Abstract
Rationale: Modulation of the activity of sarcoendoplasmic reticulum calcium ATPase (SERCA) can profoundly affect Ca2+ homeostasis. Although altered calcium homeostasis is a characteristic of cystic fibrosis (CF), the role of SERCA is unknown.
Objectives: This study provides a comprehensive investigation of expression and activity of SERCA in CF airway epithelium. A detailed study of the mechanisms underlying SERCA changes and its consequences was also undertaken.
Methods: Lung tissue samples (bronchus and bronchiole) from subjects with and without CF were evaluated by immunohistochemistry. Protein and mRNA expression in primary non-CF and CF cells was determined by Western and Northern blots.
Measurements and Main Results: SERCA2 expression was decreased in bronchial and bronchiolar epithelia of subjects with CF. SERCA2 expression in lysates of polarized tracheobronchial epithelial cells from subjects with CF was decreased by 67% as compared with those from subjects without CF. Several non-CF and CF airway epithelial cell lines were also probed. SERCA2 expression and activity were consistently decreased in CF cell lines. Adenoviral expression of mutant F508 cystic fibrosis transmembrane regulator gene (CFTR), inhibition of CFTR function pharmacologically (CFTRinh172), or stable expression of antisense oligonucleotides to inhibit CFTR expression caused decreased SERCA2 expression. In CF cells, SERCA2 interacted with Bcl-2, leading to its displacement from caveolae-related domains of endoplasmic reticulum membranes, as demonstrated in sucrose density gradient centrifugation and immunoprecipitation studies. Knockdown of SERCA2 using siRNA enhanced epithelial cell death due to ozone, hydrogen peroxide, and TNF-α.
Conclusions: Reduced SERCA2 expression may alter calcium signaling and apoptosis in CF. These findings decrease the likelihood of therapeutic benefit of SERCA inhibition in CF.
Keywords: cystic fibrosis, SERCA2, pulmonary epithelium, ER
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) inhibitors are being tested to enhance epithelial Cl− transport; however, potential beneficial effects are unproven. The status of SERCA2 expression and activity in cystic fibrosis airway is unknown.
What This Study Adds to the Field
SERCA2 expression and activity are decreased in cystic fibrosis airway epithelium, resulting in enhanced susceptibility to oxidants.
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane regulator gene (CFTR), an epithelial anion channel, resulting in the common autosomal recessive disease. Relentless progressive lung infection and excessive secondary inflammation remains the principal cause of death in CF. The most frequent CF-associated mutation, accounting for about 70% of CF alleles, is deletion of phenylalanine 508 (ΔF508 CFTR). ΔF508 CFTR has reduced chloride channel activity, impaired processing, and decreased stability at the cell surface. Abnormal processing leads to its retention in the endoplasmic reticulum (ER) and rapid intracellular degradation. For these reasons, ΔF508 CFTR fails to function as a cAMP-activated Cl− channel (1). Previous reports have indicated that sarcoendoplasmic reticulum calcium ATPase (SERCA) inhibitors can decrease calcium concentrations within the ER and thereby interfere with the ability of calcium-dependent chaperone proteins to retain misfolded ΔF508 CFTR within the ER (2). These investigators have suggested that blockade of this chaperone interaction by the use of SERCA inhibitors allows misfolded ΔF508 CFTR to escape the ER, reach the cell surface, and function as a Cl− channel (3). These findings precipitated a remarkable number of investigations and resulted in several papers, indicating that SERCA pump inhibitors like curcumin and/or thapsigargin can (4–9) or cannot (10–13) enhance ΔF508 CFTR trafficking to the plasma membrane and apical epithelial chloride transport.
The release of calcium ions from ER regulates essential cellular functions, including secretion, contraction, gene transcription, and survival (14). Because SERCA is responsible for (re)loading of ER calcium after such signaling events, its function can be important at the level of the whole organ and organism. For example, SERCA2 is the only SERCA isoform expressed in cardiac muscle, and decreased SERCA2 expression is a key event in congestive heart failure (15). Its importance is further demonstrated by the lethal phenotype of SERCA2 knockout mice and the loss of calcium regulation in cells with only one copy of the SERCA2 gene (16, 17). In CF, release of ER calcium by activation of purinergic receptors can allow activation of Ca2+-activated chloride channels, a principal, albeit partial, compensatory mechanism for impaired chloride secretion in CF that could diminish excessive sodium reabsorption by ENaC (18). Hence, ER Ca2+ stores can have direct importance in the adaptation of CF epithelium.
Despite the abundant research into potential effects of SERCA inhibitors on ΔF508 CFTR trafficking and function, the expression of SERCA isoforms in CF and non-CF airway epithelium has not been systematically evaluated. Herein, we report that expression of the principal lung isoform, SERCA2, was consistently down-regulated in CF airways, primary cultures of polarized CF airway epithelial cells, in response to genetic or pharmacologic inhibition of wild-type CFTR expression or function and upon expression of ΔF508 CFTR in non-CF epithelium. Even though the low-affinity SERCA3 isoform was up-regulated, total SERCA activity was decreased. In CF cells, SERCA2 was associated with Bcl-2 in ER membranes, and SERCA/Bcl-2 interaction was previously reported to cause inactivation and displacement of SERCA from membrane microdomains, referred to as caveolae-related domains (CRDs) (19, 20). Knockdown of SERCA2 using siRNA revealed that it is required for the survival of airway epithelial cells during oxidative stress. Not only do these findings indicate additional reasons why the strategy of SERCA inhibition should not be of benefit in CF, but they also have potential implications for altered calcium homeostasis and epithelial cell response in the disease pathogenesis. Some of the results of these studies have been previously reported in the form of abstracts (21, 22).
MATERIALS AND METHODS
Cell Culture
Primary human bronchial epithelial cells (HBEs) were obtained from non-CF and CF lungs under a protocol and consent form approved by the University of North Carolina School of Medicine Committee on the Protection of the Rights of Human Subjects. Epithelial cells were removed from the lower trachea and bronchi by protease XIV digestion, and cells were plated in bronchial epithelium growth medium on collagen-coated dishes as described previously (23). Approximately 5 × 105 passage 2 cells were seeded onto 12-mm-diameter type VI collagen (Sigma, St. Louis, MO)–coated Millicell CM inserts (0.4 μM pore size) (Millipore Corporation, Bedford, MA) and, after confluence on Days 4 and 5, were maintained at an air–liquid interface (ALI). Additional primary human bronchial epithelial cells were isolated in our laboratory from donor airway tissues obtained from the National Disease Research Interchange, with approval of National Jewish Institutional Committee for the Protection of the Rights of Human Subjects. The airway epithelial cell lines used were IB3–1 and a “corrected” cystic fibrosis (CF) cell line that was derived from IB3–1 cells stably transfected with wild-type CFTR (C-38) (ATCC, Manassas, VA). IB3–1 and C-38 cells were grown in LHC-8 media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin/ streptomycin. CFBE41o- (CF41o-) and CFBE45o- (CF45o-) and a wild-type airway epithelial cell line, 16HBE14o- (16HBEo-), were provided by Prof. D. Gruenert (California Pacific Medical Center Research Institute, University of California at San Francisco). These cell lines were cultured in Eagle's minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, l-glutamine, and penicillin/streptomycin at 37°C under 5% CO2. 16HBEo- cells with stable expression of sense (16HBE-S) and antisense CFTR (16HBE-AS) oligonucleotides were cultured as described previously (24). Minimally transformed primary bronchial epithelial UNCN3T from the lab of Dr. Randell was also used (25). All experiments comparing primary non-CF and CF cells were performed with polarized cultures grown simultaneously and matched for passage number, the number of cells plated, and days in culture.
Adenoviral Transduction
Transduction of wild-type CFTR, mutated CFTR (ΔF508), and GFP-encoding adenoviral vectors was performed as described previously (26). The vectors (H5′0.040CMVGFP-CFTR and H5′0.040CMVGFP-ΔF508) were provided by Vector Core at the University of Pennsylvania as described elsewhere (27). The recombinant viruses were added to the cell cultures (multiplicity of infection, 10:1) on Day 3 for 17 hours. The transduction efficiency was estimated by observing green fluorescence of adenoviral GFP-transduced cells.
SERCA2 Gene Silencing Using siRNA
Predesigned human ‘SMARTPOOL’ SERCA2 siRNAs were purchased from Dharmacon (Lafayette, CO). Primary airway epithelial cells not at ALI were transfected with 50 nM siRNA using DharmaFECT2 siRNA transfection reagent (Dharmacon) according to the manufacturer's instructions. Silencer negative control siRNA was used as a nonspecific siRNA, and mock transfection was used as a negative control. Transfection of siRNA in primary human airway epithelial cells has previously been described (28).
Statistical Analysis
All statistical calculations were performed with JMP and SAS software (SAS Institute, Cary, NC). Means were compared by two-tailed t test for comparison between two groups or one-way analysis of variance followed by the Tukey-Kramer test for multiple comparisons for analyses involving three or more groups. P < 0.05 was considered significant.
RESULTS
Expression of SERCA2 in CF Airway Cell Lines
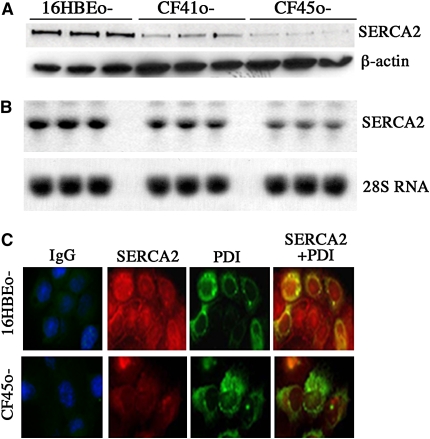
SERCA2 expression was determined by Western blot in non-CF and CF cell lysates. Expression of SERCA2 protein was decreased in two CF cell lines (CF41o- and CF45o-) as compared with the non-CF 16HBEo- cells (Figure 1A). Quantitation of SERCA2/β-actin intensity yielded 3.51 ± 0.27, 1.76 ± 0.23, and 2.59 ± 0.29 arbitrary units for 16HBEo-, CF41o-, and CF45o- cells, respectively. A statistically significant decrease in SERCA2 protein was observed in IB3–1 cells versus C-38 cells (0.22 ± 0.02 vs. 1.24 ± 0.01 SERCA2/β-actin intensity units, respectively; figure not shown) and in JME/CF15 versus Calu-3 cells (0.38 ± 0.01 vs. 0.62 ± 0.01 SERCA2/β-actin intensity units, respectively; figure not shown). Decreased SERCA2 mRNA was also observed (Figure 1B) in CF41o- and CF45o- cells relative to that found in non-CF 16HBEo- cells.
Figure 1.
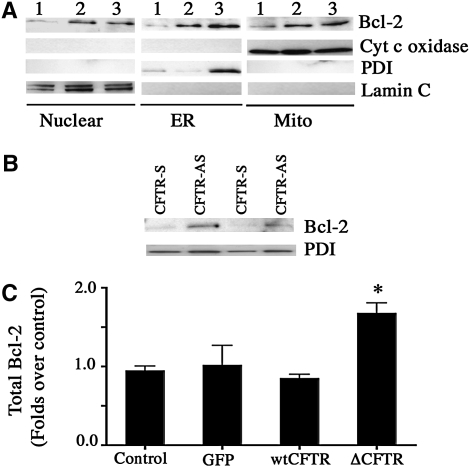
Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA)2 protein and RNA expression in non-cystic fibrosis (CF) and CF cell lines. Lysates from cultures of 16HBEo-, CF41o-, and CF45o- cells were analyzed for SERCA2 protein and RNA expression using Western and Northern blot as indicated in Methods. (A and B) Representative Western (experiments repeated six times) and Northern blots (experiments repeated three times) are shown, respectively. To localize SERCA2, cells grown on glass coverslips were fixed and coimmunostained for SERCA2 (red) and the endoplasmic reticulum (ER)-specific protein protein disulphide isomerase (PDI, green) (C). The figure represents one data set from an experiment performed in duplicate. The individual experiment was repeated three times.
Experiments were also performed to immunolocalize SERCA2. The results indicated diminished SERCA2 expression in CF cells relative to non-CF cells (Figure 1C). In non-CF cells, SERCA2 was located in ER, as demonstrated by the enhanced “merged” staining for the ER marker protein disulfide isomerase (PDI) and SERCA2 in these cells, whereas CF cells demonstrated decreased overall SERCA2 staining intensity, preservation of ER staining for PDI, and diminished merged signal for PDI and SERCA2 co-staining.
ER Density and SERCA2 Protein and Activity in CF Cells and Their Microsomal Fractions
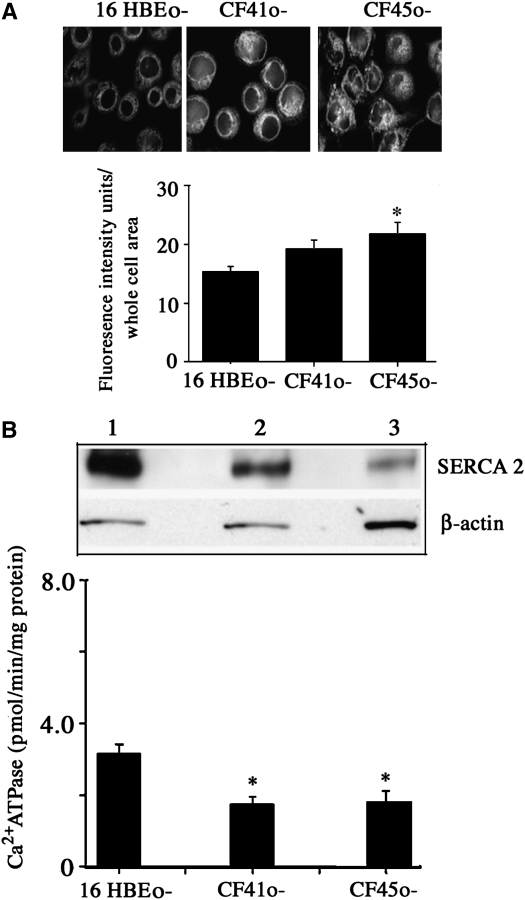
The decreased expression of SERCA2 in CF cells was not caused by diminished ER mass in these cells. ER density was quantified in CF and non-CF cell lines using an ER-specific fluorescent dye (ER-Tracker Blue-White DPX; Molecular Probes, Carlsbad, CA) (Figure 2A, upper panel). Similar to what was seen with PDI staining, CF cell cultures showed similar or greater ER staining intensity with the fluorescent dye. Specifically, CF45o- cells had significantly greater staining intensity than 16HBEo- cells, and CF41o- cells showed a similar, nonsignificant trend (Figure 2A, lower panel). These findings are consistent with previous reports of increased apical ER density in CF cells (29).
Figure 2.
Estimation of endoplasmic reticulum (ER) content (A) and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA2) (protein and activity) in purified microsomal membranes (B). Live cells cultured in chambered coverglass were stained using ER-Tracker Blue-White DPX (Molecular Probes) (A). The lower panel shows the quantification of fluorescence intensity per whole cell area. For each of three cell lines, about 20 cells were analyzed. Results show means of the data. *Significant difference (P < 0.05) from non-cystic fibrosis (CF) 16HBEo- cells (n = 3). (B, top panel) Representative result of SERCA2 expression in purified microsomal membranes of 16HBEo- (lane 1), CF41o- (lane 2), and CF45o- (lane 3) cells. Microsomal extracts (20 μg) were loaded on 7.5% polyacrylamide gel, and Western blot analysis was performed for SERCA2 expression. (B, lower panel) Thapsigargin (2 μM)-sensitive Ca2+ATPase activity in microsomal membranes of normal and CF cells. *Significant difference from 16HBEo- cells (P < 0.05) (n = 3; represents three individual experiments).
In microsomal membrane (ER) preparations, CF cell lines CF41o- and CF45o- had decreased SERCA2 protein expression in absolute terms and per β-actin protein expressed relative to non-CF cell line 16HBEo- (Figure 2B, upper panel). In these microsomal membrane preparations, total thapsigargin-inhibitable Ca2+ATPase (SERCA) activity was decreased by approximately 50% in the two CF cell lines relative to the non-CF cells (Figure 2B, lower panel). Because thapsigargin is a specific inhibitor of SERCA, these findings indicate that diminished SERCA2 expression resulted in a substantial overall decrease of SERCA activity in CF cells.
SERCA2 Expression in Primary Polarized Non-CF and CF Airway Epithelial Cells
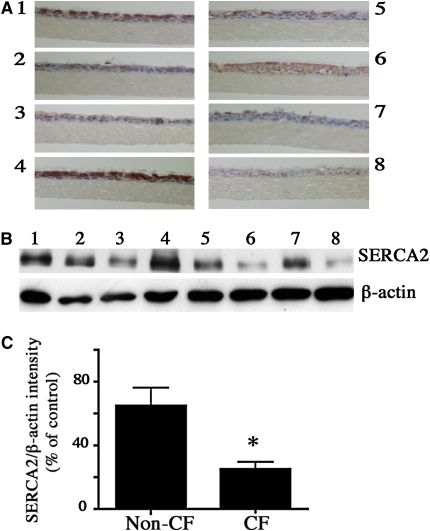
SERCA2 expression also was estimated in ALI cultures of differentiated primary non-CF (14 donors) and CF (eight donors) cells using immunohistochemistry and Western blot analysis. Using either technique, expression of SERCA2 in epithelial cells varied from donor to donor (demographics provided in Table E1 in the online supplement), despite comparable passage number and days in culture (Figures 3A and 3B). However, mean SERCA2 protein expression (Western blot) was decreased by 67% in airway epithelial cells from CF donors relative to non-CF (Figure 3C). We also analyzed SERCA2 expression at 7 days of culture, a stage at which cells are undifferentiated, and at 30 days of culture, at which time cells are more differentiated (30), in primary non-CF and CF airway epithelial cells from four different donors to determine if the extent of differentiation could affect SERCA2 expression. CF airway epithelial cells had decreased SERCA2 expression at 7 days and at 30 days of culture as compared with non-CF cells (data not shown). Thus, SERCA2 protein expression in primary polarized CF airway epithelial cells differed from the non-CF cells to a similar extent as seen in CF versus non-CF cell lines.
Figure 3.
Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA2) expression in air–liquid interface (ALI), 17–42 days of cultures of primary non-cystic fibrosis (CF) and CF airway epithelial cells. (A) Representative images of in situ immunohistochemistry for SERCA2 expression in cultures fixed with 4% paraformaldehyde (PFA) and stained with 3,3-diaminobenzidine and H2O2 for detection of horeseradish peroxidase–coupled mouse secondary antibody. Data shown are from cells from four individual (1–4) non-CF donors and four individual (5–8) CF donors (one of three separate experiments). Identical staining conditions were used for staining non-CF and CF sections. For Western blot, lysates from the ALI cultures of the cells from the above donors were analyzed by SDS PAGE on 4 to 15% gradient gels, and the proteins were transferred electrophoretically onto nitrocellulose membranes. The membranes were probed with the SERCA2 antibodies at a 1/1,000 dilution (B). (C) Quantification of Western blots for SERCA2 expression in ALI cultures of cells from non-CF and CF donors (14 non-CF and 8 CF) analyzed in three separate experiments. The mean of the non-CF group is the control value; > 60% of CF donors were F508 double mutant, and ∼ 90% had F508 mutation for one allele in the CFTR gene. The bars represent means of data. * Significant difference (P < 0.05) from non-CF cells (results of three individual experiments).
SERCA2 Expression in Proximal and Distal Airways from Human CF and Non-CF Lungs
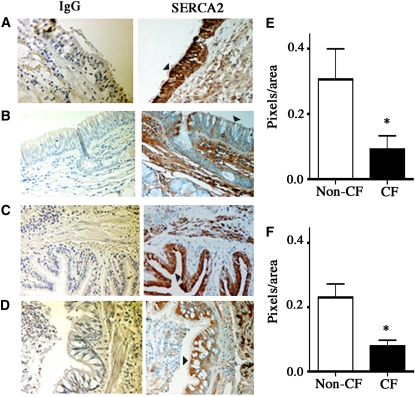
Immunohistochemistry was used to estimate SERCA2 expression in lung tissue from subjects with and without CF. SERCA2 staining was predominantly localized to airway epithelium and to smooth muscle cells in airway and arterial/arteriolar walls. SERCA2 staining was decreased in bronchial and bronchiolar epithelium of subjects with CF compared with subjects without CF (Figures 4A–4D). CF airway epithelium also differed from non-CF airway epithelium in that the extent of mucus cell hyperplasia was greater in the former than in the latter. Quantitation of SERCA2 staining (excluding the mucus-containing regions) revealed a significant decrease in bronchial and bronchiolar epithelium of subjects with CF compared with subjects without CF (Figures 4E and 4F). The demographics of the individuals with and without CF are provided in Table E2.
Figure 4.
Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA2) expression in the epithelium of proximal and distal cystic fibrosis (CF) and non-CF airways. Immunohistochemical localization of SERCA2 was performed as described in Methods using identical conditions for non-CF and CF tissue. Left panel: Nonspecific IgG control. Right panel: SERCA2 staining in epithelium (arrowheads). SERCA2 staining was found predominantly in the epithelium of non-CF bronchi (n = 5 donors) (A) and bronchioles (C), and it was significantly less intense in the epithelium of CF airways (n = 5 donors) (B and D). (E) Quantitation of SERCA2 staining (SERCA2-IgG) in the non-CF and CF bronchi. The regions containing mucus were excluded during quantitation. For each tissue, two SERCA2 and two IgG-stained sections were analyzed, and 10 nonmucus areas per section were randomly selected for quantitation using Image-Pro Plus version 4.0 (Media Cybernetics, Silver Spring, MD). Similarly, SERCA2 staining in the non-CF and CF bronchioles was quantified (F).
SERCA3 expression in CF and non-CF airway epithelial cells and cell lines
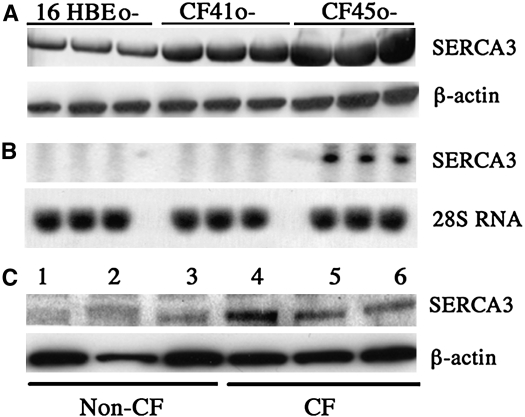
SERCA3 is the only other known SERCA isoform expressed in lung. It differs from SERCA2 in that it has a low affinity for calcium. By contrast to SERCA2, SERCA3 protein expression was increased in both CF cell lines, CF41o- and CF45o-, relative to that seen in the non-CF cell line 16HBEo- (Figure 5A). Steady-state SERCA3 mRNA expression was not consistently elevated in CF cell lines, being elevated in CF45o- but not in CF41o- relative to 16HBEo- (Figure 5B). However, SERCA3 protein expression was increased in primary polarized airway epithelial cells from CF versus non-CF donors (Figure 5C). These findings suggest a compensatory response of SERCA3 expression to the down-regulation of SERCA2 in CF.
Figure 5.
Expression of low-affinity sarcoendoplasmic reticulum calcium ATPase (SERCA) isoform SERCA3 in cystic fibrosis (CF) and non-CF airway epithelial cells. (A) Western blot for SERCA3 from whole cell lysates (20 μg of protein/lane) from non-CF and CF bronchial airway epithelial cell lines. Membranes were also probed for β-actin to verify equal loading of protein. (B) Northern blot for SERCA3 mRNA expression. About 15 μg of RNA was subjected to Northern blot analysis, and SERCA3 was identified using 32P-labeled cDNA probe. Before hybridization membranes were analyzed with ultraviolet light exposure for visualization of 18S and 28S RNA bands to verify equality of RNA loading and transfer. Equal loading was further established by 28S RNA (bottom panel) analysis. Cell lysates from air-liquid interface cultures of primary airway epithelial cells from three non-CF (1–3) and three CF (4–6) subjects were also assessed for SERCA3 expression by Western blot (C). Results represent three individual experiments.
SERCA2 Protein Expression Is Decreased by Pharmacologic Inhibition of CFTR Function
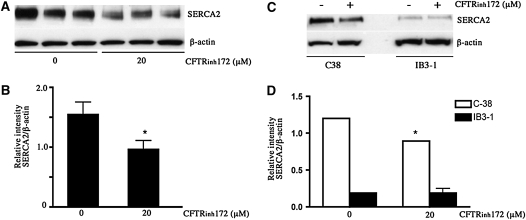
To investigate the role of CFTR function in mediating SERCA2 expression, we used the specific CFTR inhibitor CFTRinh172. Relatively short-term (24-hour) incubation of primary HBE (ALI; Figure 6A) and the wt-CFTR–corrected C-38 (Figure 6C) cells with CFTRinh172 (20 μM) caused a significant decrease in SERCA2 protein levels. By contrast, incubation of the parent CF cell line IB3–1 with the same concentration of the inhibitor did not cause a further decrease in its expression of SERCA2 (Figure 6C). Exposure of these cells to the inhibitor for this duration did not cause cytotoxicity.
Figure 6.
Effect of cystic fibrosis transmembrane regulator gene inhibitor (CFTR)inh172 on sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA2) protein expression. Primary bronchial epithelial cells were cultured for short-term duration on collagen-coated inserts and then treated with 20 μM CFTRinh172 for 24 hours. Cell lysates were prepared, and Western blot analysis was performed (20 μg protein loaded). (A) SERCA2 expression in CFTRinh172–treated primary bronchial epithelial cells (lanes 1–3 are untreated control; lanes 4–6 are CFTRinh172–treated cells). (B) Quantitative data for SERCA2 expression with and without CFTRinh172 treatment. The bars represent means of data. *Significant difference (P < 0.05) from non-CF cells (results of three individual experiments are shown). (C) Representative blot showing the effect of CFTRinh172 on SERCA2 expression treatment in CF IB3–1 cells and CFTR-corrected C-38 cells. (D) Quantitative data. Bars represent means of data. *Significant difference (P < 0.05) from untreated cells; n = 3 (experiment repeated twice).
SERCA2 Protein Expression Is Decreased by Genetic Inhibition of CFTR Function
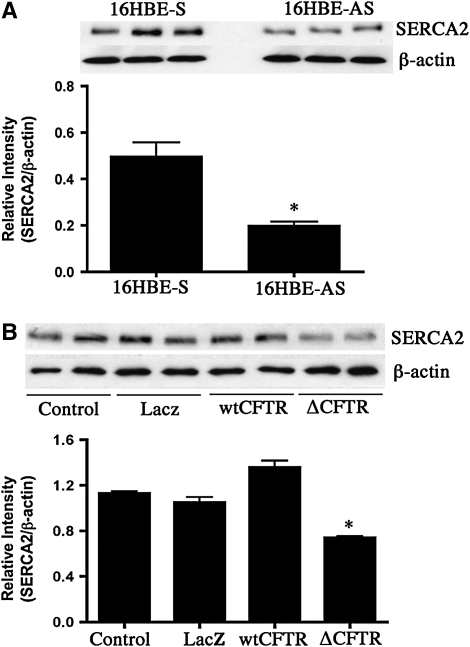
The effect of interference with CFTR function was evaluated by an additional specific genetic approach. SERCA2 expression was measured in 16HBEo- cells with stable expression of sense (16HBE-S) and antisense CFTR (16HBE-AS) oligonucleotides and grown at ALI. cAMP-dependent chloride conductance was absent in antisense CFTR oligonucleotides (16HBE-AS)-containing cells (Figure E1A). SERCA2 protein, corrected for β-actin expression, was diminished in CFTR antisense-expressing cells by about two thirds relative to sense-expressing cells (Figure 7A), comparable to the extent of reduction of SERCA2 expression in primary CF relative to non-CF airway cells.
Figure 7.
Effect of inhibiting functional cystic fibrosis transmembrane regulator gene (CFTR) expression by antisense CFTR oligonucleotides (A) or by overexpressing mutated CFTR (B) on sarcoendoplasmic reticulum Ca2+-ATPase (SERCA)2 expression. Cell lysates were prepared from polarized cultures of 16HBEo- cell line stably transfected with sense (S) and antisense (AS) CFTR oligonucleotide and analyzed for SERCA2 expression by Western blot. The bars represent means of data. *Significant difference (P < 0.05) from control (16HBE-S) cells. The experiment was repeated four times. (B) Results of SERCA2 expression analysis in lysates obtained from control and adenovirally transduced minimally transformed primary human bronchial epithelial cells (UNCN3T) grown on collagen-coated culture dishes. The bars represent means of data. *Significant difference (P < 0.05) from control (LacZ) cells. The experiment was repeated three times (n = 3 per condition).
SERCA2 Protein Expression Is Decreased by Expression of Mutant ΔF508 CFTR
The ΔF508 CFTR mutation causes diminished channel activity, impaired processing, and reduced CFTR protein stability at the cell surface. In addition, this mutation inhibits gating of CFTR channels, resulting in a diminished rate of opening (31). Because this is the most common CFTR mutation, we sought to determine the effect of its overexpression in airway epithelial cells on SERCA2 expression. We conducted studies in minimally transformed primary human bronchial epithelial cells (UNCN3T) transiently transduced using adenoviral vectors. Compared with nontransduced, LacZ-overexpressing, and wt-CFTR–overexpressing cells (increased CFTR expression by Ad.wt-CFTR is shown by Western blot in Figure E1B), ΔF508 CFTR-overexpressing cells had decreased SERCA2 protein expression (Figure 7B). Taken together, the results from use of these three strategies for CFTR functional inhibition demonstrate a link between diminished CFTR function and SERCA2 expression.
Bcl-2–induced displacement of SERCA2 from CRDs in CF cells.
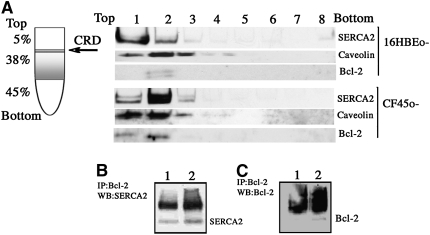
Interaction of Bcl-2 with SERCA2 has been described previously (20, 32). Bcl-2 binds to and inactivates SERCA and causes SERCA displacement from the CRDs of the ER membrane (19, 20). Sucrose density gradient fractionation of purified microsomes from non-CF 16HBEo- cells and CF45o- cells was performed to localize SERCA2 in the CRDs. For the detection of SERCA2 distribution in the sucrose density fractions, each fraction was loaded onto the gel for Western blot analysis (Figure 8A). Fractions were collected from the top of the gradient, where fraction 1 represents the initial CRD fraction. SERCA2 of 16HBEo- cells primarily localized in the CRD fraction, whereas the major SERCA2 band of CF45o- cells migrated deeper into the gradient. Thus, sucrose density gradient centrifugation of microsomes from CF preparations showed that SERCA2 was predominantly recovered in fraction 2, whereas non-CF samples showed SERCA2 predominantly in fraction 1. SERCA2 coimmunoprecipitated with Bcl-2 in 16HBEo- and CF45o- cells (Figure 8B). However, an increased amount of Bcl-2 was detected in CRDs and the immunoprecipitates from CF cell microsomes. These figures indicate the presence of increased Bcl-2 expression and Bcl-2–SERCA2 complexes in CF. The increase in Bcl-2–SERCA2 complexes in CF cells occurred despite the fact that SERCA2 protein was decreased by about 50% in the CF cell line. These findings indicate a mechanism for decline in ER-associated SERCA2 expression and total SERCA activity due to Bcl-2 association.
Figure 8.
Translocation of sarcoendoplasmic reticulum Ca2+-ATPase (SERCA2) within caveolae-related domains (CRDs) from the estimation of endoplasmic reticulum (ER) of cystic fibrosis (CF) cells. Purified microsomes from 16HBEo- and CF45o- cells were lysed in ice-cold 0.5 M sodium carbonate buffer. The homogenate was adjusted to 45% (w/v) sucrose by the addition of 90% sucrose in the MBS buffer and placed in the bottom of an ultracentrifuge tube. A discontinuous sucrose gradient was established by overlaying this solution with 4 ml of 38% sucrose and 3 ml of 5% sucrose. The tubes were then centrifuged at 4°C for 16 to 18 hours at 130,000 × g, and fractions were manually collected from the top of the gradient. To determine the distribution of CRD-associated proteins within the gradient, each fraction was analyzed by SDS-PAGE, followed by Western blot analysis with SERCA2 and caveolin antibody (A). The Western blot shown is representative of findings in three separate sucrose gradient centrifugation/Western blot experiments. (B and C) Western blot of SERCA2 and Bcl-2 using immunoprecipitate of microsomal fractions from (1) 16HBEo- and (2) CF45o- using Bcl-2 antibody.
Increased Bcl-2 expression in cellular compartments of CF cells.
We have shown that Bcl-2 was present at higher levels in the microsomal Bcl-2 immunoprecipitates of CF45o- cells relative to those present in the non-CF cell line (Figure 8C). Figure 9A shows the Western blot analysis of Bcl-2 distribution in the nucleus, ER, and mitochondria of 16HBEo-, CF41o-, and CF45o- cells. Increased Bcl-2 protein was observed in each of these cellular compartments of the two CF, relative to the non-CF, cell lines. Analysis of total Bcl-2 content in the ER membrane (M) of 16HBEo- and CF45o- cells using ELISA (Table 1) confirmed findings obtained by Western blot. Bcl-2 contents of whole cell lysates from differentiated primary human non-CF and CF bronchial epithelial cells showed a pattern of increase in CF cells, but the difference was not significantly different (Table 1).
Figure 9.
Increased Bcl-2 expression in the cellular compartments of cystic fibrosis (CF) cells. Nuclear, endoplasmic reticulum (ER), and mitochondrial fractions were prepared from (1) 16HBEo-, (2) CF41o-, and (3) CF45o- cells. Western blots were performed using antibodies against Bcl-2, cytochrome c oxidase (mitochondrial marker), protein disulphide isomerase (PDI, ER-specific protein) and lamin C (nuclear marker) (A). (B) Bcl-2 expression in the ER fraction of 16HBEo- cell line stably transfected with sense (S) and antisense (AS) CFTR oligonucleotide. (C) Total Bcl-2 content in cellular lysates of control and adenovirally transduced primary human bronchial epithelial cells grown on collagen-coated culture dishes. The bars represent means of data of two individual experiments (n = 4). *Significant difference (P < 0.05) from control.
TABLE 1.
TOTAL BCL-2 EXPRESSION IN NON–CYSTIC FIBROSIS AND CYSTIC FIBROSIS BRONCHIAL EPITHELIAL CELLS
| Cells | Total Bcl-2 (ng/mg protein) |
|---|---|
| Differentiated primary non-CF bronchial epithelial cells (WCL) (n = 5 donors) | 7.74 ± 1.41 |
| Differentiated primary non-CF bronchial epithelial cells (WCL) (n = 4 donors) | 10.55 ± 1.50 |
| 16HBEo- (M) | 8.90 ± 0.11 |
| CF45o- (M) | 14.50 ± 0.12* |
Definition of abbreviations: CF = cystic fibrosis; M = microsomal membrane preparations; WCL = whole cell lysate.
Significant difference from non–cystic fibrosis 16HBEo- cells (P < 0.05).
Role of diminished CFTR expression in increased Bcl-2 expression.
To determine if decreased CFTR function plays a role in the enhanced expression of Bcl-2, we isolated ER microsomes from 16HBEo- cell line stably transfected with sense (S) and antisense (AS) CFTR oligonucleotide. Increased expression of Bcl-2 was observed in cells in which CFTR was decreased by expression of antisense CFTR oligonucleotides (Figure 9B). This indicates that deficient CFTR expression is sufficient to increase Bcl-2 expression. Bcl-2 analysis was also performed in lysates obtained from control and adenovirally transduced primary human bronchial epithelial cells using ELISA. Cells expressing ΔF508 CFTR showed an approximately 2-fold increase in Bcl-2 expression as compared with the control cells (Figure 9C). Direct and/or indirect effects of altered CFTR function may lead to persistent endogenous activation of NF-κB in CF airway epithelial cells (33, 34). NF-κB regulates the transcription of Bcl-2 (35, 36). We determined Bcl-2 content and NF-κB activation (by measuring nuclear p65) of control and TNF-treated primary HBE cells that were adenovirally transduced with ΔF508 CFTR (Figure E2A). There was no change in CFTR expression with and without TNF treatment in the lacZ, wtCFTR, and ΔF508 CFTR-expressing cells. The nuclear lysates, when assessed by Western blot, showed increased p65 in the ΔF508 CFTR-expressing cells in both conditions of ΔF508 CFTR, with or without TNF treatment. Quantitative estimation of p65 in the nuclear lysates using ELISA revealed statistically significant increased nuclear p65 upon TNF treatment in the adenoviral lacZ and wtCFTR transduced cells (Figure E2B). The ΔF508 CFTR-expressing cells had increased basal nuclear p65 that was further enhanced upon TNF treatment. The cells that had increased nuclear p65 had increased Bcl-2 expression (Figure E2C).
SERCA2 is required for cell survival in oxidative stress.
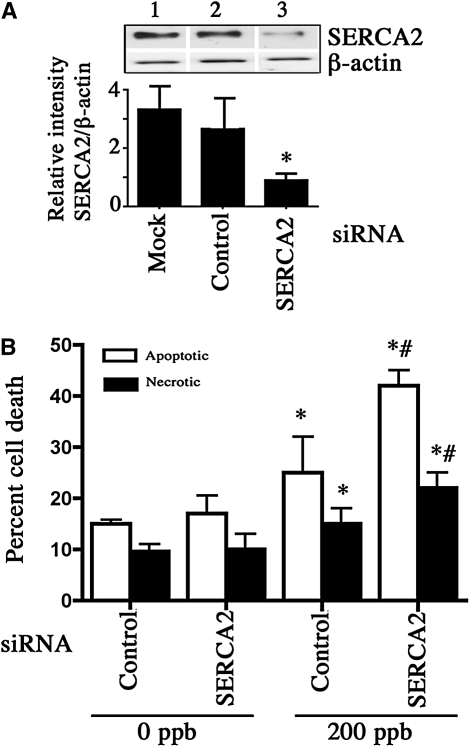
CF airway epithelial cells are continuously exposed to oxidative stress presented directly or indirectly by air pollutants, bacterial endotoxins, proinflammatory cytokines, and neutrophils. For this reason, we sought to determine the potential impact of diminished SERCA2 expression on airway epithelial cell survival during challenge by three relevant prooxidant stimuli: ozone (O3), hydrogen peroxide (H2O2), and TNF-α. SERCA2 siRNA was used to investigate the effect of SERCA2 expression on O3–induced cell death in primary airway epithelial cells cultured on collagen coated 6-well plates. First, we verified that SERCA2 protein levels were reduced in the cells expressing SERCA2 siRNA (Figure 10A). Transfection with nonspecific siRNA had no effect on SERCA2 protein expression, whereas SERCA2 siRNA–treated cells had SERCA2 protein expression decreased by 67% relative to nonspecific siRNA-treated control cells. Bcl-2 expression was not affected by SERCA2 knockdown in these cells (data not shown). Increased apoptotic and necrotic cell death was observed in the control siRNA-transfected cells upon exposure to 200 ppb O3 for 18 hours (Figure 10B). SERCA2 siRNA increased apoptotic and necrotic cell death induced by O3 (25.0 ± 7.0% vs. 42.0 ± 3.0% apoptotic cell death in control siRNA vs. SERCA2 siRNA–expressing cells). Besides O3 as an environmental factor, we assessed the toxicity of TNF-α and H2O2 because these are abundant in CF lungs. With 10 ng/ml TNF-α, a concentration close to those found in CF airways (37), there was enhanced cell death in SERCA2 siRNA–expressing cells, but the extent of death was minimal (4.17 ± 0.30% in control vs. 7.15 ± 0.50% total cell death in SERCA2 siRNA–expressing cells). However, when TNF-α was combined with IL-1β, another cytokine abundant in CF airways, there was increased apoptotic cell death in control and 1.7-fold still greater apoptosis in SERCA2 siRNA–treated cells (Table 2). The extent of necrosis was not different in the two groups. Similarly, treatment with H2O2 caused enhanced apoptotic and necrotic cell death in SERCA2 siRNA–expressing cells when compared with those expressing control siRNA (Table 2).
Figure 10.
Sarcoendoplasmic reticulum Ca2+-ATPase (SERCA2) is essential for cell survival during oxidative stress. (A) SERCA2 knockdown using SERCA2 siRNA. Top: representative Western blot of SERCA2 expression in primary human bronchial epithelial cells (grown on collagen-coated culture dishes) (1) mock-transfected or transfected with (2) control or (3) SERCA2 siRNA. Bottom: Quantitation of SERCA2 knockdown. The bars represent means of data. *Significant difference (P < 0.05) from control siRNA-transfected cells (n = 3; three individual experiments). For assessing ozone toxicity, primary human airway epithelial cells were transfected either with a control or SERCA2 siRNA for 24 hours and exposed to 0 ppb or 200 ppb ozone for 18 hours. After exposure, cells were analyzed for cell death using Vybrant apoptosis assay kit. The Alexa Fluor-488–labeled annexin-positive apoptotic and Sytox green-positive dead cells were quantified by flow cytometry (B). The columns represent means of data. *Significant difference (P < 0.05) from 0 ppb control cells. #Significant difference from 200 ppb control cells (n = 3). This experiment was repeated four times.
TABLE 2.
SERCA2 KNOCKDOWN ENHANCES CELL DEATH
| Control siRNA
|
SERCA2 siRNA
|
|||
|---|---|---|---|---|
| Treatment | Apoptosis (%) | Necrosis (%) | Apoptosis (%) | Necrosis (%) |
| TNF + IL-1β (10 ng/ml for 18 h) | 17.53 ± 1.83 | 8.54 ± 0.55 | 29.46 ± 0.72* | 9.53 ± 0.39 |
| H2O2 (500 μM for 18 h) | 9.66 ± 0.33 | 14.9 ± 0.85 | 14.00 ± 1.00* | 28.33 ± 1.66* |
Definition of abbreviation: SERCA2 = sarcoendoplasmic reticulum Ca2+-ATPase; TNF = tumor necrosis factor.
Significant difference from control siRNA-treated cells (P < 0.05). n = 3, experiment repeated three times.
DISCUSSION
Our study on the expression of SERCA2 in CF airways relative to expression in respiratory cells and tissues of non-CF individuals reveals that SERCA2 protein expression is decreased in CF airway epithelial cell lines and primary polarized airway epithelial cells grown at ALI and in vivo in distal and proximal airway epithelial tissue of subjects with CF. CFTR dysfunction-induced enhanced NF-κB activation and increased Bcl-2, which interacted with SERCA2 to translocate it from the caveolae-related domains (CRDs), were potential mechanisms causing decreased SERCA2 expression.
In the series of experiments involving primary cells grown at ALI, although there was considerable variability in expression from donor to donor, the overall extent of SERCA2 protein expression was diminished in primary CF cells. Individual variability in the primary cells could have resulted from variations in genotype, types of differentiated cells present (e.g., mucus, ciliated), the types and/or severity of infection(s) previously present, the presence of additional disease states (e.g., diabetes) in the donor (38), and/or the types of medications previously administered (39). SERCA2 protein expression was quantitatively diminished, as detected by immunohistochemistry, in epithelial cells of large and smaller airways in lungs of patients with CF. This supports the potential clinical relevance of these findings. Previous studies indicated that altered calcium homeostasis in airway epithelial cells of patients with CF is mainly a result of inflammation and infection (40, 41). The airway epithelial cell lines used in the present studies were maintained in the absence of infection and inflammation for months to years, and the primary cells examined were cultured in the absence of infection and inflammatory cells for 1 to 6 weeks. Despite the differences in cell environment, decreased SERCA2 expression was a constant feature of CF airway epithelium, suggesting that it is an intrinsic feature of the disease. This is further supported by IHC studies using lung tissues of patients with CF. These tissue samples were not free of infection and chronic inflammation.
ER plays an important role in CF disease pathogenesis. The decreased SERCA2 expression was not due to a diminished ER mass in CF cells. In fact, as indicated by staining for the ER-specific protein PDI and an ER-specific fluorescent dye, CF airway epithelial cells contained similar or greater quantities of ER. These findings are generally in agreement with previous reports of expanded ER in CF (40). Although abnormal ΔF508 CFTR can be retained and degraded in ER, and transient adenoviral overexpression of ΔF508 CFTR was sufficient to cause decreased SERCA2 expression in non-CF airway epithelial cells, the findings in our study (e.g., antisense CFTR oligonucleotide expression) do not indicate that the presence of mutant CFTR(s) in the ER is the principal cause of diminished SERCA2 expression in CF airway epithelial cells.
Recent investigations on the modulation of SERCA2 activity have reported the presence of an important interaction between SERCA2 and Bcl-2, resulting in SERCA2's translocation from CRD to membrane domains of higher density, partial unfolding, and inactivation (19, 20). Bcl-2 is capable of interaction with SERCA1 or SERCA2. In purified sarcoplasmic reticulum preparations, the addition of Bcl-2 causes significant loss of SERCA activity (20). Because of relevant reports in CF (42, 43), we determined if such an interaction occurs in CF airway epithelium. Bcl-2 protein expression and association with SERCA2 was elevated in CF relative to non-CF airway epithelial cells. Given that Bcl-2 binding to SERCA2 in other tissues can displace it from CRD and inactivate it, our findings suggest that similar mechanisms could act in lung epithelium to diminish SERCA2 protein expression and activity in the ER. We do not know whether SERCA2 is also chemically modified such that its interaction with Bcl-2 and/or its inactivation is enhanced.
A previous study in relation to CF and Bcl-2 expression indicated that Bcl-2 expression increased mucus cell metaplasia of airway epithelial cells and that insufflation of bacterial endotoxin could cause such changes in the airways of rats (42). Our studies of CF and non-CF airway epithelial cell lines and of CFTR sense and antisense oligonucleotide-expressing cells were done in the absence of bacterial infection, inflammation, and endotoxin, and these cells were maintained in the absence of these factors for extended periods of time. In addition, the cells in these experiments were not cultured at ALI. Thus, unlike our studies of primary cell cultures grown at ALI, they could not undergo differentiation to mucus-expressing cells and could not have had altered Bcl-2 expression in association with mucus cell differentiation. In this case, such culture conditions were considered advantageous because they prevented potential Bcl-2 changes due to differentiated phenotype. Some variability in SERCA2 expression in CF primary airway epithelial cells from different donors could have related to differences in mucus cell differentiation of such cultures, but this could not have occurred in the various transformed CF and non-CF cell lines we studied. Based on the absence of infection, inflammation, bacterial endotoxin, and mucus cell differentiation in our experiments pertinent to Bcl-2, our findings indicate that increased Bcl-2 expression is an inherent feature of CF airways epithelium.
CFTR dysfunction is associated with aberrations in a number of signaling pathways, including those related to inflammation and infection. The specific link(s) between CFTR dysfunction and increased NF-κB activation in CF cells remains undefined. However, there are several factors that could cause this to occur (43). Our experiment with overexpression of mutant CFTR provides further evidence that CFTR dysfunction causes increased NF-κB activation p65 mobilization to the nucleus. Increased NF-κB–mediated proinflammatory gene transcription has been reported elsewhere (24) in the cells expressing CFTR antisense oligonucleotide (16HBE-AS), which is another model we studied. NF-κB is a transcriptional regulator of Bcl-2 and other proteins of the Bcl-2 family (35, 44). Thus, increased Bcl-2 in cells with CFTR dysfunction could result, at least in part, from enhanced NF-κB activation.
Increased expression of Bcl-2 in CF airway epithelium could have beneficial and/or detrimental effects. It could increase proliferation and diminish cell death via its antiapoptotic action. Increased proliferation in the submucosal gland and in basal cells of CF airways has been described elsewhere (45), and this could have an important role in airway epithelial regeneration and repair. Furthermore, osmotic challenges routinely faced by CF airway epithelial cells might be better tolerated in the presence of Bcl-2 overexpression. CF epithelial cells have increased susceptibility to osmotic stress and blunted regulatory volume decrease responses (46, 47), and Bcl-2 overexpression can stimulate such responses and promote survival (48). Moreover, by inhibiting apoptosis in the presence of oxidative stress and/or infection, Bcl-2 expression at high levels could favor necrosis of airway epithelium and enhance the release of cell contents like DNA and proteolytic enzymes, worsening airways damage and obstruction. Such effects of Bcl-2 may be site specific. In this context and in the context of the present study, it is noteworthy that others recently described a paradoxical proapoptotic effect of high-level Bcl-2 expression when Bcl-2 is localized to the nucleus (49). Although Bcl-2 expression often acts to oppose apoptosis and cell death, it might do otherwise in the context of CF, if, for example, its effects on SERCA2 secondarily alter other functions, like alternate chloride channels, that could themselves influence cell fate.
Members of the Bcl-2 family regulate ER Ca2+ homeostasis. Bcl-x(L) binds to the inositol trisphosphate receptor (InsP(3)R) Ca2+ release channel to enhance Ca2+ release, resulting in reduced ER [Ca2+], increased oscillations of cytoplasmic Ca2+ concentration ([Ca2+]i), and resistance to apoptosis (50). Bcl-2 also increases emptying of endoplasmic reticulum Ca2+ stores during photodynamic therapy–induced apoptosis (51). Emptying of ER stores after SERCA inhibition with thapsigargin causes cells to undergo growth arrest or apoptotic cell death (52). Thus, Bcl-2 and Bcl-2–related proteins regulate apoptosis by altering ER [Ca2+] via modulation of InsP(3)R and/or SERCAs (20, 53). However, it is not clear whether reduced ER [Ca2+] or enhanced [Ca2+]i signaling is most relevant for apoptosis protection.
Although the literature is mixed on the ability of CF cells to undergo apoptosis, recent reports indicated that CF airway epithelial cells are more prone to apoptosis and/or necrosis than non-CF cells (54, 55). Despite the antiapoptotic effects that Bcl-2 could have in the CF airway, we sought to define whether down-regulation of SERCA2 in airway epithelial cells, whether via Bcl-2 or other mechanisms, affects cell survival there. We specifically asked whether diminished SERCA2 expression could enhance apoptosis and/or cell death. Primary airway epithelial cells expressing SERCA2 siRNA showed enhanced toxicity to three different stimuli, each of which can contribute, directly and indirectly, to oxidative stress in CF airways (56). These included O3, H2O2, and TNF-α + IL-1β. These studies indicated the capacity of SERCA2 down-regulation to modulate susceptibility to cell death due to oxidative stress.
There are multiple examples in the cardiovascular literature in which SERCA modification can potentially affect the disease process. Oxidative stress may modify SERCA structure and/or function (57). In CF airways, oxidative stress may be innate and/or secondary to inflammation. Cholesterol also can affect SERCA expression (58) and may accumulate excessively in CF lung (59). In one model, heterozygous SERCA2 knockout (SERCA2a+/−) mice showed substantially enlarged infarction after cardiac ischemia (60). In addition, they showed impaired postischemic myocardial relaxation and reduced postischemic myocardial contractile function. These animals had higher diastolic intracellular calcium without oxidative stress and higher intracellular calcium after the oxidative stress of reperfusion. The level of reactive oxygen species production was not increased in the SERCA2 knockouts, but the level of dysfunction related to oxidative stress was increased. Thus, there are precedents for substantial alterations in cell survival and function in the heart with comparable changes in SERCA2 expression to those seen in CF, relative to non-CF, lung cells.
Intracellular calcium signaling depends upon SERCA activity. [Ca2+]i can regulate a number of important functions pertinent to airway epithelial cells, including mucus secretion, ciliary contraction and motility, signal transduction, and cell survival. Diminished SERCA activity in CF could dampen such intracellular calcium signals and blunt adaptive chloride export. Novel purinergic compounds in development for treatment of CF are directed at stimulating intracellular calcium signals by acting on extracellular ATP receptors that activate this process (61). It was recently reported that ATP-induced increased short circuit currents were lower in CF than non-CF small airway epithelial cell cultures (62). Taken together, these data suggest that SERCA inhibitor therapy would not likely be beneficial in CF and that SERCA inhibition may worsen airway epithelial cell survival under oxidative stress and decrease alternative calcium-dependent chloride transport.
Hence, investigation of an alternate approach using SERCA-stimulating interventions in clinically relevant models of CF may be warranted by the present study.
Supplementary Material
Acknowledgments
The authors thank Leslie Fulcher for expert technical advice on primary airway cell culture; Dr. A.S. Verkman, University of California, San Francisco, CA, for providing the CFTRinh172; Dr. P.B. Davis, Case Western Reserve University Cleveland, OH, for the CFTR-S and CFTR-AS expressing 16HBEo- cells; Barbara K. Schneider for assistance with immunofluorescence experiments and Stacy Miller for assistance with the siRNA experiments; Tara Hendry-Hofer for assistance in the quantitation of the IHCs; and Starsa Duskin for assistance with toxicity determinations.
Supported by R01-ES014448 from the National Institute of Environmental Health Sciences, National Institutes of Health, and by the Max and Yetta Karasik Family Foundation (C.W.W.); R01-HL080322 and CF Foundation Resources Development Program Grant RDP CORE C: R026-R02 (S.R.); P01AG12993 from the National Institute of Aging (C.S.); K12 KL2RR025779 from the National Center for Research Resources (S.A.); by the Scientist Development Grant award (0830418N) from the American Heart Association (A.A.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200807-1104OC on February 6, 2009
Conflict of Interest Statement: S.A. holds a provisional patent for use of SERCA activators in CF. This patent was filed on October 2, 2008, after the orginal submission of this manuscript. No funds have been received as a result of this patent filing. A.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.S.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. X.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.N.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A-L.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H.R. serves as a consultant for Gilead and Novartis. C.W.W. holds a provisional patent for use of SERCA activators in CF. This patent was filed on October 2, 2008, after the orginal submission of this manuscript. No funds have been received as a result of this patent filing.
References
- 1.Ratjen F. Recent advances in cystic fibrosis. Paediatr Respir Rev 2008;9:144–148. [DOI] [PubMed] [Google Scholar]
- 2.Egan ME, Glockner-Pagel J, Ambrose C, Cahill PA, Pappoe L, Balamuth N, Cho E, Canny S, Wagner CA, Geibel J, et al. Calcium-pump inhibitors induce functional surface expression of delta F508-CFTR protein in cystic fibrosis epithelial cells. Nat Med 2002;8:485–492. [DOI] [PubMed] [Google Scholar]
- 3.Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004;304:600–602. [DOI] [PubMed] [Google Scholar]
- 4.Berger AL, Randak CO, Ostedgaard LS, Karp PH, Vermeer DW, Welsh MJ. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl- channel activity. J Biol Chem 2005;280:5221–5226. [DOI] [PubMed] [Google Scholar]
- 5.Harada K, Okiyoneda T, Hashimoto Y, Oyokawa K, Nakamura K, Suico MA, Shuto T, Kai H. Curcumin enhances cystic fibrosis transmembrane regulator expression by down-regulating calreticulin. Biochem Biophys Res Commun 2007;353:351–356. [DOI] [PubMed] [Google Scholar]
- 6.Lipecka J, Norez C, Bensalem N, Baudouin-Legros M, Planelles G, Becq F, Edelman A, Davezac N. Rescue of deltaF508-CFTR (cystic fibrosis transmembrane conductance regulator) by curcumin: involvement of the keratin 18 network. J Pharmacol Exp Ther 2006;317:500–505. [DOI] [PubMed] [Google Scholar]
- 7.Norez C, Noel S, Wilke M, Bijvelds M, Jorna H, Melin P, DeJonge H, Becq F. Rescue of functional delF508-CFTR channels in cystic fibrosis epithelial cells by the alpha-glucosidase inhibitor miglustat. FEBS Lett 2006;580:2081–2086. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem 2007;282:4533–4544. [DOI] [PubMed] [Google Scholar]
- 9.Wang W, Li G, Clancy JP, Kirk KL. Activating cystic fibrosis transmembrane conductance regulator channels with pore blocker analogs. J Biol Chem 2005;280:23622–23630. [DOI] [PubMed] [Google Scholar]
- 10.Grubb BR, Gabriel SE, Mengos A, Gentzsch M, Randell SH, Van Heeckeren AM, Knowles MR, Drumm ML, Riordan JR, Boucher RC. SERCA pump inhibitors do not correct biosynthetic arrest of deltaF508 CFTR in cystic fibrosis. Am J Respir Cell Mol Biol 2006;34:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dragomir A, Bjorstad J, Hjelte L, Roomans GM. Curcumin does not stimulate cAMP-mediated chloride transport in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun 2004;322:447–451. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Sonawane ND, Salinas D, Qian L, Pedemonte N, Galietta LJ, Verkman AS. Evidence against the rescue of defective deltaF508-CFTR cellular processing by curcumin in cell culture and mouse models. J Biol Chem 2004;279:40629–40633. [DOI] [PubMed] [Google Scholar]
- 13.Loo TW, Bartlett MC, Clarke DM. Thapsigargin or curcumin does not promote maturation of processing mutants of the ABC transporters, CFTR, and p-glycoprotein. Biochem Biophys Res Commun 2004;325:580–585. [DOI] [PubMed] [Google Scholar]
- 14.Ashby MC, Tepikin AV. ER calcium and the functions of intracellular organelles. Semin Cell Dev Biol 2001;12:11–17. [DOI] [PubMed] [Google Scholar]
- 15.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure: a possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res 1993;72:463–469. [DOI] [PubMed] [Google Scholar]
- 16.Shull GE. Gene knockout studies of Ca2+-transporting ATPases. Eur J Biochem 2000;267:5284–5290. [DOI] [PubMed] [Google Scholar]
- 17.Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun 2004;322:1192–1203. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber R, Kunzelmann K. Purinergic P2Y6 receptors induce Ca2+ and CFTR dependent Cl- secretion in mouse trachea. Cell Physiol Biochem 2005;16:99–108. [DOI] [PubMed] [Google Scholar]
- 19.Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA). Biochem J 2004;383:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dremina ES, Sharov VS, Schoneich C. Displacement of SERCA from SR lipid caveolae-related domains by Bcl-2: a possible mechanism for SERCA inactivation. Biochemistry 2006;45:175–184. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad S, Ahmad A, Guo X, Schneider BK, Jones T, McConville G, Tatreu J, Perraud A, Randell SH, White CW. Differential expression of sarco-endoplasmic reticulum calcium ATPases (SERCAs) in cystic fibrosis (CF) epithelium. Presented at 21st annual North American Cystic Fibrosis Conference, Anaheim, CA, October 3–7, 2007.
- 22.Ahmad S, Ahmad A, Dremina ES, Sharov VS, Perraud A, Schoneich C, Randell SH, White CW. Bcl-2 suppresses SERCA2 expression in cystic fibrosis airway epithelial cells. Presented at Lung Epithelium and Disease, FASEB Summer Research Conference, Saxtons River, VT, August 3–8, 2008.
- 23.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 2005;107:183–206. [DOI] [PubMed] [Google Scholar]
- 24.Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 2001;280:L493–L502. [DOI] [PubMed] [Google Scholar]
- 25.Fulcher ML, Gabriel SE, Olsen JC, Tatreau JR, Gentzsch M, Livanos E, Saavedra MT, Salmon P, Randell SH. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am J Physiol Lung Cell Mol Physiol 2009;296:L82–L91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad A, Ahmad S, Chang LY, Schaack J, White CW. Endothelial Akt activation by hyperoxia: role in cell survival. Free Radic Biol Med 2006;40:1108–1118. [DOI] [PubMed] [Google Scholar]
- 27.Vais H, Gao GP, Yang M, Tran P, Louboutin JP, Somanathan S, Wilson JM, Reenstra WW. Novel adenoviral vectors coding for GFP-tagged wtCFTR and deltaF508-CFTR: characterization of expression and electrophysiological properties in A549 cells. Pflugers Arch 2004;449:278–287. [DOI] [PubMed] [Google Scholar]
- 28.Park J, Fang S, Crews AL, Lin KW, Adler KB. MARCKS regulation of mucin secretion by airway epithelium in vitro: interaction with chaperones. Am J Respir Cell Mol Biol 2008;39:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnaudeau S, Frieden M, Nakamura K, Castelbou C, Michalak M, Demaurex N. Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J Biol Chem 2002;277:46696–46705. [DOI] [PubMed] [Google Scholar]
- 30.Wu R, Zhao YH, Chang MM. Growth and differentiation of conducting airway epithelial cells in culture. Eur Respir J 1997;10:2398–2403. [DOI] [PubMed] [Google Scholar]
- 31.Ostedgaard LS, Rogers CS, Dong Q, Randak CO, Vermeer DW, Rokhlina T, Karp PH, Welsh MJ. Processing and function of CFTR-deltaF508 are species-dependent. Proc Natl Acad Sci USA 2007;104:15370–15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo TH, Kim HR, Zhu L, Yu Y, Lin HM, Tsang W. Modulation of endoplasmic reticulum calcium pump by Bcl-2. Oncogene 1998;17:1903–1910. [DOI] [PubMed] [Google Scholar]
- 33.Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl- channel function. Am J Physiol Lung Cell Mol Physiol 2001;281:L71–L78. [DOI] [PubMed] [Google Scholar]
- 34.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol 2006;291:C218–C230. [DOI] [PubMed] [Google Scholar]
- 35.Wang CY, Guttridge DC, Mayo MW, Baldwin AS Jr. NF-kappaB induces expression of the Bcl-2 homologue A1/bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol 1999;19:5923–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Togo S, Al-Mugotir M, Kim H, Fang Q, Kobayashi T, Wang X, Mao L, Bitterman P, Rennard S. NF-kappaB mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respir Res 2008;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osika E, Cavaillon JM, Chadelat K, Boule M, Fitting C, Tournier G, Clement A. Distinct sputum cytokine profiles in cystic fibrosis and other chronic inflammatory airway disease. Eur Respir J 1999;14:339–346. [DOI] [PubMed] [Google Scholar]
- 38.Vasanji Z, Dhalla NS, Netticadan T. Increased inhibition of SERCA2 by phospholamban in the type I diabetic heart. Mol Cell Biochem 2004;261:245–249. [DOI] [PubMed] [Google Scholar]
- 39.Randriamboavonjy V, Pistrosch F, Bolck B, Schwinger RH, Dixit M, Badenhoop K, Cohen RA, Busse R, Fleming I. Platelet sarcoplasmic endoplasmic reticulum Ca2+-atpase and mu-calpain activity are altered in type 2 diabetes mellitus and restored by rosiglitazone. Circulation 2008;117:52–60. [DOI] [PubMed] [Google Scholar]
-
40.Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial
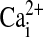 signaling: the mechanism for the larger agonist-mediated
signaling: the mechanism for the larger agonist-mediated 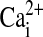 signals in human cystic fibrosis airway epithelia. J Biol Chem 2005;280:10202–10209. [DOI] [PubMed] [Google Scholar]
signals in human cystic fibrosis airway epithelia. J Biol Chem 2005;280:10202–10209. [DOI] [PubMed] [Google Scholar] - 41.Ribeiro CM. The role of intracellular calcium signals in inflammatory responses of polarised cystic fibrosis human airway epithelia. Drugs R D 2006;7:17–31. [DOI] [PubMed] [Google Scholar]
- 42.Harris JF, Fischer MJ, Hotchkiss JR, Monia BP, Randell SH, Harkema JR, Tesfaigzi Y. Bcl-2 sustains increased mucous and epithelial cell numbers in metaplastic airway epithelium. Am J Respir Crit Care Med 2005;171:764–772. [DOI] [PubMed] [Google Scholar]
- 43.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol 2008;34:146–162. [DOI] [PubMed] [Google Scholar]
- 44.Heckman CA, Mehew JW, Boxer LM. NF-kappaB activates Bcl-2 expression in t(14;18) lymphoma cells. Oncogene 2002;21:3898–3908. [DOI] [PubMed] [Google Scholar]
- 45.Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 2005;172:1013–1018. [DOI] [PubMed] [Google Scholar]
- 46.Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Ismailov II, et al. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem 2001;276:6621–6630. [DOI] [PubMed] [Google Scholar]
- 47.Braunstein GM, Zsembery A, Tucker TA, Schwiebert EM. Purinergic signaling underlies CFTR control of human airway epithelial cell volume. J Cyst Fibros 2004;3:99–117. [DOI] [PubMed] [Google Scholar]
- 48.Shen MR, Yang TP, Tang MJ. A novel function of Bcl-2 overexpression in regulatory volume decrease: enhancing swelling-activated Ca(2+) entry and Cl(-) channel activity. J Biol Chem 2002;277:15592–15599. [DOI] [PubMed] [Google Scholar]
- 49.Portier BP, Taglialatela G. Bcl-2 localized at the nuclear compartment induces apoptosis after transient overexpression. J Biol Chem 2006;281:40493–40502. [DOI] [PubMed] [Google Scholar]
- 50.Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-x(l) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci USA 2007;104:12565–12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Granville DJ, Ruehlmann DO, Choy JC, Cassidy BA, Hunt DW, van Breemen C, McManus BM. Bcl-2 increases emptying of endoplasmic reticulum Ca2+ stores during photodynamic therapy-induced apoptosis. Cell Calcium 2001;30:343–350. [DOI] [PubMed] [Google Scholar]
- 52.Zhou YP, Teng D, Dralyuk F, Ostrega D, Roe MW, Philipson L, Polonsky KS. Apoptosis in insulin-secreting cells: evidence for the role of intracellular Ca2+ stores and arachidonic acid metabolism. J Clin Invest 1998;101:1623–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu C, Xu W, Palmer AE, Reed JC. Bi-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem 2008;283:11477–11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rottner M, Kunzelmann C, Mergey M, Freyssinet JM, Martinez MC. Exaggerated apoptosis and NF-kappaB activation in pancreatic and tracheal cystic fibrosis cells. FASEB J 2007;21:2939–2948. [DOI] [PubMed] [Google Scholar]
- 55.Ornatowski W, Poschet JF, Perkett E, Taylor-Cousar JL, Deretic V. Elevated furin levels in human cystic fibrosis cells result in hypersusceptibility to exotoxin a-induced cytotoxicity. J Clin Invest 2007;117:3489–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krunkosky TM, Fischer BM, Martin LD, Jones N, Akley NJ, Adler KB. Effects of TNF-alpha on expression of ICAM-1 in human airway epithelial cells in vitro. Signaling pathways controlling surface and gene expression. Am J Respir Cell Mol Biol 2000;22:685–692. [DOI] [PubMed] [Google Scholar]
- 57.Ying J, Sharov V, Xu S, Jiang B, Gerrity R, Schoneich C, Cohen RA. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic Biol Med 2008;45:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vangheluwe P, Raeymaekers L, Dode L, Wuytack F. Modulating sarco(endo)plasmic reticulum Ca2+ ATPase2 (SERCA2) activity: Cell biological implications. Cell Calcium 2005;38:291–302. [DOI] [PubMed] [Google Scholar]
- 59.White NM, Jiang D, Burgess JD, Bederman IR, Previs SF, Kelley TJ. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 2007;292:L476–L486. [DOI] [PubMed] [Google Scholar]
- 60.Talukder MA, Kalyanasundaram A, Zuo L, Velayutham M, Nishijima Y, Periasamy M, Zweier JL. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol 2008;294:H1426–H1434. [DOI] [PubMed] [Google Scholar]
- 61.Deterding RR, Lavange LM, Engels JM, Mathews DW, Coquillette SJ, Brody AS, Millard SP, Ramsey BW. Phase 2 randomized safety and efficacy trial of nebulized denufosol tetrasodium in cystic fibrosis. Am J Respir Crit Care Med 2007;176:362–369. [DOI] [PubMed] [Google Scholar]
- 62.Blouquit S, Regnier A, Dannhoffer L, Fermanian C, Naline E, Boucher R, Chinet T. Ion and fluid transport properties of small airways in cystic fibrosis. Am J Respir Crit Care Med 2006;174:299–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.