Abstract
Background:
Amino acids are important nutrients during fetal development, and the activity of placental amino acid transporters is crucial in the regulation of fetal growth. Leptin, an adipocyte- and placenta-derived hormone, has been proposed to act as a peripheral signal in reproduction in humans. Leptin is elevated during pregnancy and elevated further in pathologic pregnancies such as preeclampsia. However, the role of leptin in placental function has not been fully elucidated. We hypothesize that leptin plays a role in the regulation of placental amino acid transport by activation of the JAK-STAT pathway.
Methods:
Placental amino acid transport, specifically system A transport was studied in placental villous fragments using the amino acid analog, methylaminoisobutyric acid (MeAIB). Specific inhibitors of the JAK-STAT signal transduction pathway were used to further elucidate their role in leptin-mediated effects on amino acid transport activity. Western blotting was performed to identify STAT3 phosphorylation as a measure of leptin receptor activation.
Results:
Leptin significantly increased system A amino acid transporter activity by 22-42% after 1 h of incubation. Leptin activated JAK-STAT signaling pathway as evidenced by STAT3 phosphorylation, and inhibition of STAT3 or JAK2 resulted in 36-45% reduction in system A amino acid transporter activity. Furthermore, blocking endogenously produced leptin also decreased system A transport by 45% comparable to STAT3 inhibition.
Conclusions:
These data demonstrate that leptin stimulates system A by JAK-STAT dependent pathway in placental villous fragments. Our findings support the autocrine/paracrine role of leptin in regulating amino acid transport in the human placenta.
Introduction
Amino acids are an important nutrient for fetal growth and development [1, 2]. Amino acids are actively transported to the fetal circulation via the placenta and are used not only for protein synthesis but also serve as a metabolic energy source. It is estimated that as much as 20-40% of the total energy supplied to the fetal/placental unit may be derived from amino acids [3]. Blood amino acid concentrations are significantly lower in intrauterine growth restricted (IUGR) fetuses. Cord blood amino acid concentrations are significantly elevated in pregnancies complicated by preeclampsia [4-6]. Fetal growth is associated with specific changes in nutrient transporters, including the placental system A amino acid transporter [7-11]. System A is a highly regulated sodium-dependent transport system capable of transporting small, non-branched amino acids such as alanine, glutamine, glycine, and serine. It is regulated by many effectors, including insulin, glucagon, cortisol, pH and oxygen levels [12-14]. Placental system A amino acid transporter activity is significantly lower in pregnancies complicated by IUGR and elevated in diabetic pregnancies [7, 15-17].
Leptin, the product of the Lep gene, was initially discovered as an adipocyte hormone involved in appetite regulation and energy balance [18]. However, over the past several years additional evidence has accumulated demonstrating that leptin has other important biological activities including a role in reproduction [19, 20]. Specifically, leptin concentrations provide a peripheral signal of adequate nutritional status via body fat stores and leptin is involved in the initiation of puberty. Circulating leptin concentrations during pregnancy are elevated 2 to 3 fold above that observed in nonpregnant women, leptin concentrations are further elevated in the pregnancy complication preeclampsia and are lower in pregnancies complicated by IUGR [21-24]. In addition, the leptin receptor (LepR) is present in several reproductive tissues including: gonadotropin-releasing hormone neurons, ovary, endometrium, and placenta [25-29].
Previously, a study by Jansson et al. demonstrated that leptin stimulated placental amino acid transport in human placenta [30]. We hypothesized that placental leptin may act in an autocrine/paracrine fashion to influence placental system A amino acid transport, and does so by activating JAK-STAT pathways in the placental villous fragment model.
Methods
Buffers and Reagents
Experiments using placental villous tissue were carried out using Tyrodes buffer as previously described by Shibata et al. [31]. Tyrodes buffer consisted of 135mM NaCl (or 135mM choline chloride for sodium-free Tyrodes buffer), 5mM KCl, 1.8mM CaCl2, 1.0mM MgCl2, 10mM HEPES, and 5.6mM glucose, at pH 7.4 (adjusted with NaOH for sodium containing buffer or KOH for sodium-free buffer). All tissue incubations and dissections were carried out in a buffer consisting of one volume of Dulbecco's Modified Eagle's Medium (DMEM, containing 5.6mM glucose, amino acids, vitamins and minerals) mixed with three volumes of Tyrodes buffer; DMEM/Tyrodes (1:3 v/v). Because DMEM contains high amino acid concentrations, DMEM/Tyrodes (1:3 v/v) was used to achieve a more physiologic concentration of amino acids. Leptin (human), insulin (human), angiotensin II, were purchased from Sigma-Aldrich (St. Louis, MO,), JSI-124 (inhibitor of STAT3) and 1,2,3,4,5,6-Hexabromocyclohexane (inhibitor of JAK2) were from Calbiochem (Gibbstown, NJ) . 14C-Methylaminoisobutyric Acid (MeAIB; 50.5mCi/mmol) was purchased from NEN Life Science Products (Boston, MA). Antibodies against STAT3, pSTAT3, leptin receptor and anti-goat FITC were from Santa Cruz Biotechnology (Santa Cruz, CA), anti-leptin and SOCS3 antibody were from Abcam (Cambridge, MA), and anti- β-actin antibody was from Sigma (St. Louis, MO).
Treatment solutions
Leptin was diluted in 15mM HCl and 7.5mM NaOH and stored as a stock solution at −20°C. Experimental concentrations of leptin (100-1000ng/ml) were achieved by diluting stock solutions in DMEM/Tyrodes medium and were chosen in accordance with published literature [30]. Insulin (in buffer consisting of 0.1% BSA, 1.6% glycerol) was freshly prepared for each experiment to a final concentration of 300ng/ml [30]. Angiotensin II (100 nM), JSI-124 (0.005-0.5 μM) and 1,2,3,4,5,6-Hexabromocyclohexane (50 μM) were reconstituted in DMSO and stored as stock solutions at −20°C.
Placental villous fragment preparation
Fresh placental tissue was obtained within 10 min after delivery from full-term, uncomplicated pregnancies as described previously [31]. Labor was not found to significantly influence system A activity in this experimental system (labored, 1.21 vs. non-labored, 1.22 fold change in MeAIB/mg protein/40 min after leptin treatment, P=0.7). This study was approved by the University of Pittsburgh Institutional Review Board and all women gave written informed consent. Biopsies were collected from the maternal side of the placenta, after removal of the decidua, from a central part of cotyledons between the umbilical cord insertion site and the peripheral edge of the placenta that was free of infarcts. Placental tissue was washed 3x at room temperature in PBS and then transported to the laboratory at room temperature in DMEM/Tyrodes buffer (1:3 v/v). Placental tissue was further dissected into small fragments (approximately 1mm3) in DMEM/Tyrodes buffer (1:3 v/v). Single villous fragments were tied with # 4-0 silk suture and hung on specially designed hooks capable of holding three individual villous fragments [31]. All dissection procedures were performed at room temperature.
Measurement of system A activity
Placental system A activity was measured using the method described previously with some modifications [30, 31]. All determinations were performed in triplicate. Prepared villous fragments were pre-incubated for 1 h to 2 h in 1ml DMEM/Tyrodes (1:3 v/v) buffer in the presence or absence of leptin (or other effectors as experiments dictated) at 37°C in a shaking water bath. After pre-incubation, the villous fragments were washed twice in sodium-containing (for total uptake) or sodium-free (for sodium independent uptake) Tyrodes buffer at 37°C for 1 min each. Subsequently, the uptake of C14-MeAIB, a specific substrate transported by system A, was measured in sodium-containing and sodium-free (for non-specific uptake) Tyrodes buffer containing C14-MeAIB (1.7 μmol/L, 0.05μCi/ml) for 40 min at 37°C. Previous studies showed that the uptake of C14-MeAIB is linear up to 180 min [31, 32]. The uptake of C14-MeAIB by villous fragments was stopped by washing the fragments in ice-cold sodium-free Tyrodes buffer two times for 20 sec each. Villous fragments were incubated in 1 ml distilled H2O for 18 h to release the accumulated C14-MeAIB, and then the released C14-MeAIB measured using a scintillation counter. Each villous fragment was incubated in 0.25 ml of 0.3N NaOH overnight to denature the proteins, and the total protein amount was determined by the Bradford method. Total pmol of C14-MeAIB accumulated was calculated from the radioactive value and normalized to the protein content of its respective villous fragment. System A activity was calculated by subtracting C14-MeAIB uptake in sodium-free media from C14-MeAIB uptake in sodium-containing media and expressed as pmol C14-MeAIB/mg protein/40 min. System A activity assays were completed within 4 h after delivery in order to avoid significant loss of normal morphology and functional integrity of the villous trophoblast [30-33].
Time course experiments
Time course experiments were performed as described above after pre-incubating villous fragments for 0, 1 h and 2 h in DMEM/Tyrodes medium, DMEM/Tyrodes medium containing leptin (500 ng/ml), anti-leptin antibody (5 μg/ml), non-specific antibody (5 μg/ml, anti-goat FITC), JSI-124 (STAT3 inhibitor, 0.5 μM), or 1,2,3,4,5,6-Hexabromocyclohexane (JAK2 inhibitor, 50 μM) before C14-MeAIB uptake was performed.
LDH determination
LDH release was determined as a measure of cell death using a colorimetric assay (Sigma, St. Louis, MO) and expressed as percentage of maximum releasable LDH. Maximum releasable LDH was expressed as LDH per milligram protein after complete cell lysis and obtained by sonicating a fresh placental villous explants (equivalent in size to the explants used for experiments) from matching placentae in the same culture media used for experimentation [31].
Western blot analysis
Western blot analysis was performed according to the published protocols [34]. Total protein extracts prepared from placental villous fragments were used in Western blot analysis for total STAT3, pSTAT3, SOCS3 and β-actin protein expression and were prepared by sonication of 10 mg of villous tissue in 8 volumes of 1X Laemmli buffer (50 mM Tris HCl, pH 6.8, 2% SDS, 10% Glycerol) containing 5 mM DTT, 0.5 mM PMSF, 1 mM sodium vanadate and 1 μl per ml protease inhibitors cocktail (Calbiochem, San Diego, CA).
Fifteen micrograms of protein were separated on a 10% (STAT3, pSTAT3, SOCS3, β-actin) SDS containing polyacrylamide gel. Incubation with specific primary antibodies to the proteins of interest were performed for 1 h at room temperature (STAT3 1:1000, pSTAT3 1:200, SOCS3 1:1000). After incubation with primary antibodies, membranes were washed 3x in TBS-0.05%Tween20 buffer for 10 min each and incubated with a species specific secondary antibody (Santa Cruz, CA, 1:2500) for 30 min. Chemiluminescent detection was carried out using the CDP Star detection system as per the manufacturer's protocol (Roche, Indianapolis, IN). Membranes were exposed for different times to Kodak Bio-Max–AR film. Films were scanned and density of the proteins of interest were estimated using the software, Unscanit (Silk Scientific, Orem, UT). For the analysis of β-actin, the membranes were stripped and reprobed with anti β-actin antibody to account for protein loading variations [35].
Statistical analysis
Differences in system A activity or protein expression between individual experiments were controlled by expressing the values in experimental conditions as fold changes of activity or protein expression compared to the matched control sample for each individual experiment [36]. Data are expressed as the median fold change (interquartile range as appropriate) in system A activity or protein expression [36]. The reported number of samples represents data from individual placentas. All amino acid transport activity assays were performed in triplicate. Protein expression data were expressed as the pSTAT3/STAT3 ratio after adjusting for β-actin. Statistical analysis was performed using STATA 8 (STATA Corporation, Collage Station, TX). Data were analyzed by non-parametric Wilcoxon signed rank test or Kruskal-Wallis test as appropriate. Cuzick's test of trend was used to determine a dose response relationship between STAT3 inhibitor concentration and system A activity. Statistical significance was accepted at p value less than 0.05.
Results
Leptin activates JAK-STAT signaling pathway
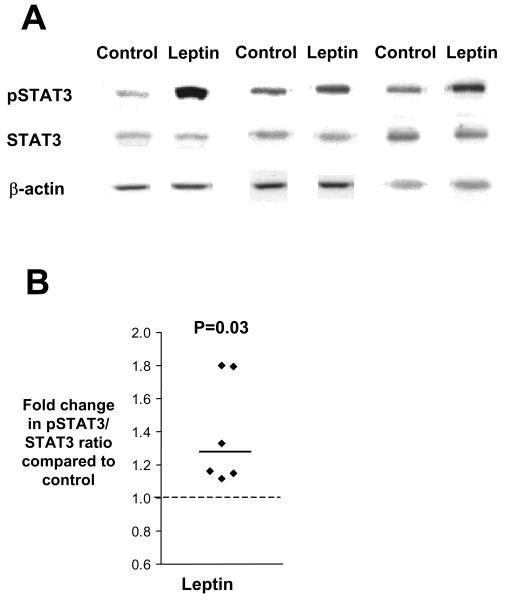
In order to confirm the presence of a functional leptin receptor in placental villous fragments we investigated the ability of leptin to activate the leptin receptor in placental villous fragments by measuring the phosphorylation of the transcription factor STAT3. As shown in figure 1, incubation of placental villous fragments with leptin (1000 ng/ml) led to a significant increase in phosphorylated STAT3 (pSTAT3) within 10 min compared to untreated villous fragments (1.24 fold higher (1.15-1.79), n=6 placentas, P<0.05).
Figure 1.
Leptin receptor activation increases STAT3 phosphorylation. A) Stimulation of STAT3 phosphorylation by leptin in placental villous fragments. Villous fragments were treated for 10 min with leptin (1000 ng/ml). Representative western Blot of pSTAT3, STAT3 and β-actin as respective loading control of three experiments on a 10% gel. B) Representative scatter plot showing increased pSTAT3/STAT3 ratio for leptin treated villous fragments as compared to control (n=6 placentas). Data are presented as fold change in pSTAT3/STAT3 ratio of leptin treated fragments compared with that of control (dashed line). The median fold change for each condition is denoted by a solid line.
Leptin increases placental system A activity
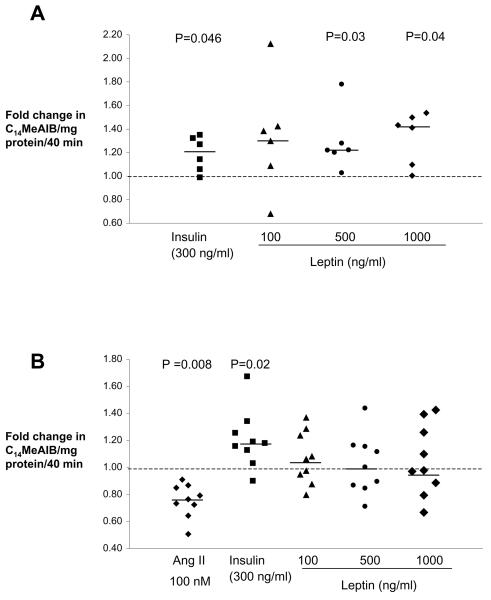
As shown in figure 2A and in the supplementary table, leptin (at 500 and 1000 ng/ml) significantly increased system A amino acid transport activity by 22-42% in placental villous fragments after a 1 h incubation compared to time control villous fragments (P<0.05). However, the effect of leptin on system A activity was lost after 2 h of incubation (figure 2B and supplementary table). In contrast, insulin (positive control) increased system A activity after both a 1 h and a 2 h incubation by 21% and 18% (figure 2A, 2B, supplementary table, P<0.05) compared to untreated time controls, and angiotensin II (negative control) significantly decreased system A activity by 24% (figure 1B, supplementary table, P<0.01).
Figure 2.
Leptin effect on placental system A activity. A) Leptin and insulin significantly increase system A amino acid transport activity in placental villous fragments after a 1 h incubation (n=6 placentas). B) After 2 h of incubation leptin does not increase system A amino acid transport activity, but insulin increases and angiotensin II (ANG II) decreases system A activity. (n=9 placentas). Data are presented as fold change in system A activity compared with that of control (dashed line). The median fold change for each condition is denoted by a solid line.
Leptin induced increase of system A activity is dependent on STAT3 phosphorylation
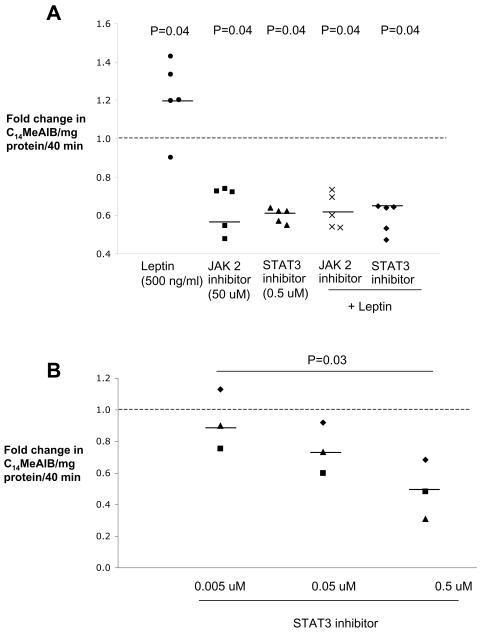
To determine whether the leptin induced increase of system A activity is dependent on activation of the JAK-STAT pathway we performed the experiments in the presence of inhibitors for JAK2 (1,2,3,4,5,6-Hexabromocyclohexane, 50 μmol/L) or STAT3 (JSI-124, 0.5 μmol/L). After a 1 h preincubation, system A activity was significantly blunted by 36-45%, (figure 3A, supplementary table, n=5 placentas, P<0.05) by both inhibitors in the presence or absence of leptin (500 ng/ml). In addition, we observed that the inhibitory effect of JSI-124 on system A activity was dose dependent (figure 3B, supplementary table, n=3, P<0.05).
Figure 3.
Leptin induced increase of system A activity is dependent on STAT3 phosphorylation. A) Inhibition of JAK2 and STAT3 phosphorylation reduces system A amino acid transport by 36-45% (n=5 placentas). B) The effect of STAT3 inhibitors on system A activity is dose dependent (n=3 placentas). Data are presented as fold change in system A activity compared with that of control (dashed line). The median fold change for each condition is denoted by a solid line.
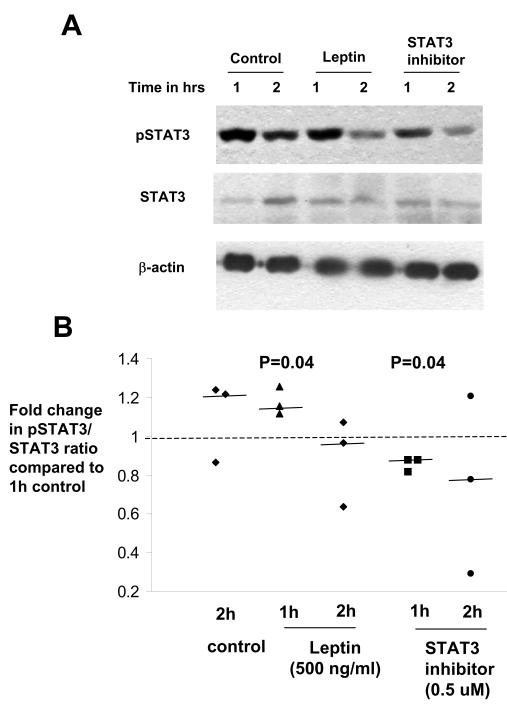
We further investigated the effect of leptin on STAT3 phosphorylation by western blot analysis. In controls, pSTAT3 expression was higher after 1-2 h incubation in DMEM/Tyrodes medium compared to after 10 min (figure 1A, 4A). Leptin (500 ng/ml) incubation for 1 h further increased STAT3 phosphorylation by 25%, and this effect was diminished by 2 h. Blocking the STAT3 pathway decreased phosphorylated STAT3 at 1 h by 18% compared to control villous fragments (figure 4, n=5 placentas, P<0.05).
Figure 4.
Western Blot analyses to detect phosphorylated STAT3 after incubation with leptin (500 ng/ml) or STAT3 inhibitor (0.5 μM). A) Representative western blot to demonstrate the relative levels of phosphorylated STAT3 (pSTAT3), STAT3, or β-actin in villous fragments incubated with control, leptin (500 ng/ml) or STAT3 phosphorylation inhibitor (0.5 μM) for 1 h and 2 h (n=5 placentas). B) Leptin treatment of villous fragments increased pSTAT3/STAT3 ratio after 1 h of incubation and STAT3 inhibitor treatment reduced pSTAT3/STAT3 ratio when compared to control at 1 h, (n=5 placentas). The median fold change for each condition is denoted by a solid line.
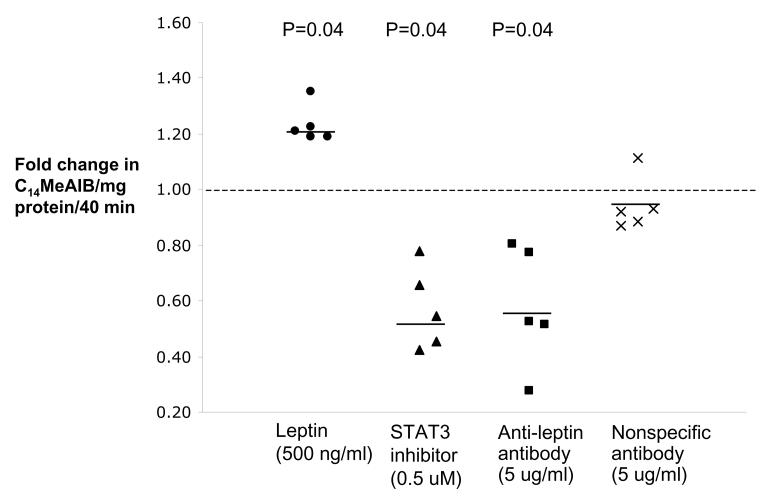
Binding of endogenous leptin reduces system activity
We next investigated whether endogenously produced leptin by villous fragments is capable of influencing system A transport activity. To answer this question, we measured system A activity after 1 h and 2 h preincubation in the absence and presence of leptin (500 ng/ml), an anti-leptin antibody (5 μg/ml) or the STAT3 inhibitor JSI-124 (0.5 μmol/L). Blocking of endogenously produced leptin with an anti-leptin antibody significantly reduced system A activity after 1 h by 45-49% (figure 5, supplementary table, n=5, P<0.05) and this effect was similar to that observed with the STAT3 inhibitor JSI-124. Importantly, system A activity was not affected after incubation with a non-specific antibody (anti-FITC) compared to the time control (Figure 5 and supplementary table). However, in contrast to the data obtained at 1 h, after 2 h of preincubation, system A activity of fragments treated with leptin, anti-leptin antibody or JSI-124 approached control levels (supplementary table).
Figure 5.
Effect of binding endogenous leptin on system A activity. Binding of endogenous leptin with an anti-leptin antibody (5 μg/ml) reduces system A amino acid transport within 1 h of incubation by 45%. A nonspecific antibody had no effect on system A activity (n=5 placentas).
Non-specific MeAIB accumulation in the tissue, as measured by MeAIB uptake in sodium-free medium was 8.9 ± 1.2 pmol/MeAIB/40min in the control group, 9.7 ± 1.4 pmol/MeAIB/40min in leptin treated samples, 9.7 ± 1.2 pmol/MeAIB/40min in JAK2 inhibitor treated samples, 10.1 ± 1.2 pmol/MeAIB/40min in STAT3 inhibitor treated group and 7.9 ± 0.97 pmol/MeAIB/40min leptin antibody treated explants (Kruskal-Wallis p=0.4).
Treatment of villous fragments did not increase LDH release into the media
Preincubation with the different treatment solutions or for different periods of time was not associated with significant LDH release into the media (p>0.05; supplementary figure). Therefore the observed effects on system A activity appear unrelated to loss of cell viability during preincubation. Also, the use of DMSO as vehicle to reconstitute JAK2 and STAT3 inhibitors did not significantly affect system A activity or LDH release by villous fragments (p>0.05).
Discussion
The hormonal regulation of placental amino acid transport activity has not been fully elucidated. The present study examined the role of leptin in placental amino acid transport using primary villous fragments as an experimental model. We observed that incubation of villous fragments with leptin stimulates phosphorylation of STAT3 and activation of the JAK-STAT pathway. We found that exogenous leptin stimulates system A amino acid uptake in primary villous fragments from the placenta, and that this effect is pronounced within the first hour of leptin exposure and is absent by 2 h. In contrast, the stimulatory effect of insulin and the inhibitory effect of angiotensin II on placental system A activity persists after 2 h of incubation. The effect of leptin on system A amino acid transporter activity was significantly reduced by blocking STAT3 or JAK2. Lastly, incubation of villous fragments with excess antibody to leptin in order to bind and inhibit endogenously produced leptin by villous tissue led to a significant reduction in system A amino acid transport compared to time controls, and the inhibitory effect observed with the anti-leptin antibody was similar to that observed with STAT3 inhibition.
Normal fetal growth and development is dependent on an adequate supply of nutrients to the fetus. Transport of nutrients across the syncytiotrophoblast cell membranes of the placenta constitutes an important step in nutrient delivery to the growing fetus. System A is a ubiquitous sodium-dependent transporter that actively transports small, zwitterionic, neutral amino acids [17]. In vitro studies have demonstrated that insulin, cortisol, IGF-I, and leptin stimulate placental system A activity at term [30, 37, 38]. Surprisingly, a study by Ericsson in first trimester villous fragments showed that system A amino acid transport is not regulated by leptin or insulin [39]. However, the present findings reinforce the hypothesis that leptin may play a role in the regulation of placental nutrient transport in the later half of pregnancy and thereby influence fetal growth. Importantly, placental amino acid transport activity is lower in pregnancies complicated by IUGR and elevated in diabetic pregnancies [7, 15, 16]. In addition, circulating leptin concentrations are elevated in diabetic pregnancies and preeclampsia [21, 40, 41]. It is interesting to speculate that leptin may be involved in regulating placental nutrient transport in these pregnancy complications. However, this will require further investigations.
The technique of using single, isolated villous fragments to study placental amino acid uptake has several potential advantages. It enables villous transport to be studied in primary (explant) tissue, maintaining microvillous membrane/basal membrane polarization and cell-cell contacts, and avoiding potential changes in transporter characteristics that may occur with passaged cells in culture. In addition, the ability to maintain intact villous tissue with functional membrane receptors allows for the investigation of the effect of exogenous factors such as growth factors and hormones including leptin. However, the use of tissue fragments does not permit the differentiation between apical and basal uptake by the syncytiotrophoblast, and non-specific uptake of solutes by the extracellular space that is not accounted for by the sodium-independent treatment group may have confounding effects on the interpretation of results. Also, the ability to retain the structural and functional integrity of the fragments is dependent on experimental conditions. It was previously shown that system A amino acid transport activity is linear up to 3 h in the fragment model and that villous fragments remain viable up to 4 h in DMEM/Tyrode's buffer, but appear vacuolated and degenerated after long term incubations [30, 31, 42]. We used the release of LDH, an intracellular enzyme, as a measure for cell integrity in our model. During short time incubations for 1 h and 2 h, which have been used before in other studies [30, 31, 36], there was no significant difference in LDH release between the experimental groups indicating that differences in amino acid transport are unlikely to be the result of changes in cell viability.
In this study we observed that leptin enhances system A amino acid transport after a 1 h incubation. These data confirm the findings of a previous study where leptin concentrations higher than usually seen in the circulation resulted in a stimulation of this amino acid transporter in the villous fragment model [30]. However, this effect was lost after 2 h of incubation, which was not the case for 2 h incubations with insulin (300 ng/ml) or angiotensin II (100 nM), which increase and decrease placental system A amino acid transport activity respectively [30-32, 38]. The explanation for this difference in stimulatory activity between leptin and insulin is not readily apparent. Leptin and insulin have similarities in their cell signaling activities and their receptors are functionally similar. A possible explanation may be the loss of leptin; however, this is unlikely despite the fact that the half-life of leptin is 30 min in the circulation [43], and the concentrations of leptin used in this study were higher than physiologic concentrations in pregnant women (30ng/ml)[23]. Another possibility may be the production of a soluble form of the leptin receptor by the villous fragments that is capable of binding and inactivating the exogenous leptin in the experimental system. However, the relative amount of this soluble receptor is likely too low to overcome the ex-vivo elevated leptin concentrations in our system, and therefore are unlikely to be the cause of the loss of leptin stimulation after 2 h. It is possible that the loss of leptin stimulation may be the result of feedback inhibition and the down regulation of signaling by the leptin receptor. This seems a more likely explanation given our findings of reduced STAT3 phosphorylation at 2 h, that this activity has been previously described for the leptin receptor, and this feedback inhibition is common among other cytokine like receptors [44, 45]. Additional experiments looking at suppressor of cytokine signaling 3 (SOCS3) expression did not show a difference between controls and leptin treated samples at 1 h and 2 h suggesting that SOCS3 inhibition of leptin signaling e.g. by binding to JAKs or to the leptin receptor may not be involved in the downregulation of system A transport at 2 h (SOCS3/actin ratio in control group at 1 h and 2 h: 0.63; 0.7 and leptin treated group at 1 h and 2 h: 0.69; 0.68; P=0.97). However, this mechanism will require further investigation. Lastly, the fact that insulin and angiotensin II stimulate prolonged activation or reduction of system A activity would suggest that changes in the transporter proteins themselves may not be involved in the down-regulatory phase seen with leptin stimulation.
Leptin binding to its cell surface receptor results in activation of JAK and the transmission of subsequent downstream phosphotyrosine-dependent signaling [46]. In the current experimental system we find that leptin stimulation leads to the phosphorylation of STAT3. In addition, blocking the JAK-STAT pathway led to a significant dose-dependent decrease in system A transporter activity. However, the observation of increasing system A transporter activity in controls and the increasing STAT3 phosphorylation over time (10 min vs. 1 hr) opens new questions about the release of endogenous factors that stimulate amino acid transport and STAT signaling pathways. While it is unlikely that factors present in DMEM/Tyrodes medium drive these changes (buffer with electrolytes, physiologic amino acid concentrations) we speculate that endogenous leptin produced by villous tissue may be one factor that maintains basal system A activity and increases activity over time in vitro. In accordance with this hypothesis, we observed a significant reduction in system A activity in leptin blocking experiments comparable to inhibition of the JAK-STAT pathway.
While the exact mechanism(s) for acute upregulation of system A transporter by leptin in the placenta are unclear, it has been shown in skeletal muscle and adipose tissue that translocation of pre-formed SNAT proteins to the cell membrane can be stimulated by insulin via the PIK3 pathway [47, 48]. Leptin and insulin activate many of the same pathways, such as MAPK, STAT1 and 3, and PIK3. Therefore it is also possible that leptin signaling via PIK3 activates translocation of intracellular system A proteins to the microvillous membrane of the syncytiotrophoblast.
In summary, we showed that placental villous fragments have functional leptin receptors and that leptin can activate the STAT3 signaling pathway in the placental villous fragment model. We have confirmed that leptin is capable of stimulating placental system A amino acid transport in a time specific manner and that this is dependent on activation of JAK-STAT signaling pathway. In addition, we found that endogenously produced leptin has an autocrine/paracrine affect on placental system A activity. The present study supports the hypothesis that leptin is capable of influencing placental nutrient transport, and that leptin may play an important role in regulating fetal growth.
Supplementary Material
LDH release into media by villous fragments across the treatment groups. Data are shown as median percentages of maximum releasable LDH ± SEM. Median LDH release into media was not different across the treatment groups (p=0.23).
Acknowledgements
We are grateful to the staff of the Clinical Data Core and the nurses of Magee-Womens Hospital for invaluable assistance in obtaining placental samples.
This project was supported by National Institutes of Health grant number P01-HD30367, the Pennsylvania Department of Health, the Magee-Womens Research Institute & Foundation, and by funds received from the “Rotationsprogramm” of the Faculty of Medicine, RWTH Aachen University, Germany.
References
- 1.Moe AJ. Placental amino acid transport. American Journal of Physiology. 1995;268:C1321–1331. doi: 10.1152/ajpcell.1995.268.6.C1321. [DOI] [PubMed] [Google Scholar]
- 2.Regnault TR, Friedman JE, Wilkening RB, Anthony RV, Hay WW., Jr Fetoplacental transport and utilization of amino acids in IUGR--a review. Placenta. 2005;26(Suppl A):S52–62. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Bauer MK, Harding JE, Bassett NS, Breier BH, Oliver MH, Gallaher BH, Evans PC, Woodall SM, Gluckman PD. Fetal growth and placental function. Molecular & Cellular Endocrinology. 1998;140:115–120. doi: 10.1016/s0303-7207(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 4.Cetin I, Corbetta C, Sereni LP, Marconi AM, Bozzetti P, Pardi G, Battaglia FC. Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. American Journal of Obstetrics & Gynecology. 1990;162:253–261. doi: 10.1016/0002-9378(90)90860-a. [DOI] [PubMed] [Google Scholar]
- 5.Bajoria R, Sooranna SR, Ward S, D'Souza S, Hancock M. Placental transport rather than maternal concentration of amino acids regulates fetal growth in monochorionic twins: implications for fetal origin hypothesis. American Journal of Obstetrics & Gynecology. 2001;185:1239–1246. doi: 10.1067/mob.2001.118269. [DOI] [PubMed] [Google Scholar]
- 6.Evans RW, Powers RW, Ness RB, Cropcho LJ, Daftary AR, Harger GF, Vergona R, Finegold DN. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction. 2003;125:785–790. doi: 10.1530/rep.0.1250785. [DOI] [PubMed] [Google Scholar]
- 7.Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- 8.Jansson T, Persson E. Placental transfer of glucose and amino acids in intrauterine growth retardation: studies with substrate analogs in the awake guinea pig. Pediatric Research. 1990;28:203–208. doi: 10.1203/00006450-199009000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatric Research. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. Journal of Clinical Endocrinology & Metabolism. 1993;77:1554–1562. doi: 10.1210/jcem.77.6.8263141. [DOI] [PubMed] [Google Scholar]
- 11.Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 12.Jones HN, Ashworth CJ, Page KR, McArdle HJ. Cortisol stimulates system A amino acid transport and SNAT2 expression in a human placental cell line (BeWo) American Journal of Physiology - Endocrinology & Metabolism. 2006;291:E596–603. doi: 10.1152/ajpendo.00359.2005. [DOI] [PubMed] [Google Scholar]
- 13.Nelson DM, Smith SD, Furesz TC, Sadovsky Y, Ganapathy V, Parvin CA, Smith CH. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. American Journal of Physiology - Cell Physiology. 2003;284:C310–315. doi: 10.1152/ajpcell.00253.2002. [DOI] [PubMed] [Google Scholar]
- 14.Yao D, Mackenzie B, Ming H, Varoqui H, Zhu H, Hediger MA, Erickson JD. A novel system A isoform mediating Na+/neutral amino acid cotransport. Journal of Biological Chemistry. 2000;275:22790–22797. doi: 10.1074/jbc.M002965200. [DOI] [PubMed] [Google Scholar]
- 15.Dicke JM, Henderson GI. Placental amino acid uptake in normal and complicated pregnancies. American Journal of the Medical Sciences. 1988;295:223–227. doi: 10.1097/00000441-198803000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatric Research. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Johnson LW, Smith CH. Neutral amino acid transport systems of microvillous membrane of human placenta. American Journal of Physiology. 1988;254:C773–780. doi: 10.1152/ajpcell.1988.254.6.C773. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 19.Cervero A, Dominguez F, Horcajadas JA, Quinonero A, Pellicer A, Simon C. The role of the leptin in reproduction. Current Opinion in Obstetrics & Gynecology. 2006;18:297–303. doi: 10.1097/01.gco.0000193004.35287.89. [DOI] [PubMed] [Google Scholar]
- 20.Hoggard N, Hunter L, Trayhurn P, Williams LM, Mercer JG. Leptin and reproduction. Proceedings of the Nutrition Society. 1998;57:421–427. doi: 10.1079/pns19980061. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy JF, Misra DN, Roberts JM. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. American Journal of Obstetrics & Gynecology. 1999;180:731–736. doi: 10.1016/s0002-9378(99)70280-2. [DOI] [PubMed] [Google Scholar]
- 22.Yildiz L, Avci B, Ingec M. Umbilical cord and maternal blood leptin concentrations in intrauterine growth retardation. Clinical Chemistry & Laboratory Medicine. 2002;40:1114–1117. doi: 10.1515/CCLM.2002.195. [DOI] [PubMed] [Google Scholar]
- 23.Laivuori H, Gallaher MJ, Collura L, Crombleholme WR, Markovic N, Rajakumar A, Hubel CA, Roberts JM, Powers RW. Relationships between maternal plasma leptin, placental leptin mRNA and protein in normal pregnancy, pre-eclampsia and intrauterine growth restriction without pre-eclampsia. Molecular Human Reproduction. 2006;12:551–556. doi: 10.1093/molehr/gal064. [DOI] [PubMed] [Google Scholar]
- 24.Catov JM, Patrick TE, Powers RW, Ness RB, Harger G, Roberts JM. Maternal leptin across pregnancy in women with small-for-gestational-age infants. American Journal of Obstetrics & Gynecology. 2007;196:558.e551–558. doi: 10.1016/j.ajog.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141:333–339. doi: 10.1210/endo.141.1.7260. [DOI] [PubMed] [Google Scholar]
- 26.Karlsson C, Lindell K, Svensson E, Bergh C, Lind P, Billig H, Carlsson LM, Carlsson B. Expression of functional leptin receptors in the human ovary. Journal of Clinical Endocrinology & Metabolism. 1997;82:4144–4148. doi: 10.1210/jcem.82.12.4446. [DOI] [PubMed] [Google Scholar]
- 27.Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- 28.Ebenbichler CF, Kaser S, Laimer M, Patsch JR. Polar expression and phosphorylation of human leptin receptor isoforms in paired, syncytial, microvillous and basal membranes from human term placenta. Placenta. 2003;24:800–801. doi: 10.1016/s0143-4004(03)00109-7. [DOI] [PubMed] [Google Scholar]
- 29.Bodner J, Ebenbichler CF, Wolf HJ, Muller-Holzner E, Stanzl U, Gander R, Huter O, Patsch JR. Leptin receptor in human term placenta: in situ hybridization and immunohistochemical localization. Placenta. 1999;20:677–682. doi: 10.1053/plac.1999.0431. [DOI] [PubMed] [Google Scholar]
- 30.Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. Journal of Clinical Endocrinology & Metabolism. 2003;88:1205–1211. doi: 10.1210/jc.2002-021332. [DOI] [PubMed] [Google Scholar]
- 31.Shibata E, Powers RW, Rajakumar A, von Versen-Hoynck F, Gallaher MJ, Lykins DL, Roberts JM, Hubel CA. Angiotensin II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation. American Journal of Physiology - Endocrinology & Metabolism. 2006;291:E1009–1016. doi: 10.1152/ajpendo.00134.2006. [DOI] [PubMed] [Google Scholar]
- 32.Shibata E, Powers RW, Hubel CA, Rajakumar A, von Versen-Hoynck F, Roberts JM. Placental System A amino acid transport activity is decreased in pregnancies with SGA infants but not preeclampsia with or without SGA infants. Journal of the Society for Gynecologic Investigation. 2006;13:77A. [Google Scholar]
- 33.Sooranna SR, Oteng-Ntim E, Meah R, Ryder TA, Bajoria R. Characterization of human placental explants: morphological, biochemical and physiological studies using first and third trimester placenta. Human Reproduction. 1999;14:536–541. doi: 10.1093/humrep/14.2.536. [DOI] [PubMed] [Google Scholar]
- 34.Rajakumar A, Doty K, Daftary A, Harger G, Conrad KP. Impaired oxygen-dependent reduction of HIF-1alpha and -2alpha proteins in pre-eclamptic placentae. Placenta. 2003;24:199–208. doi: 10.1053/plac.2002.0893. [DOI] [PubMed] [Google Scholar]
- 35.Rajakumar A, Jeyabalan A, Markovic N, Ness RB. Placental HIF-1 alpha, HIF-2 alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2007;293(2):R766–74. doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- 36.Parrott MS, von Versen-Hoeynck F, Ness RB, Markovic N, Roberts JM. System A amino acid transporter activity in term placenta is substrate specific and inversely related to amino acid concentration. Reproductive Sciences. 2007;14:687–693. doi: 10.1177/1933719107306895. [DOI] [PubMed] [Google Scholar]
- 37.Karl PI. Insulin-like growth factor-1 stimulates amino acid uptake by the cultured human placental trophoblast. Journal of Cellular Physiology. 1995;165:83–88. doi: 10.1002/jcp.1041650111. [DOI] [PubMed] [Google Scholar]
- 38.Karl PI, Alpy KL, Fisher SE. Amino acid transport by the cultured human placental trophoblast: effect of insulin on AIB transport. American Journal of Physiology. 1992;262:C834–839. doi: 10.1152/ajpcell.1992.262.4.C834. [DOI] [PubMed] [Google Scholar]
- 39.Ericsson A, Hamark B, Jansson N, Johansson BR, Powell TL, Jansson T. Hormonal regulation of glucose and system A amino acid transport in first trimester placental villous fragments. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2005;288:R656–662. doi: 10.1152/ajpregu.00407.2004. [DOI] [PubMed] [Google Scholar]
- 40.Xiong X, Demianczuk NN, Buekens P, Saunders LD. Association of preeclampsia with high birth weight for age. American Journal of Obstetrics & Gynecology. 2000;183:148–155. doi: 10.1067/mob.2000.105735. [DOI] [PubMed] [Google Scholar]
- 41.Teppa RJ, Ness RB, Crombleholme WR, Roberts JM. Free leptin is increased in normal pregnancy and further increased in preeclampsia. Metabolism: Clinical & Experimental. 2000;49:1043–1048. doi: 10.1053/meta.2000.7707. [DOI] [PubMed] [Google Scholar]
- 42.Siman CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2001;280:R1116–1122. doi: 10.1152/ajpregu.2001.280.4.R1116. [DOI] [PubMed] [Google Scholar]
- 43.Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB, Chrousos GP, Karp B, Allen C, Flier JS, Gold PW. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nature Medicine. 1997;3:575–579. doi: 10.1038/nm0597-575. [DOI] [PubMed] [Google Scholar]
- 44.Dunn SL, Bjornholm M, Bates SH, Chen Z, Seifert M, Myers MG., Jr Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Molecular Endocrinology. 2005;19:925–938. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- 45.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Molecular Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 46.Pai R, Lin C, Tran T, Tarnawski A. Leptin activates STAT and ERK2 pathways and induces gastric cancer cell proliferation. Biochemical & Biophysical Research Communications. 2005;331:984–992. doi: 10.1016/j.bbrc.2005.03.236. [DOI] [PubMed] [Google Scholar]
- 47.Hyde R, Christie GR, Litherland GJ, Hajduch E, Taylor PM, Hundal HS. Subcellular localization and adaptive up-regulation of the System A (SAT2) amino acid transporter in skeletal-muscle cells and adipocytes. Biochemical Journal. 2001;355:563–568. doi: 10.1042/bj3550563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyde R, Peyrollier K, Hundal HS. Insulin promotes the cell surface recruitment of the SAT2/ATA2 system A amino acid transporter from an endosomal compartment in skeletal muscle cells. Journal of Biological Chemistry. 2002;277:13628–13634. doi: 10.1074/jbc.M108609200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LDH release into media by villous fragments across the treatment groups. Data are shown as median percentages of maximum releasable LDH ± SEM. Median LDH release into media was not different across the treatment groups (p=0.23).