Abstract
Rationale: Ambient air pollution has been associated with heart failure morbidity and mortality. The mechanisms responsible for these associations are unknown but may include the effects of traffic-related pollutants on vascular or autonomic function.
Objectives: We assessed the cross-sectional relation between long-term air pollution, traffic exposures, and important end-organ measures of alterations in cardiac function—left ventricular mass index (LVMI) and ejection fraction—in the Multi-Ethnic Study of Atherosclerosis, a multicenter study of adults without previous clinical cardiovascular disease.
Methods: A total of 3,827 eligible participants (aged 45–84 yr) underwent cardiac magnetic resonance imaging between 2000 and 2002. We estimated air pollution exposures using residential proximity to major roadways and interpolated concentrations of fine particulate matter (less than 2.5 microns in diameter). We examined adjusted associations between these exposures and left ventricular mass and function.
Measurements and Main Results: Relative to participants living more than 150 m from a major roadway, participants living within 50 m of a major roadway showed an adjusted 1.4 g/m2 (95% CI, 0.3–2.5) higher LVMI, a difference in mass corresponding to a 5.6 mm Hg greater systolic blood pressure. Ejection fraction was not associated with proximity to major roadways. Limited variability in estimates of fine particulate matter was observed within cities, and no associations with particulate matter were found for either outcome after adjustment for center.
Conclusions: Living in close proximity to major roadways is associated with higher LVMI, suggesting chronic vascular end-organ damage from a traffic-related environmental exposure. Air pollutants or another component of roadway proximity, such as noise, could be responsible.
Keywords: epidemiology, particulate matter, hypertrophy, heart failure, magnetic resonance imaging
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Although air pollution has been associated with cardiovascular disease morbidity and mortality, including heart failure hospitalizations, a relationship between air pollution and left ventricular mass or function has not previously been observed.
What This Study Adds to the Field
Traffic exposure, as measured by very close (less than 50 m) home proximity to major roadways, is associated with higher left ventricular mass in adults, corresponding to the effect of a 6 mm Hg increase in systolic blood pressure. Although air pollutants are the likely cause, other traffic-derived environmental conditions should be considered.
Short-term and long-term exposures to air pollution have been consistently linked to cardiovascular disease morbidity and mortality. However, the mechanisms generating these associations and the impact of air pollution on specific subtypes of cardiovascular disease, such as heart failure (HF), remain to be fully understood. Although recent evidence suggests that air pollution may increase hospitalizations (1, 2), acute myocardial infarctions (3), and mortality (4, 5) in individuals with HF, it remains unknown whether air pollution is associated with the development of HF itself.
HF affects nearly 6 million people in the United States (6). Although long-term age-adjusted rates do not appear to be increasing, the aging of the population and decreases in HF mortality predict an increasing prevalence of HF in the coming years (7). HF-related hospital discharges in the United States have increased by nearly a factor of three over the past 20 years, and the magnitude of the problem appears equally striking in Europe (6, 8). Thus, the identification of factors contributing to the onset of HF has considerable worldwide public health importance.
Cardiac magnetic resonance imaging (MRI) provides a very precise measure of left ventricular (LV) mass that predicts the development of HF. In the Cardiovascular Health Study, increased baseline LV mass measured by echocardiography (a less precise measure) was an independent risk factor for the development of depressed ejection fraction (EF) 5 years later (9). The Multi-Ethnic Study of Atherosclerosis (MESA) is the first large epidemiologic cohort to take advantage of cardiac MRI to assess the effects of air pollution.
METHODS
Participants
MESA is a multiethnic longitudinal epidemiologic study of 6,814 participants aged 45 to 84 years that was initiated in July 2000 to understand the importance of subclinical atherosclerosis measures as well as other factors in individuals without known clinical cardiovascular disease (10). Full MESA eligibility criteria are available online at http://www.mesa-nhlbi.org. This prospective cohort study includes individuals from six United States communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; New York, New York; and St. Paul, Minnesota) and consists of 38% white, 28% African American, 22% Hispanic, and 12% Asian (of Chinese descent) subjects. For the present study, we included all participants who underwent cardiac MRI at the first examination (n = 5,004), had accurate address information for exposure assignment (n = 4,569), and had full covariate data, leaving 3,827 participants for analysis.
Procedures
Medical history, address, anthropometric measurements, physical examination, and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000 to August 2002) (10). Information about age, sex, ethnicity, and medical history were obtained by questionnaires administered at the screening and the first examination. The protocol was approved by institutional review boards at all participating centers, and all participants gave informed consent.
Participants' residential addresses were assigned geographic coordinates using ArcGIS 9.1 software (ESRI, Redlands, CA) in conjunction with the Dynamap/2000 street network and geocoding database (Tele Atlas, Boston, MA). Addresses were manually cleaned prior to geocoding, and matches with a geocoding score less than or equal to 80% were checked manually for accuracy.
Proximity to traffic was calculated by measuring the distance from a geocoded home address to the nearest major roadway (interstate, state, or county highway or major arterial), with a maximum search radius of 150 m. Exposure groups were divided into less than 50 m, 50 to 100 m, 101 to 150 m, and greater than 150 m from the nearest major roadway. These groups were defined to reflect varying levels of exposure based on the observed distribution of specific near-roadway air pollutants, with many pollutants approaching background levels by 150 to 200 m from large roadways (11, 12). For comparison purposes with previous studies of traffic effects and as a sensitivity analysis, we also used methods developed for the Netherlands Cohort Study on Diet and Cancer, assessing exposure as an indicator of living either within 100 m of a highway or within 50 m of a major arterial (13).
We also investigated the impacts of regional levels of ambient air pollution based on interpolated concentrations of particulate matter less than 2.5 μm in aerodynamic diameter (PM2.5) at each participant's home. Although more accurate estimates will ultimately be available through ongoing ancillary research in MESA, this early analysis relied on average concentrations for the year 2000 for each U.S. Environmental Protection Agency (EPA) Air Quality System monitor sited for population, general, or background pollution, with at least 90 days of valid observations for the year. Using ArcGIS Geostatistical Analyst, we then calculated interpolated PM2.5 exposures for each city using ordinary kriging estimates. We compared the results obtained using these kriged estimates with results obtained on a subset of participants using the PM2.5 value of the nearest EPA monitor selected based on criteria previously described by our group (14).
LV mass, LV end-diastolic volume (EDV), and LV end-systolic volume (ESV) were obtained by cardiac MRI. Images were acquired by 1.5 Tesla MRI scanners (SIGNA LX and CVi, GE Healthcare, Buckinghamshire, UK, and Somatom Vision and Sonata, Siemens Medical Solutions, Berlin, Germany), using a protocol described recently (15). All MRI data were submitted to the MESA MRI Reading Center at Johns Hopkins Hospital for centralized processing using MASS software, version 4.2 (Medis, Leiden, Netherlands). LV mass index (LVMI, g/m2) was calculated using the DuBois formula for body surface area. EF was calculated by dividing stroke volume (SV, EDV minus ESV) by EDV.
Statistical Analysis
All data analyses were performed using Stata 9.2 (StataCorp, College Station, TX). Univariate and bivariate summaries for all covariates were initially examined. We then used linear regression models to examine the associations. To avoid spurious correlation (16) introduced by the use of ratios in regression analyses, we initially used LV mass as the outcome in a regression model that adjusted for height and weight rather than calculated LVMI. Similarly, for LV function analyses, we initially studied SV adjusted for EDV, height, and weight rather than LVEF. For ease of clinical understanding, and because the results remain unchanged, we present here the results of models using LVMI and EF as outcomes. We then examined for the presence of effect modification by cardiovascular risk factors and geographic covariates.
Covariates were chosen a priori based on either a presumed relationship with both the exposures and the outcomes in each model, or a presumed ability to increase precision. These included age, sex, race/ethnicity, household income, highest educational attainment level, center, systolic and diastolic blood pressure, antihypertensive medication use, low-density lipoprotein and high-density lipoprotein cholesterol, lipid-lowering medication use, physical activity, weekly alcohol consumption, current smoking and pack-year history of smoking, hours per week of secondhand smoke exposure, diabetes status by fasting blood glucose criteria, and use of diabetes medications. Because several of these parameters (particularly blood pressure) may lie in the hypothesized causal biologic pathway between air pollutants and LV mass or function, we examined several partially adjusted models to evaluate the impact of such covariates. Both outcomes were modeled as continuous variables. We assessed model fit and found no significant evidence of non-normality or heteroscedasticity of residuals.
Sensitivity analyses included omitting from the model variables not observed to have strong relationships with the exposures, adding adjustment for individual classes of antihypertensive medications, adding center by race and center by income interactions, and adding neighborhood-level measures of socioeconomic status described previously (17). We also evaluated the impact of different inclusion/exclusion criteria based on years lived at current residence, MRI quality, missing data, and using different forms of the exposure variables.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Participant Characteristics
Table 1 shows the participants' characteristics at the initial examination of MESA. Age ranged from 45 to 84 years. Although all MESA participants were free of clinical cardiovascular disease at baseline, 35% were on antihypertensive therapy and 16% were on lipid-lowering therapy.
TABLE 1.
BASELINE PARTICIPANT CHARACTERISTICS, MULTI-ETHNIC STUDY OF ATHEROSCLEROSIS AND AIR POLLUTION, 2002
| N (%) or Mean (SD) | N (%) or Mean (SD) | ||
|---|---|---|---|
| Sex | Age, years | ||
| Female | 2,035 (53) | 45–54 | 1,166 (31) |
| Race | 55–64 | 1,088 (28) | |
| African American | 891 (23) | 65–74 | 1,116 (29) |
| white | 1,539 (40) | 75–84 | 457 (12) |
| Chinese | 549 (14) | Education | |
| Hispanic | 848 (22) | Did not complete high school | 586 (15) |
| Center | Completed high school | 1,220 (32) | |
| St. Paul | 584 (15) | Associate/technical degree | 461 (12) |
| New York | 684 (18) | Bachelor's degree | 755 (20) |
| Los Angeles | 806 (21) | Graduate/professional degree | 805 (21) |
| Chicago | 712 (19) | Household income, $ | |
| Winston-Salem | 459 (12) | <25,000 | 1,105 (29) |
| Baltimore | 582 (15) | 25,000–49,999 | 1,091 (29) |
| Smoking | 50,000–74,999 | 683 (18) | |
| Never smoker | 2,201 (58) | 75,000–99,999 | 369 (10) |
| Former, <20 pack-years | 913 (24) | ≥100,000 | 579 (15) |
| Former, ≥20 pack-years | 581 (15) | Fasting blood glucose, mg/dl | |
| Current, <20 pack-years | 68 (2) | <100 | 2,361 (62) |
| Current, ≥20 pack-years | 64 (2) | 100–125 | 1,009 (26) |
| Second-hand smoke, hours/week | >125, untreated | 129 (3) | |
| <1 | 2,276 (60) | >125, treated | 328 (9) |
| 1 | 543 (14) | On lipid-lowering therapy | 626 (16) |
| 2–5 | 493 (13) | On HTN therapy | 1,340 (35) |
| 6–10 | 200 (5) | Alcohol, drinks/week | |
| >10 | 315 (8) | 0 | 2,479 (65) |
| Blood pressure, mm Hg | 1–14 | 1,265 (33) | |
| Systolic <130 and diastolic <85 | 2,389 (62) | >14 | 93 (2) |
| Systolic 130–139 or diastolic 85–89 | 566 (15) | BMI (kg/m2) | |
| Systolic 140–159 or diastolic 90–99 | 658 (17) | 18.5–22.9 | 1,197 (31) |
| Systolic ≥160 or diastolic ≥100 | 214 (6) | 23–27.5 | 1,549 (41) |
| LVMI, g/m2 | 77.2 (16) | 27.6–40 | 1,010 (26) |
| Ejection fraction, % | 69.4 (7) | >40 | 71 (2) |
| Total | 3,827 |
Definition of abbreviations: BMI = body mass index; HTN = hypertension; LVMI = left ventricular mass index.
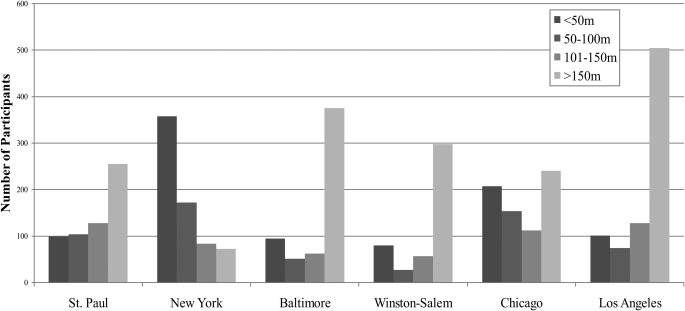
Overall, more than 50% of participants lived within 150 m of a major roadway (Figure 1). Of those participants who lived within 150 m of a major roadway, 86% lived near major arterials rather than highways (data not presented). Distance to a major roadway was strongly patterned by center: for example, 38% of New York participants lived within 50 m of a major roadway compared with only 8% in Winston-Salem, North Carolina. Mean kriged PM2.5 values were also strongly associated with center, with the highest values being observed in Los Angeles and the lowest in St. Paul (Figure 2). As illustrated in Figure 2, the majority of the variability in PM2.5 was between-city.
Figure 1.
Number of participants in each category of residential proximity to major roadway, by Multi-Ethnic Study of Atherosclerosis center. Multi-Ethnic Study of Atherosclerosis and Air Pollution, 2002.
Figure 2.
Box plots of interpolated residential PM2.5 concentration (μg/m3) for each participant, by Multi-Ethnic Study of Atherosclerosis center. Multi-Ethnic Study of Atherosclerosis and Air Pollution, 2002.
On bivariate analysis, distance to roadway was also associated with race, income, and systolic blood pressure. African Americans and Hispanics, and those with lower income and lower systolic blood pressure, were more likely to live near major roadways than others (data not shown). The relationships between proximity to roadways and race and proximity to roadways and systolic blood pressure, but not the relationship between roadway proximity and income, were markedly attenuated by adjustment for center. Before adjustment for center, PM2.5 exposures were strongly associated with nearly every covariate (age, race, systolic blood pressure, smoking and second-hand smoke exposure, income and education, and both outcomes). After adjustment for center, whites and Hispanics, older individuals, smokers, and those with lower education and income had slightly higher PM2.5 exposures than others.
Unadjusted Analysis
In unadjusted analyses, a positive difference of 2.1 g/m2 (95% CI, 0.9–3.4) in LVMI over the first 50 m of roadway proximity compared with living more than 150 m away, but no difference in EF, were observed (Tables 2 and 3). In this unadjusted analysis, the kriged PM2.5 values demonstrated an unexpected inverse relationship with LVMI and a positive relationship with EF such that a positive 10 μg/m3 difference in PM2.5 was associated with a −6.0 g/m2 (95% CI, −7.8 to −4.2) difference in LVMI and a 3.0% (95% CI, 1.5–3.4) difference in EF.
TABLE 2.
DIFFERENCE IN LEFT VENTRICULAR MASS INDEX ASSOCIATED WITH PROXIMITY TO TRAFFIC AND PM2.5 EXPOSURE, MULTI-ETHNIC STUDY OF ATHEROSCLEROSIS AND AIR POLLUTION, 2002
| Model | Analysis | Exposure (m) | Difference in LVMI (g/m2) (95% CI) | P Value | Exposure | Difference in LVMI (g/m2) per 10 μg/m3 PM2.5 | P Value |
|---|---|---|---|---|---|---|---|
| >150 | Referent | ||||||
| 1 | Unadjusted | 101–150 | 1.2 (−0.4, 2.7) | 0.13 | Annual PM2.5 | −6.0 (−7.8, −4.2) | <0.0001 |
| 50–100 | 1.0 (−0.5, 2.5) | 0.19 | |||||
| <50 | 2.1 (0.9, 3.4) | 0.001 | |||||
| 2 | All covariates except center, blood pressure | 101–150 | 1.9 (0.6, 3.2) | 0.003 | Annual PM2.5 | −6.1 (−7.8, −4.4) | <0.0001 |
| 50–100 | 1.6 (0.4, 2.9) | 0.01 | |||||
| <50 | 2.3 (1.2, 3.4) | <0.001 | |||||
| 3 | All covariates except blood pressure | 101–150 | 1.0 (−0.3, 2.2) | 0.14 | Annual PM2.5 | 3.7 (−6.0, 13.4) | 0.46 |
| 50–100 | 0.4 (−1.0, 1.7) | 0.58 | |||||
| <50 | 1.4 (0.2, 2.5) | 0.02 | |||||
| 4 | Full model | 101–150 | 1.0 (−0.3, 2.2) | 0.13 | Annual PM2.5 | 4.6 (−4.7, 13.9) | 0.33 |
| 50–100 | 0.5 (−0.8, 1.7) | 0.48 | |||||
| <50 | 1.4 (0.3, 2.5) | 0.01 | |||||
| 5 | Full model + center/race interaction | 101–150 | 1.0 (−0.3,2.2) | 0.13 | Annual PM2.5 | 3.8 (−6.1, 13.7) | 0.45 |
| 50–100 | 0.5 (−0.8, 1.7) | 0.47 | |||||
| <50 | 1.4 (0.3, 2.5) | 0.01 |
Full model includes age, sex, race/ethnicity, household income, highest educational attainment level, antihypertensive medication use, low-density lipoprotein and high-density lipoprotein cholesterol, lipid-lowering medication use, physical activity, weekly alcohol consumption, current smoking and pack-year history of smoking, hours per week of secondhand smoke exposure, diabetes status by fasting blood glucose criteria, use of diabetes medications, center, and systolic and diastolic blood pressure.
Definition of abbreviations: LVMI = left ventricular mass index; PM2.5 = particulate matter less than 2.5 μm in aerodynamic diameter.
TABLE 3.
DIFFERENCE IN LEFT VENTRICULAR EJECTION FRACTION ASSOCIATED WITH PROXIMITY TO TRAFFIC AND PM2.5 EXPOSURES, MULTI-ETHNIC STUDY OF ATHEROSCLEROSIS AND AIR POLLUTION, 2002
| Model | Analysis | Exposure (m) | Difference in LVEF (%) (95% CI) | P Value | Exposure | Difference in LVEF (%) per 10 ug/m3 PM2.5 | P Value |
|---|---|---|---|---|---|---|---|
| >150 | Referent | ||||||
| 1 | Unadjusted | 101–150 | −0.5 (−1.2, 0.2) | 0.17 | Annual PM2.5 | 3.0 (2.2, 3.8) | <0.0001 |
| 50–100 | −0.3 (−0.9, 0.4) | 0.45 | |||||
| <50 | −0.1 (−0.6, 0.5) | 0.79 | |||||
| 2 | All covariates except center, blood pressure | 101–150 | −0.5 (−1.2, 0.1) | 0.10 | Annual PM2.5 | 1.4 (0.5, 2.2) | 0.001 |
| 50–100 | −0.3 (−0.9, 0.4) | 0.40 | |||||
| <50 | 0.03 (−0.5, 0.6) | 0.92 | |||||
| 3 | All covariates except blood pressure | 101–150 | −0.5 (−1.2, 0.1) | 0.10 | Annual PM2.5 | −1.1 (−5.8, 3.7) | 0.66 |
| 50–100 | −0.4 (−1.0, 0.3) | 0.23 | |||||
| <50 | −0.1 (−0.6, 0.5) | 0.84 | |||||
| 4 | Full model | 101–150 | −0.49 (−1.12, 0.1) | 0.12 | Annual PM2.5 | −1.3 (−6.0, 3.5) | 0.60 |
| 50–100 | −0.37 (−1.02, 0.3) | 0.25 | |||||
| <50 | −0.01 (−0.58, 0.6) | 0.97 | |||||
| 5 | Full model + center/race interaction | 101–150 | −0.5 (−1.2, 0.1) | 0.10 | Annual PM2.5 | −3.0 (−8.0, 2.0) | 0.24 |
| 50–100 | −0.4 (−1.1, 0.2) | 0.22 | |||||
| <50 | −0.1 (−0.6, 0.5) | 0.83 |
Full model includes age, sex, race/ethnicity, household income, highest educational attainment level, antihypertensive medication use, low-density lipoprotein and high-density lipoprotein cholesterol, lipid-lowering medication use, physical activity, weekly alcohol consumption, current smoking and pack-year history of smoking, hours per week of secondhand smoke exposure, diabetes status by fasting blood glucose criteria, use of diabetes medications, center, and systolic and diastolic blood pressure.
Definition of abbreviations: LVEF = left ventricular ejection fraction; PM2.5 = particulate matter less than 2.5 μm in aerodynamic diameter.
Adjusted Models
In our fully adjusted models, we observed a relationship between close proximity (within 50 m) to a major roadway compared with living more than 150 m away for LVMI but not EF (Tables 2 and 3). Living within 50 m of a major roadway was associated with a 1.4 g/m2 higher (95% CI, 0.3–2.5) LVMI compared with living more than 150 m away. In addition, there was some evidence (P = 0.02) of a graded association of proximity on LVMI across the four categories of distance to major roadways, modeled as a single ordinal term (using covariates in model 4).
There was no evidence of an association between PM2.5 and LVMI or EF in the fully adjusted model. Because most of the variability in PM2.5 relates to differences between cities (Figure 2), adjustment for city caused a large reduction in the amount of variability in the PM2.5 estimates, and consequently also in the precision of the PM2.5 association estimates. Each 10 μg/m3 higher level of PM2.5 was associated with a 4.6 g/m2 (95% CI, −4.7 to 13.9) higher LVMI and a −1.3% (95% CI, −6.0 to 3.5) lower EF.
Effect Modification Analyses
The observed association between close proximity to major roadways (<50 m compared with >150 m) and LVMI was examined for effect modification by important risk factors and demographics: age, race, center, smoking status, diabetes, sex, body mass index categories, systolic blood pressure, and hypertensive medication therapy. Although evidence of overall effect modification was not observed (multiple partial F test), several modifiers showed some differences in the associations. Associations between close proximity to roadways and LVMI were more pronounced among men (2.33 g/m2; 95% CI, 0.8–3.9) than women (0.6 g/m2; 95% CI, −0.9 to 2.0; P for interaction = 0.09), and in New York (4.3 g/m2; 95% CI, 1.1–7.5), St. Paul (3.6 g/m2; 95% CI, 0.6–6.6), and Baltimore (1.9 g/m2; 95% CI, −1.0 to 4.8) compared with Chicago (0.5 g/m2; 95% CI, −1.9 to 2.9), Winston-Salem (−0.6 g/m2; 95% CI, −3.7 to 2.6), and Los Angeles (−0.9 g/m2; 95% CI, −3.6 to 1.9; P for interaction = 0.08).
There was no evidence of effect modification for participants with hypertension (1.7 g/m2; 95% CI, 0–3.3) compared with those without hypertension (1.2 g/m2; 95% CI, −0.2 to 2.6) (P for interaction = 0.69) or for those on blood pressure medication (1.3 g/m2; 95% CI, −0.5 to 3.1) compared with those not on blood pressure medication (1.4 g/m2; 95% CI, 0.1–2.8) (P for interaction = 0.89).
Sensitivity Analyses
As described above, adjustment for center had the greatest impact on estimates of the association between both outcomes and both exposures (Tables 2 and 3, compare models 2 and 3). Examining several models with restricted sets of covariates demonstrated that including all other confounders except blood pressure had minimal impact (compare models 1 and 2), with even small increases in estimates of the association between proximity to major roadways and LVMI after adjustment. Additional adjustment for blood pressure (compare models 3 and 4) also slightly increased estimates of association. Further adjusting for interactions between center and race (compare models 4 and 5), interactions between income and center, and neighborhood-level socioeconomic status (data not shown) did not significantly alter the results.
Adjustment for individual classes and number of hypertension medications using indicator variables for ACE inhibitors, angiotensin-2 receptor blockers, diuretics by class, calcium channel blockers, β blockers, vasodilators, and α blockers marginally increased the magnitude and precision of the overall findings regarding the relationship between traffic proximity and LVMI.
Restricting the analysis to participants with at least 1 year residence in their present neighborhood (91.4% of participants), restricting the analysis to participants with at least minimally adequate MRI image quality (99.6% of participants), and using the nearest monitor techniques described above rather than kriging to estimate average PM2.5 exposure for a subset of participants had no substantial impact on the result of this analysis. Using the alternate exposure method developed for the Netherlands Cohort Study on Diet and Cancer, living either within 100 m of a highway or within 50 m of a major arterial was associated with a 0.9 g/m2 difference in LVMI (95% CI, −0.1 to 1.9). We also reanalyzed the data with a more limited set of covariates including all participants with cardiac MRI data who had been excluded because of missing data, and obtained results that did not differ meaningfully from the findings presented here.
Although not the primary goal of this analysis, we also explored the fully-adjusted relationship between proximity to major roadways and blood pressure (models based on model 3 with the additional covariate LVMI) to understand whether the observed association between proximity and LVMI might be mediated by an impact of roadway proximity on blood pressure. In this exploratory analysis, we found no association between close (<50 m) proximity to major roadways and systolic blood pressure (−0.6 mm Hg associated with close proximity; 95% CI, −2.1 to 0.9; P = 0.4) or diastolic blood pressure (0.3 mm Hg; 95% CI, −0.5 to 1.1; P = 0.5).
DISCUSSION
This study observed an association between residential proximity to major roadways and higher LVMI but no difference in EF in a large, multiethnic, geographically dispersed population.
Although PM2.5 and traffic-related pollutants have previously been associated with cardiovascular disease morbidity and mortality, and increased susceptibility to cardiovascular morbidity and mortality has been shown in those with existing HF, there are few data regarding associations with HF itself. In fact, this is the first epidemiologic study to directly examine the relationship between air pollution exposure and these measures of LV mass and function. Such an analysis was possible because we had access to cardiac MRI, which provides highly precise, reproducible measurements of LV mass and EF (18).
LVMI and EF both provide insight as to preclinical cardiac function with a special focus on HF disease. Increased LV mass is strongly related to HF severity in individuals with HF, and increased LV mass in individuals without HF may predict the development of both systolic and diastolic HF. As described above, increased LV mass in the Cardiovascular Health Study was an independent risk factor for the longitudinal development of depressed EF (a measure of systolic dysfunction) (9). Similarly, a subgroup of MESA participants who underwent tagged cardiac MRI demonstrated worsening impairment of myocardial relaxation in individuals with higher LV mass (19), thus indicating that MRI characterization of LV mass is a marker of diastolic dysfunction. LV mass is also an important predictor of adverse cardiac outcomes other than HF, including sudden cardiac death and arrhythmia. EF itself is a direct measure of cardiac systolic function, one which predicts HF disease severity and clinical outcomes.
In this investigation, we observed a 1.4 g/m2 difference in LVMI associated with living in close (<50 m) proximity to a major roadway. The size of this difference in LVMI corresponds to the association of LVMI with a positive 5.6 mm Hg difference in SBP in this cohort. Provided that these results are confirmed by future investigations, and given that a relatively large number of individuals in this cohort live near major roadways, the public health implications of an association of this magnitude may be substantial (20).
The apparent lack of an association between roadway proximity and EF in this population may relate to the relative insensitivity of global EF to detect early, preclinical changes compared with LV mass (21).
Although this analysis has advantages over previous approaches, our exposure assessment remains necessarily limited at present. The fine-scale indicators of proximity to major roadway are somewhat crude measures of traffic-related air pollution exposure, and they do not take into account vertical distance from roadways, although they have gained increasing use in air pollution epidemiology studies (13, 22, 23) for a number of reasons. They possess much greater ability to capture micro-scale, within-city variability that cannot be captured using traditional modeling approaches, and they provide useful indicators of long-term traffic exposure. Given the very strong relationships between center and both exposures and outcomes, and the large impact of center adjustment on the PM2.5 results compared with other confounders (Tables 2 and 3), the unexpected unadjusted relationship between PM2.5 and both outcomes is likely due to strong confounding by center. Because of the large amount of confounding related to center in this study, and the relatively small amount of within-city spatial variability in PM2.5 left after adjusting for center, such exposure metrics as these proximity measures provide much better approximations of the variability of within-city air pollution concentrations.
There was somewhat less within-city variability in PM2.5 exposures in this study compared with previous studies (14, 24). This study was limited to participants in the original MESA cohort with cardiac MRI data at the initial examination. Future studies of pollutant exposures in MESA will be able to take advantage of improved classification of exposures through a dense network of monitors, incorporation of further geographic characteristics, and modeling methods that use the full spatiotemporal extent of the data. The recruitment of 257 additional participants, based on geographic location and known PM2.5 levels, will also help to increase within-city variability in PM2.5 exposures in future MESA studies.
The geocoding methods used in this study have been commonly used in studies implicating traffic-related air pollution because of the advantages described above (13, 23, 22). Accuracy of this method of geocoding and measuring distances depends to a large extent on the underlying accuracy of the road network used for these procedures, as well as the availability of accurate address information (25). We chose a geocoding database (Tele Atlas Dynamap/2000) with a very high level of positional accuracy and performed extensive manual address cleaning and checking prior to exposure assignment. Despite the general high level of accuracy of these methods, they are known to involve varying degrees of measurement error (26). It is possible that such measurement error may cause an underestimation of the observed health effects of exposures (27, 28).
In general, this study benefited from excellent data on potential confounding factors. Although our final model included a simple indicator of hypertension medication usage, additional adjustment for individual classes and number of hypertension medications did not alter our overall findings. Specific data on dosages of medication used or intensity of therapy was not available, although this limitation appears unlikely to have altered the findings, especially given the small increases in the magnitude and precision of the associations seen with additional adjustment for medication use.
Small differences in the associations between proximity to major roadways and LVMI between cities may be related to differences in the amount of traffic represented by road type from city to city (differential exposure misclassification), meteorological effects on the distribution of near-roadway air pollution that differ by city, or other unmeasured characteristics of city. Subjects' activities and time spent away from the home may also contribute to misclassification of exposure in this analysis but are unlikely to have introduced a bias that would explain the findings. Future work in MESA, including an intensive monitoring program, the development of a full statistical model that includes integration of point source data (including detailed traffic data), meteorological data, and time patterns in pollutant concentrations, will be able to explore the effect of further improved exposure classification on these findings.
The somewhat larger association between roadway proximity and LVMI in men than in women bears further investigation. Previous studies of air pollution and cardiovascular disease have demonstrated conflicting results with respect to the susceptibilities of men and women, although LVMI has not been investigated as an outcome, and (as in this study) sex differences could often have been explained by chance (23, 24). However, women do have an increased prevalence of HF without systolic dysfunction compared with men, suggesting that the most common pathophysiology of HF (and potentially, underlying susceptibilities) may differ by sex (29).
It is difficult to characterize, based on this analysis alone, which characteristics of proximity to roadways are responsible for the observed association. The pattern of association (over the nearest 150 m) very closely mirrors the approximate distribution of ultrafine particulate matter and oxides of nitrogen near major roadways in the studies that have examined this distribution, but other pollutants and even non-air pollutant (e.g., traffic noise) exposures may follow similar distributions. Noise in particular, related to both traffic and aircraft proximity, has been associated with elevated blood pressure and hypertension in a number of epidemiologic studies (30–32). Although the relationship between roadway proximity and LVMI in this analysis appeared to be independent of blood pressure (as described below), lack of information on noise exposure makes it difficult to assess the portion of this association attributable to specific attributes of proximity to roadways. Current work is underway in MESA and elsewhere to begin to disentangle these other important traffic-related exposures.
Social or economic characteristics may be associated with residential proximity to major roadways. Adjustment for individual and neighborhood-level socioeconomic factors, including interactions between center and race and center and income, had little influence on these findings, and adjustment for these factors even marginally increased the magnitude of the observed associations.
Because this was a cross-sectional study, it is difficult to make causal inferences about these associations. Plans for longitudinal studies of these relationships, including follow-up cardiac MRI examinations in a subset of MESA participants, are underway. Although we used a single residential address to determine exposures in this study, the residential stability of the MESA population (with more than 90% of participants living more than 1 yr at their home address), and the fact that our results did not change when restricted to participants living more than 1 year at their address, suggest that this limitation had minimal impact on the overall findings.
Epidemiologic and experimental evidence suggest that air pollution may be associated with elevated blood pressure (33–35) and atherosclerotic cardiovascular disease (23, 24), two of the most critical risk factors for the development of increasing LV mass and HF (36). Although in this cross-sectional study there was no association between roadway proximity and blood pressure, and adjustment for measurement of blood pressure at a single examination had little effect on the associations, the relationship between LV mass and traffic proximity observed here might relate to longer-term (rather than cross-sectional) alterations in blood pressure or atherosclerotic changes (37). Other mechanisms that have been observed in experimental and epidemiologic studies, including changes in vascular tone (38–40) or autonomic function (41), could be responsible for these effects.
Conclusion
This large epidemiologic study suggests that residential proximity to major roadways leads to important increases in LVMI in a pattern consistent with an effect of traffic-related air pollution. Evidence to date regarding the potential mechanisms by which air pollution may affect LV mass implicates alterations in vascular tone, inflammation, oxidative stress, and blood pressure. Although the specific mechanism for the association between traffic proximity and LV mass is not known, long-term alterations in vascular tone could plausibly explain this association. These results, suggesting chronic end-organ effects of residing close to traffic, provide an important insight but demand complementary experimental studies and further epidemiologic investigation with better classification of exposure, using time-activity patterns of individuals, more precise traffic measures, and direct air pollution monitoring to clarify this association.
The MESA study is supported by National Heart, Lung, and Blood Institute contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169. MESA Air is supported by U.S. Environmental Protection Agency STAR Grant RD831697. J.D.K. was supported by National Institute of Environmental Health Sciences grant K24ES013195.
Originally Published in Press as DOI: 10.1164/rccm.200808-1344OC on January 22, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006;295:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among Medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol 2005;161:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Ippoliti D, Forastiere F, Ancona C, Agabiti N, Fusco D, Michelozzi P, Perucci CA. Air pollution and myocardial infarction in Rome: a case-crossover analysis. Epidemiology 2003;14:528–535. [DOI] [PubMed] [Google Scholar]
- 4.Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology 2001;12:355–357. [DOI] [PubMed] [Google Scholar]
- 5.Pope CA, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004;109:71–77. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics−2009 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:480–486. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KKL, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397–1402. [DOI] [PubMed] [Google Scholar]
- 8.Bleumink GS, Knetsch AM, Sturkenboom MCJM, Straus SMJM, Hofman A, Deckers JW, Witteman JCM, Stricker BHC. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure the Rotterdam Study. Eur Heart J 2004;25:1614–1619. [DOI] [PubMed] [Google Scholar]
- 9.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol 2004;43:2207–2215. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. Journal of the Air & Waste Management Association 2002;52:1032–42. [DOI] [PubMed] [Google Scholar]
- 12.Hoek G, Meliefste K, Cyrys J, Lewne M, Bellander T, Brauer M, Fischer P, Gehring U, Heinrich J, van Vliet P, et al. Spatial variability of fine particle concentrations in three European areas. Atmos Environ 2002;36:4077–4088. [Google Scholar]
- 13.Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet 2002;360:1203–1209. [DOI] [PubMed] [Google Scholar]
- 14.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med 2007;356:447–458. [DOI] [PubMed] [Google Scholar]
- 15.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JAC, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186:S357–S365. [DOI] [PubMed] [Google Scholar]
- 16.Kronmal RA. Spurious correlation and the fallacy of the ratio standard revisited. J R Stat Soc Ser A Stat Soc 1993;156:379–392. [Google Scholar]
- 17.Diez Roux AV, Auchincloss AH, Franklin TG, Raghunathan T, Barr RG, Kaufman J, Astor B, Keeler J. Long-term exposure to ambient particulate matter and prevalence of subclinical atherosclerosis in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2008;167:667–675. [DOI] [PubMed] [Google Scholar]
- 18.Tseng WY. Magnetic resonance imaging assessment of left ventricular function and wall motion. J Formos Med Assoc 2000;99:593–602. [PubMed] [Google Scholar]
- 19.Edvardsen T, Rosen BD, Pan L, Jerosch-Herold M, Lai S, Hundley WG, Sinha S, Kronmal RA, Bluemke DA, Lima JAC. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging–the Multi-Ethnic Study of Atherosclerosis. Am Heart J 2006;151:109–114. [DOI] [PubMed] [Google Scholar]
- 20.Atilla K, Vasan RS. Prehypertension and risk of cardiovascular disease. Expert Rev Cardiovasc Ther 2006;4:111–117. [DOI] [PubMed] [Google Scholar]
- 21.Nishikage T, Nakai H, Lang RM, Takeuchi M. Subclinical left ventricular longitudinal systolic dysfunction in hypertension with no evidence of heart failure. Circ J 2008;72:189–194. [DOI] [PubMed] [Google Scholar]
- 22.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, Lurmann F, Avol E, Kunzli N, Jerrett M, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet 2007;369:571–577. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann B, Moebus S, Möhlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation 2007;116:489–496. [DOI] [PubMed] [Google Scholar]
- 24.Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, Thomas D, Peters J, Hodis HN. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect 2005;113:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longley P. Geographical information systems and science. Chichester: Wiley; 2005.
- 26.Zandbergen PA, Green JW. Error and bias in determining exposure potential of children at school locations using proximity-based GIS techniques. Environ Health Perspect 2007;115:1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Roosbroeck S, Li R, Hoek G, Lebret E, Brunekreef B, Spiegelman D. Traffic-related outdoor air pollution and respiratory symptoms in children: the impact of adjustment for exposure measurement error. Epidemiology 2008;19:409–416. [DOI] [PubMed] [Google Scholar]
- 28.Whitsel EA, Quibrera PM, Smith RL, Catellier DJ, Liao D, Henley AC, Heiss G. Accuracy of commercial geocoding: assessment and implications. Epidemiol Perspect Innov 2006;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol 2003;41:217–223. [DOI] [PubMed] [Google Scholar]
- 30.Babisch W. Transportation noise and cardiovascular risk: updated review and synthesis of epidemiological studies indicate that the evidence has increased. Noise Health 2006;8:1–29. [DOI] [PubMed]
- 31.Eriksson C, Rosenlund M, Pershagen G, Hilding A, Ostenson C, Bluhm G. Aircraft noise and incidence of hypertension. Epidemiology 2007;18:716–721. [DOI] [PubMed] [Google Scholar]
- 32.Jarup L, Babisch W, Houthuijs D, Pershagen G, Katsouyanni K, Cadum E, Dudley M, Savigny P, Seiffert I, Swart W, et al. Hypertension and exposure to noise near airports: the HYENA Study. Environ Health Perspect 2008;116:329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation 2004;110:2184–2189. [DOI] [PubMed] [Google Scholar]
- 34.Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health 2001;91:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect 2005;113:1052–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med 2003;138:10–16. [DOI] [PubMed] [Google Scholar]
- 37.Auchincloss AH, Roux AVD, Dvonch JT, Brown PL, Barr RG, Daviglus ML, Goff DC, Kaufman JD, O'Neill MS. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect 2008;116:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 2002;105:1534–1536. [DOI] [PubMed] [Google Scholar]
- 39.Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, Carlsten C, Wilkinson CW, Gill EA, Kaufman JD. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect 2008;116:937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dales R, Liu L, Szyszkowicz M, Dalipaj M, Willey J, Kulka R, Ruddy TD. Particulate air pollution and vascular reactivity: the bus stop study. Int Arch Occup Environ Health 2007;81:159–164. [DOI] [PubMed] [Google Scholar]
- 41.Liao D, Duan Y, Whitsel EA, Zheng Z, Heiss G, Chinchilli VM, Lin H. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol 2004;159:768–777. [DOI] [PubMed] [Google Scholar]