Abstract
Rationale: Maternal vitamin D intake during pregnancy has been inversely associated with asthma symptoms in early childhood. However, no study has examined the relationship between measured vitamin D levels and markers of asthma severity in childhood.
Objectives: To determine the relationship between measured vitamin D levels and both markers of asthma severity and allergy in childhood.
Methods: We examined the relation between 25-hydroxyvitamin D levels (the major circulating form of vitamin D) and markers of allergy and asthma severity in a cross-sectional study of 616 Costa Rican children between the ages of 6 and 14 years. Linear, logistic, and negative binomial regressions were used for the univariate and multivariate analyses.
Measurements and Main Results: Of the 616 children with asthma, 175 (28%) had insufficient levels of vitamin D (<30 ng/ml). In multivariate linear regression models, vitamin D levels were significantly and inversely associated with total IgE and eosinophil count. In multivariate logistic regression models, a log10 unit increase in vitamin D levels was associated with reduced odds of any hospitalization in the previous year (odds ratio [OR], 0.05; 95% confidence interval [CI], 0.004–0.71; P = 0.03), any use of antiinflammatory medications in the previous year (OR, 0.18; 95% CI, 0.05–0.67; P = 0.01), and increased airway responsiveness (a ≤8.58-μmol provocative dose of methacholine producing a 20% fall in baseline FEV1 [OR, 0.15; 95% CI, 0.024–0.97; P = 0.05]).
Conclusions: Our results suggest that vitamin D insufficiency is relatively frequent in an equatorial population of children with asthma. In these children, lower vitamin D levels are associated with increased markers of allergy and asthma severity.
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Maternal vitamin D intake during pregnancy has been inversely associated with asthma symptoms in early childhood. However, no study has examined the relationship between measured vitamin D levels and markers of asthma severity in childhood.
What This Study Adds to the Field
This study provides evidence of an inverse relationship between vitamin D levels and measures of allergy and asthma severity in Costa Rican children with asthma.
For unclear reasons, the prevalence of allergic diseases and asthma increased from a period likely preceding the 1960s to the 1990s (1). Worldwide surveys have shown that asthma prevalence is highest in industrialized nations (2). Although there is a trend for increased prevalence of asthma in countries farthest from the equator, some nations near the equator have high asthma prevalence (e.g., Costa Rica) (2).
Results of some, but not all, epidemiologic studies suggest that vitamin D deficiency is associated with an increased incidence of asthma symptoms (3–5). Our group has shown that higher maternal intakes of vitamin D during pregnancy are associated with decreased risks for recurrent wheeze in young children (6, 7), suggesting that vitamin D may play a role in the development of asthma. However, among those with established asthma, vitamin D may have a role in the manifestation of the disorder. Findings from in vitro studies (8) suggest that vitamin D may reverse steroid resistance in individuals with asthma, thus suggesting that vitamin D may play a role in the control of asthma. However, there has been no epidemiologic study of the relation between serum vitamin D levels and markers of asthma severity. The aim of the current analysis, therefore, was to examine the relationship between 25-hydroxyvitamin D (25[OH] vitamin D; the major circulating form of vitamin D) and markers of allergy (e.g., serum total and specific IgE) and markers of disease control and severity (e.g., hospitalizations, lung function) among 616 Costa Rican children with asthma.
METHODS
Study Population
Children included in the study were index cases for a family-based genetic study of asthma in Costa Rica. Subject recruitment and study procedures have been described in detail elsewhere (9, 10). From February 2001 to December 2006, short questionnaires were sent to the parents of 13,125 children ages 6 to 14 years who were enrolled in 113 schools in Costa Rica. Of the 7,282 children whose parents returned screening questionnaires, 2,714 had asthma (defined as physician-diagnosed asthma and at least two respiratory symptoms or asthma attacks in the previous year). Of these 2,714 children, 616 (22.7%) unrelated children had high probability of having at least 6 great-grandparents born in the Central Valley of Costa Rica and were willing to participate in our study. There was no significant difference in sex or grade in school between children who did and did not agree to participate in the study.
Written consent was obtained from the parents of participating children, from whom assent was also obtained if at least 8 years old. The study was approved by the Institutional Review Boards of the Hospital Nacional de Niños (San José, Costa Rica) and Brigham and Women's Hospital (Boston, MA).
Questionnaires
Parents of each participating child completed slightly modified versions of questionnaires used in the Collaborative Study on the Genetics of Asthma (11) and the International Study of Asthma and Allergies in Childhood (ISAAC) (12).
Pulmonary Function Testing
Spirometry was conducted in accordance with American Thoracic Society recommendations (13), using a Survey Tach spirometer (Warren E. Collins, Braintree, MA). The best FEV1 and FVC were selected for data analysis. After completing baseline spirometry, subjects were given 200 μg of an albuterol metered-dose inhaler and spirometry was repeated after 15 minutes.
Methacholine Challenge Testing
Subjects whose FEV1 was at least 65% of predicted on baseline spirometry underwent methacholine challenge testing at a separate visit, using a slightly modified version of the Chatham protocol (14).
Allergy Skin Testing
Skin testing was performed according to the ISAAC protocol. In addition to histamine and saline controls, the following antigens were applied to the volar surface of the forearm: Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blatella germanica, Periplaneta americana, cat dander, dog dander, mixed grass pollen, mixed tree pollen, and Alternaria tenuis. A test was considered positive if the maximal diameter of the wheal was 3 mm after subtraction of the maximal diameter of the negative control.
Serum Total and Allergen-specific IgE, and Peripheral Blood Eosinophil Count
Serum total and allergen-specific IgE levels were determined with the UniCAP 250 system (Pharmacia and Upjohn, Kalamazoo, MI), with samples measured in duplicate. Total serum IgE levels were transformed to a log10 scale for data analysis. Serum was assayed for IgE to each of three allergens: cockroach (Blatella germanica [Bla g 1]), dust mite (Dermatophagoides pteronyssinus [Der p 1]), and Ascaris lumbricoides. Peripheral blood eosinophil count was measured by Coulter counter techniques and then transformed to a log10 scale for data analysis.
Serum 25-Hydroxyvitamin D3
Serum levels of 25-hydroxyvitamin D (hereafter referred to as vitamin D) are considered the best circulating biomarker of vitamin D metabolic status and reflect contributions from all sources of vitamin D (i.e., diet and sun exposure) (15, 16). A single measurement of vitamin D was obtained from all 616 subjects, using a radioimmunoassay method, in the laboratory of B. Hollis at the Medical University of South Carolina (Charleston, SC) (17, 18). Vitamin D levels were transformed to a log10 scale for data analysis. In descriptive analyses, we also categorized vitamin D levels as deficient (<20 ng/ml), insufficient (≥20 and <30 ng/ml), and sufficient (≥30 ng/ml) on the basis of previous recommendations (19–21).
Statistical Analysis
A descriptive analysis of univariate predictors was performed using quartiles of vitamin D. P values were calculated by means of the Cochran-Armitage test for trend for binary predictors, and by linear regression for continuous variables. We examined the relation between log10 vitamin D and the following continuous outcomes, using linear regression: total IgE, eosinophil count, log10 dose–response slope to methacholine, baseline FEV1, and bronchodilator responsiveness. We examined the relation between log10 vitamin D and the following binary outcomes, using logistic regression: use of antiinflammatory medications (inhaled corticosteroids or leukotriene inhibitors) in the previous year, any hospitalization within the past year, any unscheduled visit for asthma (to a physician's office, an emergency department, a health care center, or a nebulization room) within the past year, and PD20 (the provocative dose causing a 20% fall from baseline FEV1) ≤ 8.58 μmol of methacholine. Finally, we used negative binomial regression to examine the relation between vitamin D and number (count) of hospitalizations over the past year.
A stepwise approach was used to build all multivariate models. All of the final models included vitamin D level and potential confounders of the relationship between vitamin D and asthma, including age, sex, body mass index (BMI) z-score, and parental education (as a surrogate for socioeconomic status). Other variables remained in the final models if they were significant at P < 0.05 or if they satisfied a change in estimate criterion (≥10%) in the parameter estimate (e.g., odds ratio). Additional variables examined as potential confounders of the relation between vitamin D levels and the outcomes of interest are listed in the online supplement. All analyses were performed with SAS version 9.1 and JMP 7 (both from SAS Institute, Cary, NC).
RESULTS
Characteristics of the Study Population
Table 1 shows the main characteristics of study participants, stratified by vitamin D quartile. Elevated total IgE, increased eosinophil count, and skin test reactivity to dust mite were common among participating children. A high proportion of children had an unscheduled visit for asthma but only a relatively small proportion of these children were hospitalized for asthma in the previous year. Less than half of the participating children were taking either an inhaled corticosteroid or a leukotriene inhibitor in the previous year. There were statistically significant trends toward younger age, lower BMI, lower proportion of females, lower IgE, and lower absolute bronchodilator response with increasing quartiles of vitamin D. There were no statistically significant differences in eosinophil count, skin test reactivity to allergens, or allergen-specific IgE levels across quartiles of vitamin D.
TABLE 1.
CHARACTERISTICS OF STUDY PARTICIPANTS
| Quartiles of Vitamin D
|
||||||
|---|---|---|---|---|---|---|
| Characteristics | All Children* | 1st Quartile† (12.5–29.0) | 2nd Quartile (29.1–35.7) | 3rd Quartile (35.8–43.0) | 4th Quartile (43.1–98.1) | P Value for Trend‡ |
| Participants, no. | 616 | 154 | 156 | 152 | 154 | |
| Age, years | 8.7 (7.6–10.5) | 9.3 | 9.3 | 8.7 | 8.9 | 0.008 |
| Female sex | 246 (40%) | 71 (46%) | 65 (42%) | 55 (36%) | 55 (36%) | 0.04 |
| BMI | 16.9 (15.5–19.5) | 18.2 | 18.3 | 17.5 | 17.5 | 0.02 |
| Total IgE, IU/ml | 392 (114–932) | 810 | 737 | 624 | 618 | 0.03 |
| Eosinophil count, cells/mm3 | 520 (270–790) | 634 | 588 | 605 | 552 | 0.14 |
| Prebronchodilator lung function | ||||||
| FEV1, L | ||||||
| Absolute | 1.68 (1.42–1.99) | 1.8 | 1.8 | 1.7 | 1.7 | 0.03 |
| Predicted§ | 1.70 (1.40–2.04) | 1.9 | 1.8 | 1.7 | 1.7 | |
| FVC, L | 2.0 (1.7–2.4) | 2.2 | 2.2 | 2.1 | 2.0 | 0.01 |
| FEV1/FVC ratio | 83% (79–87) | 82% | 83% | 83% | 82% | 0.51 |
| Maternal history of asthma | 188 (31%) | 48 (31%) | 45 (29%) | 47 (31%) | 48 (31%) | 0.91 |
| Paternal history of asthma | 138 (23%) | 39 (26%) | 36 (23%) | 32 (21%) | 31 (20%) | 0.21 |
| Health care use | ||||||
| Hospitalizations for asthma in the previous year | 35 (5.7%) | 11 (7%) | 6 (4%) | 14 (9%) | 4 (3%) | 0.32 |
| Unscheduled visits for asthma in the previous year | 562 (91%) | 136 (88%) | 140 (89%) | 142 (93%) | 144 (94%) | 0.26 |
| Absolute bronchodilator response, ml | 70 (10–150) | 109 | 98 | 79 | 72 | 0.01 |
| Use of antiinflammatory medication in the last year‖ | 238 (39%) | 66 (43%) | 64 (41%) | 51 (34%) | 57 (36%) | 0.15 |
| Allergen-specific IgE responses¶ | ||||||
| Positive IgE to Ascaris | 234 (38%) | 59 (38%) | 60 (39%) | 51 (34%) | 64 (42%) | 0.78 |
| Positive IgE to Der p | 463 (75%) | 119 (77%) | 120 (77%) | 113 (74%) | 111 (72%) | 0.24 |
| Positive IgE to Bla g | 243 (40%) | 65 (42%) | 67 (43%) | 45 (30%) | 66 (43%) | 0.52 |
| Skin test reactivity** | ||||||
| Dust mite | 494 (81%) | 126 (82%) | 126 (81%) | 118 (78%) | 124 (81%) | 0.57 |
| Cockroach | 356 (58%) | 90 (59%) | 90 (58%) | 84 (56%) | 92 (60%) | 0.96 |
| Outdoor allergens†† | 119 (19%) | 33 (22%) | 32 (21%) | 23 (15%) | 31 (20%) | 0.51 |
Definition of abbreviations: Bla g = Blatella germanica; BMI = body mass index; Der p = Dermatophagoides pteronyssinus.
Decimal values were approximated to the closest integer for ease of exposition.
Values are given as median (interquartile range) for continuous variables or as number (%) for binary variables.
Values for quartiles are the mean of the subgroup for continuous variables, and the number (%) for binary variables. Vitamin D levels are expressed as nanograms per milliliter.
The Cochran-Armitage trend test was calculated for binary variables, and linear regression was used to calculate a trend test for continuous variables.
Predicted values are for Mexican Americans from Hankinson and colleagues (42).
Includes the use of inhaled corticosteroids and/or leukotriene inhibitors.
Allergen-specific IgE level ≥ 0.35 IU/ml.
Skin test was reactive if the maximal diameter of the wheal was 3 mm after subtraction of the maximal diameter of the negative control.
Outdoor allergens include mixed grass pollen, mixed tree pollen, and Alternaria tenuis.
Distribution and Predictors of Serum Vitamin D Levels in the Study Population
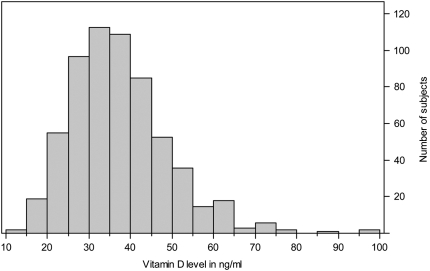
Figure 1 shows the distribution of serum vitamin D levels among participating children. The graph is skewed to the right and has a range of 12.5 to 98.1 ng/ml. Of the 616 participants, 21 (3.4%) had levels less than 20 ng/ml (considered deficient), and an additional 152 (24.6%) had levels between 20 and 30 ng/ml (considered insufficient) (19–21).
Figure 1.
Distribution of serum vitamin D in Costa Rican children with asthma. Of the 616 participants, 21 (3.4%) had levels less than 20 ng/ml (considered deficient), and an additional 152 (24.6%) had levels between 20 and 30 ng/ml (considered insufficient).
Measures of Allergy
The results of the bivariate and multivariate analyses of the relationship between serum vitamin D and continuous measures of allergy are shown in Table 2. In bivariate analyses, serum vitamin D was inversely associated with both serum total IgE and peripheral blood eosinophil count. This association remained significant after adjustment for age, sex, parental education, and BMI z-score. As an example, a 10-ng/ml increase in serum vitamin D (starting from an mean value of 35.7 ng/ml) in a male subject of average characteristics would predict a 25-IU/ml decrease in serum total IgE and a 29-cell/m3 decrease in eosinophil count. In addition, an increment in vitamin D from a borderline level of 20 ng/ml to a sufficient level of 30 ng/ml would predict a 90-IU/ml decrease in total IgE and a 54-cell/m3 decrease in eosinophil count. Vitamin D levels were also significantly associated with reductions in levels of IgE to dust mite and in the size of skin test reactivity to dust mite, even after adjustment for dust mite allergen levels in the home, in addition to age, sex, parental education, and BMI z-score. There was no significant association between vitamin D levels and serum IgE to cockroach or Ascaris.
TABLE 2.
SERUM VITAMIN D AND CONTINUOUS MEASURES OF ALLERGY AND DISEASE SEVERITY IN COSTA RICAN CHILDREN WITH ASTHMA
| Beta Coefficient* (95% CI) (P Value)
|
||
|---|---|---|
| Outcome | Unadjusted | Multivariate Model† |
| Total IgE, IU/ml‡ | −0.43 (−0.82 to −0.04) (0.03) | −0.47 (−0.86 to −0.08) (0.02) |
| Dust mite–specific IgE‡ | −1.20 (−2.11 to −0.29) (0.01) | −1.17 (−2.10 to −0.24) (0.01) |
| Size of skin test reaction to dust mite§ | −0.17 (−0.32 to −0.04) (0.01) | −0.16 (−0.30 to −0.02) (0.03) |
| Eosinophil count, cells/m3†‡ | −0.23 (−0.44 to −0.02) (0.03) | −0.26 (−0.48 to −0.05) (0.02) |
| Baseline FEV1 | −0.34 (−0.63 to −0.05) (0.02) | −0.07 (−0.28 to 0.13) (0.47) |
| Dose–response slope to methacholine, μmol | −0.26 (−0.61 to 0.07) (0.12) | −0.30 (−0.65 to 0.04) (0.08) |
| Absolute response to bronchodilator, ml | −107 (−190 to −23) (0.01) | −109 (−191 to −27) (0.01) |
Boldface entries indicate significance at α < 0.05.
Beta coefficient is for each log10 increase in vitamin D level.
Multivariate models were adjusted for age, sex, BMI z-score, and parental education for all outcomes. All models for bronchodilator responsiveness and airway hyperreactivity were additionally adjusted for prebronchodilator FEV1. Dust mite–specific IgE and dust mite skin test reaction were additionally adjusted for dust mite levels in the home.
Outcome was transformed to a log10 scale.
Defined as maximal skin test reaction to D. pteronyssinus minus maximal diameter of control, transformed to a log10 scale.
Spirometric Measures of Lung Function, Airway Responsiveness, and Bronchodilator Responsiveness
The results of the bivariate and multivariate analyses of the relation between serum vitamin D and categorical and continuous measures of lung function, airway responsiveness to methacholine, and bronchodilator responsiveness are shown in Table 2. Although serum vitamin D was inversely associated with baseline FEV1 in the bivariate analysis, this association was not significant after adjusting for age, sex, parental education, and BMI z-score (Table 2). Serum vitamin D level was significantly and inversely associated with increased airway responsiveness (PD20 ≤ 8.58 μmol of methacholine) in a multivariate analysis (Table 3). A similar but statistically nonsignificant trend for an inverse association with serum vitamin D was observed for airway responsiveness (Table 2). Serum vitamin D was significantly associated with a reduction in bronchodilator responsiveness in both bivariate and multivariate models (Table 2). For example, a 10-ng/ml increase in vitamin D levels (e.g., from 20 to 30 ng/ml) would result in a 20-ml reduction in absolute bronchodilator response, from 124 to 104 ml.
TABLE 3.
SERUM VITAMIN D LEVELS AND CATEGORICAL MEASURES OF ALLERGY AND DISEASE SEVERITY IN COSTA RICAN CHILDREN WITH ASTHMA
| Odds Ratio* (95% CI) (P Value)
|
||
|---|---|---|
| Outcome | Unadjusted | Multivariate Model† |
| Antiinflammatory medications‡ | 0.32 (0.10–1.09) (0.07) | 0.21 (0.06–0.75) (0.02) |
| Inhaled steroid | 0.20 (0.06–0.68) (0.01) | 0.14 (0.04–0.50) (0.004) |
| PD20 ≤ 8.58 μmol of methacholine | 0.17 (0.03–1.06) (0.06) | 0.15 (0.02–0.96) (0.05) |
| Hospitalizations for asthma in the previous year | 0.06 (0.004–0.74) (0.03) | 0.05 (0.004–0.71) (0.03) |
Boldface entries indicate significance at α < 0.05.
Odds ratios are for each log10 increase in vitamin D level.
Multivariate model adjusted for age, sex, BMI z-score, and parental education for all outcomes. PD20 additionally adjusted for prebronchodilator FEV1.
Defined as use of either inhaled steroid or leukotriene inhibitor.
Asthma Exacerbations
Table 3 shows the univariate and multivariate analyses of the relation between vitamin D and any hospitalization in the previous year. In these analyses, higher vitamin D levels were associated with significantly reduced odds of hospitalization in the previous year. Table 4 shows the univariate and multivariate analyses of the relation between vitamin D and number of hospitalizations in the previous year. We found a reduction in the number of hospitalizations in the prior year with increasing vitamin D levels in both bivariate and multivariate analyses. Moreover, vitamin D was the strongest predictor of the number of hospitalizations in this model.
TABLE 4.
SERUM VITAMIN D AND NUMBER OF HOSPITALIZATIONS AMONG COSTA RICAN CHILDREN WITH ASTHMA IN THE PREVIOUS YEAR
| Beta Coefficient (95% CI) (P Value)
|
||
|---|---|---|
| Outcome | Unadjusted | Multivariate Model* |
| Vitamin D deficiency (<20 ng/ml) | 1.9 (0.2 to 3.7) (0.03) | 2.3 (0.65 to 4.0) (0.006)† |
| Age, years | −0.02 (−0.25 to 0.21) (0.9) | |
| Female sex | 0.19 (−0.68 to 1.1) (0.7) | |
| BMI z-score | 0.1 (−0.30 to 0.51) (0.6) | |
| log10 dose–response slope for methacholine | 0.76 (−0.16 to 1.7) (0.1) | |
| Parental education | −0.06 (−0.43 to 0.3) (0.7) | |
| Positive IgE to cockroach | 1.1 (0.27 to 2.0) (0.01) | |
| Antiinflammatory medication use | 0.83 (−0.03 to 1.7) (0.06) | |
Boldface entries indicate significance.
The multivariate model is the final model after stepwise removal of covariates. Age, sex, and BMI z-score were forced into the model. Other variables also remained in the final models if they were significant at P < 0.05 or if they satisfied a change in estimate criterion (≥10%) in the beta coefficient. Variables removed include maternal asthma history, paternal asthma history, positive IgE to Ascaris, and positive IgE to dust mite.
In the multivariate model, a vitamin D level less than 20 ng/ml predicts a 10-fold increase in hospitalizations over a 1-year period.
Confirmatory Analysis
To examine whether low levels of vitamin D are markers of disease severity (i.e., if children with moderate to severe disease received less sun exposure than those with mild disease), additional models were adjusted for other potential markers of disease severity. Airway responsiveness outcomes were thus additionally adjusted for use of antiinflammatory medications, while the outcome of use of antiinflammatory medications was additionally adjusted for airway responsiveness. The remaining outcomes were adjusted for both antiinflammatory medication use and airway responsiveness. Tables E1 and E2 in the online supplement show the results of these analyses. The observed associations between serum vitamin D levels and total IgE, hospitalizations for asthma in the previous year, bronchodilator responsiveness, and use of inhaled steroids remained statistically significant. On the other hand, the observed associations between vitamin D and eosinophil count and use of antiinflammatory medications (inhaled steroids and/or leukotriene inhibitors) were slightly attenuated and became statistically nonsignificant.
DISCUSSION
Maternal vitamin D intake during pregnancy has previously been associated with asthma symptoms in childhood epidemiologic (6, 7) studies, while variants in the vitamin D receptor have been associated with asthma in genetic studies (22, 23). To our knowledge, this is the first study to demonstrate an inverse association between circulating levels of vitamin D and markers of asthma severity and allergy. We found that vitamin D deficiency exists even in an equatorial population of children with asthma, and that lower levels of vitamin D are associated with increased odds of hospitalization for asthma, increased bronchodilator responsiveness, and increased eosinophil count and IgE levels.
Vitamin D does not naturally occur in most foods that humans eat. The primary sources of this vitamin are natural production in the skin secondary to sun exposure, and secondarily from fortified foods and supplements (24). Although cutaneous production due to UVB radiation is considered the most important source of vitamin D, self-reported sun exposure alone is not a reliable marker of vitamin D sufficiency (25). In fact, vitamin D deficiency has been documented in healthy subjects despite reports of abundant solar exposure in Honolulu, Hawaii (latitude 21°N) (25), Beirut, Lebanon (latitude 33°N) (26), and Australia (latitude 27–43°S) (27). This is likely due to a combination of behavioral factors (e.g., sunscreen use, increased time spent indoors, and clothing coverage) and intrinsic factors such as skin melanin content, decreased cutaneous production of vitamin D3, or increased cutaneous destruction of vitamin D3. Vitamin D deficiency is especially problematic during winter months in more northerly populations, and unlikely to be corrected without supplements (28). The mean daily intake of dietary vitamin D in healthy adolescents in Costa Rica is 185 IU/day (29), which is slightly less than the recommended 200 IU/day, and much less than the 800 to 1,000 IU/day recommended for those without adequate sun exposure (20). Our finding of insufficient vitamin D levels in 28% of asthmatic children in the Central Valley of Costa Rica (latitude 10°N) supports previous findings that deficiency occurs even in sun-replete areas of the world.
Although a definitive role for vitamin D in the pathogenesis of asthma has not been determined, vitamin D may be related to asthma severity in several ways. First, vitamin D influences the immune system through its effects on helper T cell type 1, helper T cell type 2, and regulatory T cells (30–32). For example, Xystrakis and colleagues have shown that vitamin D restores the capability of regulatory T cells from steroid-resistant patients with asthma to secrete IL-10 (a potent antiinflammatory cytokine) in response to steroids (8). Second, current vitamin D intake may influence lung function in patients with asthma, similar to its potential effects in nonasthmatics (33), although we did not observe this in our cohort. Third, vitamin D stimulation has been shown to influence microarray gene expression signatures in bronchial smooth muscle cells. The most significantly affected pathways involved cell movement, growth, and survival, and may suggest a role for vitamin D in airway remodeling (34). Fourth, polymorphisms in the gene encoding the vitamin D receptor (VDR) have been associated with asthma phenotypes in two studies (22, 23). Of note, none of these polymorphisms resulted in an amino acid change in the translated protein, suggesting that the mechanism of increased asthma susceptibility is related to regulation of VDR expression. Finally, vitamin D intake during pregnancy may have effects on lung growth and development in neonates (6, 7).
In our cohort, only 39% of children were receiving inhaled corticosteroids or leukotriene inhibitors, despite a socialized health care system. Although this value may be lower than what might be expected based on current guidelines (35), it is similar to usage rates seen in Latin America (36), Europe (37), Asia (38), and the United States (39). Current evidence suggests that antiinflammatory medications are underutilized even in environments in which the ability to afford these medications is not an issue (40), which is the case in Costa Rica. Although urgent visits for asthma were high in this population, this is because this variable includes unscheduled visits not only to emergency departments but also to a physician's office, a nebulization room, or a health care center. On the other hand, the rate of hospitalization in this cohort (5%) is similar to that seen in Europe (7%) and likely indicates a more severe asthma exacerbation (37).
There is debate regarding what constitutes a normal circulating vitamin D level. Historical levels were derived from a normal distribution from humans who were presumed to be disease free (41), but this value is believed to be inaccurate as these individuals are likely sun deprived because of confounding factors such as clothing, sunscreen, and skin pigmentation (16). Other biomarkers such as parathyroid hormone, bone mineral density, and calcium absorption have been proposed as adjuvant studies to determine vitamin D sufficiency as it relates to bone health. These studies suggest that vitamin D deficiency occurs at 25(OH)vitamin D levels less than 32 ng/ml (15). Still others categorize 25(OH) vitamin D less than 20 ng/ml as deficient, from 20 to less than 30 ng/ml as insufficient, and 30 ng/ml or more as sufficient (19–21). In our analyses, we used vitamin D levels mostly as a continuous variable, except for the analyses presented in Table 4. It should be stated again that these cutoffs for vitamin D levels were determined with overall bone health in mind. In terms of non–bone-related outcomes and in particular for asthma and other atopic disorders, there are still insufficient data to determine an optimal level for prevention of exacerbations.
This cohort includes cases only, because of its original design as a family-based genetic study. Without a control group we are unable to make inferences regarding incident asthma. Although the role of vitamin D in the prevention of incident asthma is an interesting area for future research, on the basis of earlier studies we suspect that vitamin D influences the development of asthma at a much younger age, or even prenatally (6, 7). Therefore, we think the proper study design for that research question would be a birth (or prebirth) cohort study or clinical trial. A clinical trial of vitamin D supplementation given to pregnant women to prevent asthma in their offspring has been funded (HL091528).
We cannot establish causality of increased asthma morbidity due to low vitamin D levels because of the cross-sectional design of our study. A plausible alternative explanation for the observed association is that subjects with more severe asthma are likely to spend more time indoors, and therefore have lower vitamin D levels. However, most of the observed associations between vitamin D levels and markers of allergy and asthma severity remained significant and/or were only slightly attenuated after controlling for other markers of disease severity such as use of antiinflammatory medications and measures of airway responsiveness.
Another possible alternative explanation is that higher vitamin D levels reflect a higher intake of dietary vitamin D, which may be correlated with the intake of other nutrients that may modify asthma severity, such as vitamin E. Although we do not have nutritional information for these children, an earlier nutritional survey of adolescents in San José showed that the mean vitamin D intake was below recommended guidelines (29), suggesting that the primary source of vitamin D in this study population is sun exposure. In a different study, the effect of maternal vitamin D intake during pregnancy on subsequent wheezing by their children was independent of maternal intake of vitamin E, zinc, and calcium (7). Higher vitamin D intake may also be related to socioeconomic status (SES). We adjusted for an indicator of SES (parental education) in all our analyses. Although there could be residual confounding of our results by SES, Costa Rica has a socialized health care system and a high literacy rate, both of which should reduce potential confounding by health care disparities related to SES.
In summary, we found a strong inverse relationship between circulating levels of vitamin D and several markers of allergy and asthma severity. Our data suggest that additional work needs to be done to determine the potential beneficial role that vitamin D might play, if any, in established human allergy and asthma. These studies should include in vitro and animal studies to further elucidate the mechanisms for the role of vitamin D, and eventual clinical trials of vitamin D supplementation to prevent exacerbations. In addition, common polymorphisms in the vitamin D receptor and other genes in the vitamin D pathway should be further characterized, especially as they relate to circulating vitamin D levels and asthma severity.
Supplementary Material
Acknowledgments
The authors thank the participating families for their collaboration, the members of the field team in Costa Rica (Ligia Sanabria, Jenny Vega, Marvin Corrales, Adriana Gonzalez, Raquel Boza, Joaquín Acuña, Laura Rojas, Ana Castillo, Gabriela Ivankovich, Marcia Solano, Herminia Solano, and Heliberto Mena), and Ms. Jaylyn Olivo for editorial assistance.
Supported by grants HL66289, HL089842, HL04370, and T32 HL07427 from the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200808-1361OC on January 29, 2009
Conflict of Interest Statement: J.M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.C.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.E.S.-Q. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.W.H. has been a consultant to DiaSorin Corporation since 1982 for advice on Aciax development and has received $10,000 per year for this activity. S.T.W. received a grant for $900,065, Asthma Policy Modeling Study, from AstraZeneca from 1997 to 2003. He has been a coinvestigator on a grant from Boehringer Ingelheim to investigate a COPD natural history model that began in 2003. He has received no funds for his involvement in this project. He has been an advisor and chair of the advisory board to the TENOR Study for Genentech and has received $10,000 for 2005–2006. He received a grant from Glaxo-Wellcome for $500,000 for genomic equipment from 2000 to 2003. He was a consultant for Roche Pharmaceuticals in 2000 and received no financial remuneration for this consultancy. He has also served as a consultant to: Pfizer (2000–2003), Schering Plough (1999–2000), Variagenics (2002), Genome Therapeutics (2003), and Merck Frost (2002). A.A.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med 2006;355:2226–2235. [DOI] [PubMed] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469–478. [DOI] [PubMed] [Google Scholar]
- 3.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 2007;120:1031–1035. [DOI] [PubMed] [Google Scholar]
- 4.Weiss ST, Litonjua AA. Maternal diet vs lack of exposure to sunlight as the cause of the epidemic of asthma, allergies and other autoimmune diseases. Thorax 2007;62:746–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hypponen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen AL, Jarvelinb MR. Infant vitamin D supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci 2004;1037:84–95. [DOI] [PubMed] [Google Scholar]
- 6.Camargo CA Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr 2007;85:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr 2007;85:853–859. [DOI] [PubMed] [Google Scholar]
- 8.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, et al. Reversing the defective induction of IL-10–secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest 2006;116:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunninghake GM, Soto-Quiros ME, Avila L, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Silverman EK, Celedon JC. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol 2007;119:654–661. [DOI] [PubMed] [Google Scholar]
- 10.Ly NP, Soto-Quiros ME, Avila L, Hunninghake GM, Raby BA, Laskey D, Sylvia JS, Celedon JC. Paternal asthma, mold exposure, and increased airway responsiveness among children with asthma in Costa Rica. Chest 2008;133:107–114. [DOI] [PubMed] [Google Scholar]
- 11.Blumenthal MN, Banks-Schlegel S, Bleecker ER, Marsh DG, Ober C; National Heart, Lung and Blood Institute. Collaborative studies on the genetics of asthma. Clin Exp Allergy 1995;25:29–32. [DOI] [PubMed] [Google Scholar]
- 12.Weiland S. Phase II modules of the International Study of Asthma and Allergies in Childhood (ISAAC; June 14, 2004–January 15, 2008); accessed March 4, 2009. Available from: http://www.uni-ulm.de/med/med-epidemiologie/forschung-in-der-epidemiologie/isaac/isaac-studie-phase-ii.html
- 13.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 14.Chatham M, Bleecker ER, Smith PL, Rosenthal RR, Mason P, Norman PS. A comparison of histamine, methacholine, and exercise airway reactivity in normal and asthmatic subjects. Am Rev Respir Dis 1982;126:235–240. [DOI] [PubMed] [Google Scholar]
- 15.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr 2005;135:317–322. [DOI] [PubMed] [Google Scholar]
- 16.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med 2005;352:515–516 [author reply 515–516]. [DOI] [PubMed] [Google Scholar]
- 17.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 1993;39:529–533. [PubMed] [Google Scholar]
- 18.Hollis BW, Napoli JL. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem 1985;31:1815–1819. [PubMed] [Google Scholar]
- 19.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006;84:18–28. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–281. [DOI] [PubMed] [Google Scholar]
- 21.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr 2007;85:649–650. [DOI] [PubMed] [Google Scholar]
- 22.Poon AH, Laprise C, Lemire M, Montpetit A, Sinnett D, Schurr E, Hudson TJ. Association of vitamin D receptor genetic variants with susceptibility to asthma and atopy. Am J Respir Crit Care Med 2004;170:967–973. [DOI] [PubMed] [Google Scholar]
- 23.Raby BA, Lazarus R, Silverman EK, Lake S, Lange C, Wjst M, Weiss ST. Association of vitamin D receptor gene polymorphisms with childhood and adult asthma. Am J Respir Crit Care Med 2004;170:1057–1065. [DOI] [PubMed] [Google Scholar]
- 24.Lamberg-Allardt C. Vitamin D in foods and as supplements. Prog Biophys Mol Biol 2006;92:33–38. [DOI] [PubMed] [Google Scholar]
- 25.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 2007;92:2130–2135. [DOI] [PubMed] [Google Scholar]
- 26.El-Hajj Fuleihan G, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, Tannous R. Hypovitaminosis D in healthy schoolchildren. Pediatrics 2001;107:E53. [DOI] [PubMed] [Google Scholar]
- 27.van der Mei IA, Ponsonby AL, Engelsen O, Pasco JA, McGrath JJ, Eyles DW, Blizzard L, Dwyer T, Lucas R, Jones G. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect 2007;115:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003;77:204–210. [DOI] [PubMed] [Google Scholar]
- 29.Kabagambe EK, Baylin A, Irwig MS, Furtado J, Siles X, Kim MK, Campos H. Costa Rican adolescents have a deleterious nutritional profile as compared to adults in terms of lower dietary and plasma concentrations of antioxidant micronutrients. J Am Coll Nutr 2005;24:122–128. [Vitamin D intake in the paper is incorrectly listed as mg/d. Correct units are IU/d (Campos H, personal communication)]. [DOI] [PubMed] [Google Scholar]
- 30.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr 2004;80:1717S–1720S. [DOI] [PubMed] [Google Scholar]
- 31.May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy 2004;3:377–393. [DOI] [PubMed] [Google Scholar]
- 32.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 2005;97:93–101. [DOI] [PubMed] [Google Scholar]
- 33.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest 2005;128:3792–3798. [DOI] [PubMed] [Google Scholar]
- 34.Bosse Y, Maghni K, Hudson TJ. 1α,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol Genomics 2007;29:161–168. [DOI] [PubMed] [Google Scholar]
- 35.National Asthma Education and Prevention Program. Expert Panel Report III: guidelines for the diagnosis and management of asthma. Bethesda, MD: National Heart, Lung, and Blood Institute, 2007. NIH publication no. 08–4051 [accessed March 4, 2009]. Available from: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm.
- 36.Neffen H, Fritscher C, Cuevas Schacht F, Levy G, Chiarella P, Soriano JB, Mechali D. Asthma control in Latin America: the Asthma Insights and Reality in Latin America (AIRLA) Survey. Rev Panam Salud Publica 2005;17:191–197. [DOI] [PubMed] [Google Scholar]
- 37.Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) Study. Eur Respir J 2000;16:802–807. [DOI] [PubMed] [Google Scholar]
- 38.Lai CKW, de Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, Trung PL, Shan Zhong N, Zainudin N, Zainudin BMZ. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol 2003;111:263–268. [DOI] [PubMed] [Google Scholar]
- 39.Adams RJ, Fuhlbrigge A, Guilbert T, Lozano P, Martinez F. Inadequate use of asthma medication in the United States: results of the Asthma in America National Population Survey. J Allergy Clin Immunol 2002;110:58–64. [DOI] [PubMed] [Google Scholar]
- 40.Lieu TA, Lozano P, Finkelstein JA, Chi FW, Jensvold NG, Capra AM, Quesenberry CP, Selby JV, Farber HJ. Racial/ethnic variation in asthma status and management practices among children in managed Medicaid. Pediatrics 2002;109:857–865. [DOI] [PubMed] [Google Scholar]
- 41.Haddad JG, Chyu KJ. Competitive protein-binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab 1971;33:992–995. [DOI] [PubMed] [Google Scholar]
- 42.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.