have shown in ex vivo models of I/R that ethanol-mediated protection is dependent upon the sustained activation of εPKC [9, 24, 44]. While it is clear that ethanol can induce the translocation of εPKC, it was not clear where the enzyme translocates to within the cell. Other cardioprotective phenomena utilizing different models have shown that protection is dependent upon εPKC activation [45–49]. Ohnuma et al. demonstrated that this cardioprotection coincided with εPKC translocation to the mitochondria [33] and we show here that similarly, ethanol administration 60 minutes prior to ischemia was sufficient to induce translocation of εPKC to cardiac mitochondria during I/R. We also show that the protective effects of ethanol and translocation of εPKC did not occur if ethanol was administered only 15 minutes prior to LAD ligation. These data further support our conclusion that the mitochondrial association of εPKC plays a critical role in cardioprotection.
Our findings further support the body of work suggesting that ethanol administration immediately prior to the ischemic period does not protect the heart from I/R-mediated injury [15, 18–20, 31]. Krenz et al. demonstrated that ethanol administrated immediately prior to ischemia did not reduce infarct size induced by I/R in a rabbit MI model [9, 19]. In the same model, when ethanol was infused one hour prior to ischemia, infarct size was reduced. Ethanol is converted to acetaldehyde via alcohol dehydrogenase, which in turn is metabolized to acetic acid mainly by ALDH2 [50]. It is possible that the accumulating acetaldehyde competes for 4-HNE metabolism by ALDH2 allowing the accumulation of reactive 4-HNE thereby increasing 4-HNE-protein-adduct formation, and causing subsequent cellular injury.
We show here that εPKC translocation to the mitochondria was not induced by either ethanol or ischemia alone, supporting the hypothesis of a delayed two-part mechanism of εPKC activation and suggesting that a priming step is necessary for εPKC translocation and protection of the ischemic heart; here this priming was induced by ethanol. We further propose that in addition to this priming step, a second activation event occurring during ischemia is necessary for εPKC translocation to the mitochondria; ethanol treatment without a subsequent ischemic event did not induce mitochondrial translocation. Recently, εPKC has been shown to be activated by mild reactive oxygen species (ROS) [51]. We therefore propose that ROS, which is generated during ischemia [52] may oxidatively modify εPKC [53], resulting in its entry into the mitochondria in a mechanism that has yet to be determined. If the initial priming step does not occur and if sufficient time is not allowed for this second step to occur, εPKC cannot translocate into the mitochondria, and cardiac protection is not afforded.
Translocation of εPKC to cardiac mitochondria suggests that these organelles may play a role in ethanol-mediated cardioprotection. It is well established that mitochondria can regulate cardiac injury during ischemia and reperfusion through increased ROS generation [52, 54, 55], the release of pro-apoptotic molecules [56] and alterations in energy utilization [57, 58]. Additionally, there are several mitochondrial targets of εPKC which protect the heart from I/R injury through mechanisms including ROS generation [59, 60], opening of the mitochondrial permeability transition pore (MPTP) [61, 62], regulation of the mitochondrial kATP channels (reviewed in [63, 64]) and activation of mitochondrial ALDH2 [29]. One key mediator of cellular injury that accumulates during I/R is the toxic aldehyde 4-HNE [40]. Under physiological conditions, 4-HNE may act as a signaling molecule [65]. However, under conditions of oxidative stress, accumulated 4-HNE modifies and regulates enzymes involved in mitochondrial energy production [39], resulting in increased ROS generation[66], diminished protein degradation [35] and increased pro-apoptotic signaling [42, 66]. Because of the reactivity of this and other aliphatic and aromatic aldehydes, the cell has developed mechanisms to detoxify these molecules [65]. In addition to glutathione-S transferase, aldehyde dehydrognesase-2 (ALDH2) is a mitochondrial enzyme that detoxifies 4-HNE and other toxic aldehydes, thereby diminishing cellular oxidative stress. Therefore, since 4-HNE accumulation during ischemia [37] can damage the heart through oxidative mechanisms and ethanol protects the heart through activation and translocation of εPKC to cardiac mitochondria, we hypothesize that εPKC might regulate ALDH2 activity to increase detoxification of 4-HNE, thereby conferring protection by diminishing oxidative stress. Interestingly, human cardiomyocytes in which ALDH2 is overexpressed are significantly protected from acetaldehyde-induced ROS accumulation and apoptosis [67], suggesting that activation of ALDH2 may be a therapeutic target to reduce ischemic damage to the heart. In a recent study, we identified a small molecule activator of ALDH2 (alda-1) that induces 60% reduction in infarct size, in vivo, an effect that is similar to the cardioprotection seen with ethanol here (Fig. 1). These data demonstrate that ALDH2 activation is required and sufficient to produce cardioprotection from ischemic injury. Together with the current study, these data suggest that εPKC translocates to cardiac mitochondria where it interacts with ALDH2, and phosphorylates and activates that enzyme to protect the heart from the injury induced by ischemia and reperfusion, in vivo. Ethanol-induced and εPKC-mediated activation of ALDH2 increase the metabolism of the reactive aldehyde, HNE, to HNA. This prevents HNE-protein adduct formation and diminishes pro-apoptotic signaling, thus improving cardiac function.
Acknowledgments
Source of support: National Institutes of Health Grant NIH AA11147 to D.M.R.
Footnotes
Disclosures: DM-R is the founder of KAI Pharmaceuticals, a company that plans to bring PKC regulators to the clinic. However, none of the work described in the study is based on or supported by the company.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaziano JM, Gaziano TA, Glynn RJ, Sesso HD, Ajani UA, Stampfer MJ, et al. Light-to-moderate alcohol consumption and mortality in the Physicians’ Health Study enrollment cohort. Journal of the American College of Cardiology. 2000 Jan;35(1):96–105. doi: 10.1016/s0735-1097(99)00531-8. [DOI] [PubMed] [Google Scholar]
- 2.Renaud SC, Gueguen R, Schenker J, d’Houtaud A. Alcohol and mortality in middle-aged men from eastern France. Epidemiology. 1998 Mar;9(2):184–8. Cambridge, Mass. [PubMed] [Google Scholar]
- 3.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991 Aug 24;338(8765):464–8. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 4.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. The New England journal of medicine. 1988 Aug 4;319(5):267–73. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 5.Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Jr, et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. The New England journal of medicine. 1997 Dec 11;337(24):1705–14. doi: 10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- 6.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Prior alcohol consumption and mortality following acute myocardial infarction. Jama. 2001 Apr 18;285(15):1965–70. doi: 10.1001/jama.285.15.1965. [DOI] [PubMed] [Google Scholar]
- 7.Muntwyler J, Hennekens CH, Buring JE, Gaziano JM. Mortality and light to moderate alcohol consumption after myocardial infarction. Lancet. 1998 Dec 12;352(9144):1882–5. doi: 10.1016/S0140-6736(98)06351-X. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Gray MO, Mochly-Rosen D. Cardioprotection from ischemia by a brief exposure to physiological levels of ethanol: role of epsilon protein kinase C. Proceedings of the National Academy of Sciences of the United States of America. 1999 Oct 26;96(22):12784–9. doi: 10.1073/pnas.96.22.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krenz M, Baines CP, Heusch G, Downey JM, Cohen MV. Acute alcohol-induced protection against infarction in rabbit hearts: differences from and similarities to ischemic preconditioning. Journal of molecular and cellular cardiology. 2001 Nov;33(11):2015–22. doi: 10.1006/jmcc.2001.1465. [DOI] [PubMed] [Google Scholar]
- 10.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992 Jun 20;339(8808):1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999 Dec 11;319(7224):1523–8. doi: 10.1136/bmj.319.7224.1523. Clinical research ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiraud A, de Lorgeril M, Boucher F, Berthonneche C, Rakotovao A, de Leiris J. Cardioprotective effect of chronic low dose ethanol drinking: insights into the concept of ethanol preconditioning. Journal of molecular and cellular cardiology. 2004 Apr;36(4):561–6. doi: 10.1016/j.yjmcc.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Miyamae M, Diamond I, Weiner MW, Camacho SA, Figueredo VM. Regular alcohol consumption mimics cardiac preconditioning by protecting against ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 1997 Apr 1;94(7):3235–9. doi: 10.1073/pnas.94.7.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Mochly-Rosen D. Opposing effects of delta and xi PKC in ethanol-induced cardioprotection. Journal of molecular and cellular cardiology. 2001 Mar;33(3):581–5. doi: 10.1006/jmcc.2000.1330. [DOI] [PubMed] [Google Scholar]
- 15.Bellows SD, Hale SL, Kloner RA. Acute Ethanol Does Not Protect Against Ischemic/Reperfusion Injury in Rabbit Myocardium. Journal of thrombosis and thrombolysis. 1996;3(3):181–4. doi: 10.1007/BF00181659. [DOI] [PubMed] [Google Scholar]
- 16.Hale SL, Kloner RA. Ethanol does not exert myocardial preconditioning in an intact rabbit model of ischemia/reperfusion. Heart disease (Hagerstown, Md. 2001 Sep–Oct;3(5):293–6. doi: 10.1097/00132580-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Itoya M, Morrison JD, Downey HF. Effect of ethanol on myocardial infarct size in a canine model of coronary artery occlusion-reperfusion. Molecular and cellular biochemistry. 1998 Sep;186(1–2):35–41. [PubMed] [Google Scholar]
- 18.Krenz M, Baines CP, Yang XM, Heusch G, Cohen MV, Downey JM. Acute ethanol exposure fails to elicit preconditioning-like protection in in situ rabbit hearts because of its continued presence during ischemia. Journal of the American College of Cardiology. 2001 Feb;37(2):601–7. doi: 10.1016/s0735-1097(00)01125-6. [DOI] [PubMed] [Google Scholar]
- 19.Krenz M, Cohen MV, Downey JM. The protective and anti-protective effects of ethanol in a myocardial infarct model. Annals of the New York Academy of Sciences. 2002 May;957:103–14. doi: 10.1111/j.1749-6632.2002.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 20.Krenz M, Yang XM, Qin Q, Downey JM, Cohen MV. Dose-response relationships of the protective and antiprotective effects of acute ethanol exposure in isolated rabbit hearts. Heart disease (Hagerstown, Md. 2002 Sep–Oct;4(5):276–81. doi: 10.1097/00132580-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Niccoli G, Altamura L, Fabretti A, Lanza GA, Biasucci LM, Rebuzzi AG, et al. Ethanol abolishes ischemic preconditioning in humans. Journal of the American College of Cardiology. 2008 Jan 22;51(3):271–5. doi: 10.1016/j.jacc.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki K, Mochly-Rosen D. DeltaPKC-mediated activation of epsilonPKC in ethanol-induced cardiac protection from ischemia. Journal of molecular and cellular cardiology. 2005 Aug;39(2):203–11. doi: 10.1016/j.yjmcc.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 23.Miyamae M, Rodriguez MM, Camacho SA, Diamond I, Mochly-Rosen D, Figueredo VM. Activation of epsilon protein kinase C correlates with a cardioprotective effect of regular ethanol consumption. Proceedings of the National Academy of Sciences of the United States of America. 1998 Jul 7;95(14):8262–7. doi: 10.1073/pnas.95.14.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou HZ, Karliner JS, Gray MO. Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. American journal of physiology. 2002 Jul;283(1):H165–74. doi: 10.1152/ajpheart.00408.2001. [DOI] [PubMed] [Google Scholar]
- 25.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circulation research. 2005 Aug 19;97(4):329–36. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 26.Jaburek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circulation research. 2006 Oct 13;99(8):878–83. doi: 10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 27.Guo D, Nguyen T, Ogbi M, Tawfik H, Ma G, Yu Q, et al. Protein kinase C-epsilon coimmunoprecipitates with cytochrome oxidase subunit IV and is associated with improved cytochrome-c oxidase activity and cardioprotection. American journal of physiology. 2007 Oct;293(4):H2219–30. doi: 10.1152/ajpheart.01306.2006. [DOI] [PubMed] [Google Scholar]
- 28.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, et al. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. The Journal of clinical investigation. 2004 Jun;113(11):1535–49. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CH, Budas G, Churchill E, Disatnik MH, Mochly-Rosen D. Activation of aldehyde dehydrogenase 2 reduces ischemic damage to the heart. Science. 2008 12 September;:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol. 1998 Nov;275(5 Pt 2):R1468–77. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- 31.Mukamal KJ, Mittleman MA. Acute ethanol exposure fails to elicit preconditioning-like protection in in situ rabbit hearts because of its continued presence during ischemia. Journal of the American College of Cardiology. 2001 Oct;38(4):1271. doi: 10.1016/s0735-1097(01)01531-5. [DOI] [PubMed] [Google Scholar]
- 32.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circulation research. 2002 Mar 8;90(4):390–7. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 33.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, et al. Opening of mitochondrial K(ATP) channel occurs downstream of PKC-epsilon activation in the mechanism of preconditioning. American journal of physiology. 2002 Jul;283(1):H440–7. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 34.Kaneda K, Miyamae M, Sugioka S, Okusa C, Inamura Y, Domae N, et al. Sevoflurane enhances ethanol-induced cardiac preconditioning through modulation of protein kinase C, mitochondrial KATP channels, and nitric oxide synthase, in guinea pig hearts. Anesthesia and analgesia. 2008 Jan;106(1):9–16. doi: 10.1213/01.ane.0000297298.93627.36. table of contents. [DOI] [PubMed] [Google Scholar]
- 35.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, et al. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. The Journal of biological chemistry. 2001 Aug 10;276(32):30057–63. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Henderson GI, Freeman GL. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. Journal of molecular and cellular cardiology. 2001 Nov;33(11):1919–27. doi: 10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]
- 37.Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. The American journal of physiology. 1999 Mar;276(3 Pt 2):H935–43. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- 38.Kristal BS, Park BK, Yu BP. 4-Hydroxyhexenal is a potent inducer of the mitochondrial permeability transition. The Journal of biological chemistry. 1996 Mar 15;271(11):6033–8. doi: 10.1074/jbc.271.11.6033. [DOI] [PubMed] [Google Scholar]
- 39.Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 1998 Jan 20;95(2):510–4. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blasig IE, Grune T, Schonheit K, Rohde E, Jakstadt M, Haseloff RF, et al. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. The American journal of physiology. 1995 Jul;269(1 Pt 2):H14–22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- 41.Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. Journal of molecular and cellular cardiology. 2003 Jun;35(6):615–21. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 42.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, et al. HNE interacts directly with JNK isoforms in human hepatic stellate cells. The Journal of clinical investigation. 1998 Dec 1;102(11):1942–50. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soh Y, Jeong KS, Lee IJ, Bae MA, Kim YC, Song BJ. Selective activation of the c-Jun N-terminal protein kinase pathway during 4-hydroxynonenal-induced apoptosis of PC12 cells. Molecular pharmacology. 2000 Sep;58(3):535–41. doi: 10.1124/mol.58.3.535. [DOI] [PubMed] [Google Scholar]
- 44.Miyamae M, Camacho SA, Zhou HZ, Diamond I, Figueredo VM. Alcohol consumption reduces ischemia-reperfusion injury by species-specific signaling in guinea pigs and rats. The American journal of physiology. 1998 Jul;275(1 Pt 2):H50–6. doi: 10.1152/ajpheart.1998.275.1.H50. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong SC, Hoover DB, Delacey MH, Ganote CE. Translocation of PKC, protein phosphatase inhibition and preconditioning of rabbit cardiomyocytes. Journal of molecular and cellular cardiology. 1996 Jul;28(7):1479–92. doi: 10.1006/jmcc.1996.0138. [DOI] [PubMed] [Google Scholar]
- 46.Kawamura S, Yoshida K, Miura T, Mizukami Y, Matsuzaki M. Ischemic preconditioning translocates PKC-delta and -epsilon, which mediate functional protection in isolated rat heart. The American journal of physiology. 1998 Dec;275(6 Pt 2):H2266–71. doi: 10.1152/ajpheart.1998.275.6.H2266. [DOI] [PubMed] [Google Scholar]
- 47.Liu H, McPherson BC, Yao Z. Preconditioning attenuates apoptosis and necrosis: role of protein kinase C epsilon and -delta isoforms. American journal of physiology. 2001 Jul;281(1):H404–10. doi: 10.1152/ajpheart.2001.281.1.H404. [DOI] [PubMed] [Google Scholar]
- 48.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. The American journal of physiology. 1994 Mar;266(3 Pt 2):H1145–52. doi: 10.1152/ajpheart.1994.266.3.H1145. [DOI] [PubMed] [Google Scholar]
- 49.Saurin AT, Pennington DJ, Raat NJ, Latchman DS, Owen MJ, Marber MS. Targeted disruption of the protein kinase C epsilon gene abolishes the infarct size reduction that follows ischaemic preconditioning of isolated buffer-perfused mouse hearts. Cardiovascular research. 2002 Aug 15;55(3):672–80. doi: 10.1016/s0008-6363(02)00325-5. [DOI] [PubMed] [Google Scholar]
- 50.Ehrig T, Bosron WF, Li TK. Alcohol and aldehyde dehydrogenase. Alcohol and alcoholism. 1990;25(2–3):105–16. doi: 10.1093/oxfordjournals.alcalc.a044985. Oxford, Oxfordshire. [DOI] [PubMed] [Google Scholar]
- 51.Kabir AM, Clark JE, Tanno M, Cao X, Hothersall JS, Dashnyam S, et al. Cardioprotection initiated by reactive oxygen species is dependent on activation of PKCepsilon. American journal of physiology. 2006 Oct;291(4):H1893–9. doi: 10.1152/ajpheart.00798.2005. [DOI] [PubMed] [Google Scholar]
- 52.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008 Feb;294(2):C460–6. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 53.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free radical biology & medicine. 2000 May 1;28(9):1349–61. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 54.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. The Journal of biological chemistry. 1993 Sep 5;268(25):18532–41. [PubMed] [Google Scholar]
- 55.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circulation research. 2005 Jul 8;97(1):78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 56.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. The Journal of biological chemistry. 2004 Nov 12;279(46):47985–91. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 57.Lewandowski ED, White LT. Pyruvate dehydrogenase influences postischemic heart function. Circulation. 1995 Apr 1;91(7):2071–9. doi: 10.1161/01.cir.91.7.2071. [DOI] [PubMed] [Google Scholar]
- 58.Churchill EN, Szweda LI. Translocation of deltaPKC to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Archives of biochemistry and biophysics. 2005 Jul 15;439(2):194–9. doi: 10.1016/j.abb.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Andrukhiv A, Costa AD, West IC, Garlid KD. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. American journal of physiology. 2006 Nov;291(5):H2067–74. doi: 10.1152/ajpheart.00272.2006. [DOI] [PubMed] [Google Scholar]
- 60.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. Journal of molecular and cellular cardiology. 1997 Jan;29(1):207–16. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 61.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovascular research. 2004 Feb 15;61(3):372–85. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 62.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004 Apr 13;109(14):1714–7. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 63.Baines CP, Cohen MV, Downey JM. Signal transduction in ischemic preconditioning: the role of kinases and mitochondrial K(ATP) channels. Journal of cardiovascular electrophysiology. 1999 May;10(5):741–54. doi: 10.1111/j.1540-8167.1999.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 64.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annual review of physiology. 2000;62:79–109. doi: 10.1146/annurev.physiol.62.1.79. [DOI] [PubMed] [Google Scholar]
- 65.Awasthi YC, Ansari GA, Awasthi S. Regulation of 4-hydroxynonenal mediated signaling by glutathione S-transferases. Methods in enzymology. 2005;401:379–407. doi: 10.1016/S0076-6879(05)01024-4. [DOI] [PubMed] [Google Scholar]
- 66.Raza H, John A, Brown EM, Benedict S, Kambal A. Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicology and applied pharmacology. 2008 Jan 15;226(2):161–8. doi: 10.1016/j.taap.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, et al. Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. Journal of molecular and cellular cardiology. 2006 Feb;40(2):283–94. doi: 10.1016/j.yjmcc.2005.11.006. [DOI] [PubMed] [Google Scholar]