Abstract
Rationale: Portopulmonary hypertension (PPHTN) occurs in 6% of liver transplant candidates. The pathogenesis of this complication of portal hypertension is poorly understood.
Objectives: To identify genetic risk factors for PPHTN in patients with advanced liver disease.
Methods: We performed a multicenter case-control study of patients with portal hypertension. Cases had a mean pulmonary artery pressure >25 mm Hg, pulmonary vascular resistance >240 dynes·s−1·cm−5, and pulmonary capillary wedge pressure ≤15 mm Hg. Controls had a right ventricular systolic pressure < 40 mm Hg (if estimated) and normal right-sided cardiac morphology by transthoracic echocardiography. We genotyped 1,079 common single nucleotide polymorphisms (SNPs) in 93 candidate genes in each patient.
Measurements and Main Results: The study sample included 31 cases and 104 controls. Twenty-nine SNPs in 15 candidate genes were associated with the risk of PPHTN (P < 0.05). Multiple SNPs in the genes coding for estrogen receptor 1, aromatase, phosphodiesterase 5, angiopoietin 1, and calcium binding protein A4 were associated with the risk of PPHTN. The biological relevance of one of the aromatase SNPs was supported by an association with plasma estradiol levels.
Conclusions: Genetic variation in estrogen signaling and cell growth regulators is associated with the risk of PPHTN. These biologic pathways may elucidate the mechanism for the development of PPHTN in certain patients with severe liver disease.
Keywords: genetic polymorphism; portal hypertension; hypertension, pulmonary
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
It is believed that inherited factors contribute to the development of certain forms of pulmonary arterial hypertension, such as that associated with portal hypertension.
What This Study Adds to the Field
Genetic polymorphisms in estrogen and other pathways are associated with the risk of portopulmonary hypertension in patients with advanced liver disease.
Pulmonary arterial hypertension (PAH) is characterized by elevated pulmonary artery pressure and pulmonary vascular resistance, right heart failure, exercise limitation, and an increased risk of death. Histopathologic examination reveals intimal proliferation, medial hypertrophy, and adventitial fibrosis in the small muscular pulmonary arteries. Plexiform lesions and in situ thrombosis are also seen. Most commonly idiopathic, PAH may also be associated with portal hypertension, termed portopulmonary hypertension (PPHTN). McDonnell and colleagues showed a prevalence of histopathologic changes of PAH of 0.61% in autopsies of patients with cirrhosis, and PPHTN was the third most common form of PAH in a population-based epidemiologic study in France (1, 2). Recent cohort studies showed that the prevalence of PPHTN in patients presenting for liver transplant evaluation is between 5 and 6% (3–5). Patients with PPHTN have an increased risk of death, even with specific PAH treatment (4, 6–8). In many cases, PPHTN greatly complicates or precludes liver transplantation, significantly affecting the course of hepatic failure in these patients (6,9,10).
The etiology of PAH in patients with portal hypertension (characterized by systemic vasodilatation) is unclear. We have shown that female sex and type of liver disease are associated with the risk of PPHTN (11). Although germline mutations in the gene that codes for bone morphogenetic protein receptor type II (BMPR2) have been associated with idiopathic and familial forms of PAH, they have not been found in patients with PPHTN (12). Genetic variation in the serotonin transporter (SERT) has been associated with the risk of PAH in some studies (13) but not in others (14, 15). We did not find an association between genetic variation at SERT loci and the risk of PPHTN (16).
We therefore hypothesized that variation in genes other than BMPR2 and SERT contribute to the risk of developing PPHTN. We performed a high-throughput candidate gene study in an attempt to identify common genetic variation associated with the risk of PPHTN in a group of patients undergoing liver transplantation evaluation. This work has been previously published in abstract form (17).
METHODS
Study Cohort and Study Sample
The Pulmonary Vascular Complications of Liver Disease (PVCLD) Study enrolled a cohort of 536 patients evaluated for liver transplantation or pulmonary hypertension at seven centers in the United States between 2003 and 2006. The only inclusion criterion was the presence of chronic portal hypertension with or without intrinsic liver disease. We excluded patients with evidence of active infection, recent (<2 wk) gastrointestinal bleeding, or who had undergone liver or lung transplantation. The institutional review boards at each of the participating centers approved this study, and informed consent was obtained.
We performed a case-control study. The study sample included newly referred patients who were evaluated with transthoracic echocardiography (routinely performed for pretransplant evaluation) during the study period. “Prevalent” patients who met the case definition (see below) were also included. We excluded patients with pulmonary function testing showing a significant obstructive or restrictive ventilatory defect (see online supplement). We also excluded patients with HIV infection or the presence of more than moderate aortic or mitral valvular disease or significant left ventricular dysfunction by transthoracic echocardiography.
Case and Control Definitions
Cases with PPHTN met the following criteria at initial evaluation: (1) mean pulmonary artery pressure > 25 mm Hg, pulmonary capillary wedge pressure (or left ventricular end-diastolic pressure) ≤ 15 mm Hg, and pulmonary vascular resistance >240 dynes·s−1·cm−5 measured by right heart catheterization; and (2) no other etiology for pulmonary hypertension. Controls met the following echocardiographic criteria at entry into the cohort: (1) right ventricular systolic pressure < 40 mm Hg (if estimable), and (2) absence of right atrial or ventricular dilation, hypertrophy, or dysfunction. Prevalent cases who had previously undergone evaluation and were subsequently being treated were also included.
Clinical Variables and Blood Sampling
Data were collected from subjects and the medical record. The Model for End-stage Liver Disease (MELD) score was calculated (18). Phlebotomy was performed and blood was collected into EDTA-containing tubes. Plasma and buffy coat layers were stored at −80°C.
Candidate Genes and Single Nucleotide Polymorphism Selection
Ninety-three genes affecting vascular function were selected by the investigators (Table 1). For this study, 1,079 single nucleotide polymorphisms (SNPs) in the 93 candidate genes were genotyped (see Table E1 in the online supplement). We genotyped an additional set of 60 SNPs (null loci) from a validated list of Ancestry Informative Markers (19) to detect potential population stratification. (See online supplement for details of gene and SNP selection.)
TABLE 1.
CANDIDATE GENES (GENE ONTOLOGY ANNOTATION)
| Pathway | Gene | RefSeq | Chr | SNPs |
|---|---|---|---|---|
| Control of bloodcirculation GO:0008015 | Angiotensin I converting enzyme (ACE) | NM_152831 | 17q23 | 15 |
| Elastin (ELN) | NM_000501 | 7q11 | 5 | |
| Endothelin 1 (EDN1) | NM_001955 | 6p24 | 7 | |
| Endothelin converting enzyme 1 (ECE1) | NM_001397 | 1p36 | 10 | |
| Endothelin receptor, nonselective type (EDNRB) | NM_000115 | 13q22 | 13 | |
| Endothelin receptor, type A (EDNRA) | NM_001957 | 4q31 | 11 | |
| Heme oxygenase 1 (HMOX1) | NM_002133 | 22q13 | 8 | |
| Natriuretic peptide precursor A (NPPA) | NM_006172 | 1p36 | 13* | |
| Natriuretic peptide precursor B (NPPB) | NM_002521 | 1p36 | 13* | |
| Nitric oxide synthase 2 (NOS2A) | NM_000625 | 17q11 | 15 | |
| Phosphodiesterase 5 (PDE5A) | NM_001083 | 4q26 | 9 | |
| Potassium channel, voltage-gated, shaker, member 5 (KCNA5) | NM_002234 | 12p13 | 9 | |
| Rho-associated protein kinase 2 (ROCK2) | NM_004850 | 2p24 | 15 | |
| Transient receptor potential cation channel, subfamily C, 6 (TRPC6) | NM_004621 | 11q21 | 18 | |
| Cell growth apoptosis GO:0008283 GO:0006915 | Activin A receptor, type II-like kinase (ACVRL1) | NM_000020 | 12q11 | 6 |
| Apolipoprotein E (APOE) | NM_000041 | 19q13 | 4 | |
| BCL2-associated X protein (BAX) | NM_138764 | 19q13 | 6 | |
| Bone morphogenetic protein receptor type 1a (BMPR1A) | NM_004329 | 10q22 | 20 | |
| Bone morphogenetic protein receptor type 2 (BMPR2) | NM_001204 | 2q33 | 12 | |
| Caveolin 1 (CAV1) | NM_001753 | 7q31 | 20* | |
| Caveolin 2 (CAV2) | NM_001233 | 7q31 | 20* | |
| Caveolin 3 (CAV3) | NM_033337 | 3p25 | 19 | |
| CD14 molecule (CD14) | NM_000591 | 5q22 | 3 | |
| Cyclin-dependent kinase inhibitor 2A (CDKN2A) | NM_000077 | 9p21 | 13 | |
| Growth differentiation factor 2 (GDF2) | NM_016204 | 10q11 | 5 | |
| Homolog of Drosophila mothers against dpp 3 (SMAD3) | NM_005902 | 15q21 | 34 | |
| Homolog of Drosophila mothers against dpp 4 (SMAD4) | NM_005359 | 18q21 | 5 | |
| Nitric oxide synthase 3 (NOS3) | NM_000603 | 7q36 | 10 | |
| Nuclear factor kappa B p100 subunit (NFKB2) | NM_001077493 | 10q24 | 5 | |
| Nuclear factor kappa B p105 subunit (NFKB1) | NM_003998 | 4q23 | 13 | |
| Nuclear factor kappa B p65 subunit (RELA) | NM_021975 | 11q13 | 4 | |
| Prostaglandin I2 synthase (PTGIS) | NM_000961 | 20q13 | 13 | |
| Protein kinase C, alpha (PRKCA) | NM_002737 | 17q22 | 33 | |
| Protein kinase C, beta 1 (PRKCB1) | NM_002738 | 16p11 | 13 | |
| Protein kinase C, gamma (PRKCG) | NM_002739 | 19q13 | 5 | |
| Transforming growth factor, Beta-1 (TGFB1) | NM_000660 | 19q13 | 5 | |
| V-AKT murine thymoma viral oncogene homolog 1 (AKT1) | NM_005163 | 14q32 | 7 | |
| Blood vessel growth anddevelopment GO:0001568 | Angiopoietin 1 (ANGPT1) | NM_001146 | 8q22 | 37 |
| Calcium-binding protein A4 (S100A4) | NM_019554 | 1q21 | 6 | |
| Endoglin (ENG) | NM_000118 | 9q34 | 15 | |
| Hypoxia-inducible factor 1, alpha subunit (HIF1A) | NM_001530 | 14q21 | 8 | |
| Plasminogen (PLG) | NM_000301 | 6q26 | 21 | |
| Runt-related transcription factor 1 (RUNX1) | NM_001754 | 21q22 | 58 | |
| Thrombospondin-1 (THBS1) | NM_003246 | 15q15 | 5 | |
| Tyrosine kinase with Ig and EGF factor homology domains (TIE1) | NM_005424 | 1p34 | 8 | |
| Vascular endothelial growth factor (VEGF) | NM_00125366 | 6p12 | 7 | |
| Inflammation GO:0006954 | Complement component 4A (C4A) | NM_007293 | 6p21 | 4 |
| C-reactive protein (CRP) | NM_000567 | 1q21 | 8 | |
| Cytochrome b-245, NADPH oxidase 2, NOX2 (CYBB) | NM_000397 | Xp21 | 6 | |
| Lipopolysaccharide binding protein (LBP) | NM_004139 | 20q11 | 7 | |
| Tumor necrosis factor (TNF) | NM_000594 | 6p21 | 5 | |
| Oxidation reduction GO:0006979 | Dual oxidase 1 (DUOX1) | NM_017434 | 15q15 | 15* |
| Dual oxidase 2 (DUOX2) | NM_014080 | 15q15 | 15* | |
| NADPH oxidase 1 (NOX1) | NM_007052 | Xq22 | 7 | |
| NADPH oxidase 4 (NOX4) | NM_016931 | 11q14 | 19 | |
| Superoxide dismutase 1, soluble (SOD1) | NM_000454 | 21q22 | 3 | |
| Superoxide dismutase 2, mitochondrial (SOD2) | NM_00636 | 6q25 | 3 | |
| Xanthine dehydrogenase (XDH) | NM_00379 | 2p23 | 24 | |
| Tissue development GO:0009888 | Homolog of Drosophila mothers against dpp 2 (SMAD2) | NM_005901 | 18q21 | 10 |
| Ikaros (IKZF1) | NM_006060 | 7p12 | 7 | |
| Peroxisome proliferator activated receptor, gamma (PPARG) | NM_005037 | 3p25 | 13 | |
| Recombination signal-binding protein 1 for J-kappa (RBPSUH) | NM_005349 | 4p15 | 13 | |
| Steroid hormone GO:0008202 GO:0030518 | Aromatase (CYP19A1) | NM_000103 | 15q21 | 24 |
| Estrogen receptor 1 (ESR1) | NM_000125 | 6q25 | 36 | |
| Estrogen receptor 2 (ESR2) | NM_001437 | 14q24 | 14 | |
| Farnesoid X receptor (NR1H4) | NM_005123 | 12q | 7 | |
| Pregnane X receptor (NR1I2) | NM_003889 | 3q13 | 13 | |
| Sex hormone binding globulin (SHBG) | NM_001040 | 17p13 | 6 | |
| Small heterodimer partner (NR0B2) | NM_021969 | 1p36 | 5 | |
| Extracellular matrix structure and regulation GO:0043062 GO:0006508 | Collagen, type XVIII, alpha-1 (COL18A1) | NM_130445 | 21q22 | 29 |
| Elastase 1 (ELA1) | NM_001971 | 12q13 | 8 | |
| Elastase 2 (ELA2) | NM_001972 | 19p13 | 4 | |
| Matrix metalloproteinase 2 (MMP2) | NM_004530 | 16q13 | 11 | |
| Matrix metalloproteinase 3 (MMP3) | NM_002422 | 11q23 | 6 | |
| Matrix metalloproteinase 9 (MMP9) | NM_004994 | 20q11 | 6 | |
| Proteinase inhibitor 3; elafin (PI3) | NM_002638 | 20q12 | 4 | |
| Tenascin C (TNC) | NM_002160 | 9q33 | 16 | |
| Coagulation GO:0050817 | Plasminogen activator inhibitor 1 (SERPINE1) | NM_000602 | 7q21 | 9 |
| Thrombomodulin (THBD) | NM_000361 | 20p11 | 4 | |
| Thromboplastin (HEMB) | NM_000133 | Xq27 | 11 | |
| Von Willebrand factor (VWF) | NM_000552 | 12p13 | 39 | |
| Serotonin GO:0006587 GO:0007210 | Serotonin 2B receptor (HTR2B) | NM_000867 | 2q36 | 8 |
| Tryptophan hydroxylase 1 (TPH1) | NM_004179 | 11p15 | 8 | |
| Tryptophan hydroxylase 2 (TPH2) | NM_173353 | 12q21 | 16 | |
| Na/bile acid transporter GO:0008508 | Solute carrier family 10, member 1 (SLC10A1) | NM_003049.1 | 14q24 | 5 |
| Solute carrier family 10, member 2 (SLC10A2) | NM_000452.1 | 13q33 | 12 | |
| Metabolism GO:0008152 | 5,10-methylenetetrahydrofolate reductase (MTHFR) | NM_005957 | 1p36 | 7 |
| Betaine-homocysteine methyltransferase (BHMT) | NM_001713 | 5q13 | 4 | |
| Cystathionine-beta-synthase (CBS) | NM_000071 | 21q22 | 6 | |
| Peroxisome proliferator activated receptor, alpha (PPARA) | NM_005036 | 22q12 | 9 | |
| Retinoic acid signaling GO:0048384 | Retinoic acid receptor, alpha (RARA) | NM_000964 | 17q21 | 4 |
| Retinoic acid receptor, beta (RARB) | NM_016152 | 3p24 | 29 | |
| Retinoic acid receptor, gamma (RARG) | NM_000966 | 12q13 | 6 |
Definition of abbreviations: Chr = chromosome; RefSeq = Reference Sequence; SNP = single-nucleotide polymorphism.
Indicates adjacent genes that were defined by a single genomic region and tagging SNPs. Thus the number of SNPs indicated refers to the total number of SNPs assayed in the region containing both genes.
Genotyping
Genomic DNA was isolated from peripheral leukocytes using standard procedures (Gentra Puregene; Qiagen, Valencia, CA). SNP genotyping was performed using the GoldenGate Assay (Illumina, Inc., San Diego, CA).
Statistical Analysis
Continuous data were summarized using mean ± standard deviation or median (interquartile range), as appropriate. Categorical variables were summarized using n (%). Unpaired Student t tests, Wilcoxon rank sum tests, chi-square tests, and Fisher exact tests were used, as appropriate.
Hardy-Weinberg equilibrium (HWE) was assessed for genetic alleles using Fisher exact tests in controls. The association of genotype with case/control status was assessed using additive models in multivariate logistic regression and expressed with odds ratios (ORs). We adjusted for sex and autoimmune liver disease (previously associated with case status [11]) in the final multivariate logistic regression models. Because the main goal of this study was hypothesis generation, adjustment for multiple comparisons was not performed. Single locus association analyses were performed using SAS/STAT (SAS Institute, Cary, NC).
For genes in which more than one SNP was associated with PPHTN, we determined haplotype structure and pairwise linkage disequilibrium between SNPs using Haploview 4.0 (20). The presence or absence of population stratification was assessed by comparing allele frequencies of the 60 null loci between cases and controls using chi-square tests (21). Sensitivity analyses assessed the potential impact of racial differences or cryptic subpopulations. P < 0.05 was considered significant for all analyses.
There was 80% power to detect odds ratios of ≥2.4 to 4.0 (or ≤0.25 to 0.42), depending on the minor allele frequency of the SNP (range, 0.45–0.05). Power analysis was performed using QUANTO 1.2 (22).
RESULTS
There were 31 cases and 104 controls. The mean age of the subjects was 52 ± 10 years, and 60 (44%) were female. One hundred and twenty-one (90%) were white and seven (5%) were black. Sixteen (13%) of the white subjects were of Hispanic ethnicity (12% of the study sample). Subjects with PPHTN had a mean right atrial pressure of 10 ± 6 mm Hg (n = 30), a mean pulmonary artery pressure of 50 ± 9 mm Hg, and pulmonary capillary wedge pressure (or left-ventricular end-diastolic pressure) of 10 ± 4 mm Hg. The cardiac output was 5.5 ± 1.8 L/min, the cardiac index was 2.9 ± 0.9 L/min/m2, and the pulmonary vascular resistance was 672 ± 374 dynes·s−1·cm−5.
Bivariate Analyses
Age, race, and severity of liver disease were similar between the groups (Table 2). Female sex and autoimmune hepatitis were associated with an increased risk for PPHTN, as previously reported in this population (11). One case and one control had α-1 antitrypsin deficiency, one control had biliary atresia, one case had sarcoid, and one case had portal vein thrombosis.
TABLE 2.
DEMOGRAPHIC AND CLINICAL DATA FOR CASES AND CONTROLS
| Variable | Cases (N = 31) | Controls (N = 104) | P Value |
|---|---|---|---|
| Age, years | 54 ± 10 | 52 ± 10 | 0.41 |
| Female sex | 20 (65%) | 40 (39%) | 0.01 |
| Race | 0.37 | ||
| White | 29 (94%) | 92 (88%) | |
| Black | 0 | 7 (7%) | |
| Other | 2 (6%) | 5 (5%) | |
| Etiology of portal hypertension | |||
| Alcohol | 14 (45%) | 45 (43%) | 0.85 |
| Hepatitis C | 6 (19%) | 51 (49%) | 0.003 |
| Autoimmune hepatitis | 7 (23%) | 4 (4%) | 0.003 |
| Nonalcoholic fatty liver disease | 1 (3%) | 8 (75%) | 0.68 |
| Hepatitis B | 1 (3%) | 6 (6%) | 1.0 |
| Primary sclerosing cholangitis | 1 (3%) | 9 (9%) | 0.45 |
| Primary biliary cirrhosis | 3 (10%) | 3 (3%) | 0.13 |
| Cryptogenic | 2 (6%) | 8 (8%) | 1.0 |
| Model for End-stage Liver Disease score | 12 ± 4 (N = 29) | 12 ± 5 (N = 103) | 0.77 |
Genetic Analyses
Nine hundred and ninety-three (of the 1,079) SNPs conformed to HWE in controls (P > 0.05) and were included in the analysis. Twenty-nine SNPs in 15 genes were significantly associated with PPHTN after adjustment for sex and liver disease etiology (autoimmune hepatitis) (Table 3). In the gene coding for estrogen receptor 1 (ESR1), 7 of 36 SNPs were associated with either a significantly decreased (OR = 0.39–0.18) or increased (OR = 2.56–2.70) risk of PPHTN. The five protective SNPs included four intronic and one synonymous Exon 4 SNP (rs1801132, P324P). Pairwise linkage disequilibrium analyses demonstrated that these five loci represented a single haplotype block (D′ = 0.71–0.84) (Table E2 and Figure E1). Distal to these protective loci, two SNPs (rs7757956 and rs3020368, D′ = 0.89) were associated with an increased risk of PPHTN. Two promoter SNPs in the gene coding for aromatase (CYP19A1), the rate-limiting enzyme in the conversion of the androgens testosterone and androstenedione to estradiol, were associated with an increased risk of PPHTN (Tables 3 and E2, Figure E2).
TABLE 3.
ADDITIVE MULTIVARIATE LOGISTIC REGRESSION MODELS FOR SINGLE NUCLEOTIDE POLYMORPHISMS AND THE RISK OF PORTOPULMONARY HYPERTENSION (ADJUSTED FOR SEX AND THE PRESENCE OF AUTOIMMUNE HEPATITIS)
| SNP
|
Risk Allele Frequency
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Chr | Gene | Identification | Location | Risk Allele | Cases | Controls | OR (95% CI) | P Value |
| 6 | Estrogen receptor 1 (ESR1) | rs1913474 | Intron 3 | A | 0.13 | 0.26 | 0.33 (0.13–0.85) | 0.022 |
| rs1801132 | P324P | C | 0.13 | 0.27 | 0.39 (0.12–0.76) | 0.011 | ||
| rs3020317 | Intron 4 | G | 0.13 | 0.26 | 0.18 (0.06–0.55) | 0.003 | ||
| rs985694 | Intron 4 | A | 0.11 | 0.23 | 0.19 (0.05–0.67) | 0.010 | ||
| rs932477 | Intron 4 | A | 0.07 | 0.16 | 0.25 (0.08–0.87) | 0.030 | ||
| rs7757956 | Intron 4 | A | 0.24 | 0.15 | 2.70 (1.19–5.88) | 0.017 | ||
| rs3020368 | Intron 5 | A | 0.19 | 0.12 | 2.56 (1.09–5.88) | 0.031 | ||
| 15 | Aromatase (CYP19A1) | rs7175922 | 5′ | A | 0.26 | 0.13 | 2.17 (1.00–4.55) | 0.050 |
| rs1902584 | Intron 1 | A | 0.15 | 0.04 | 3.85 (1.33–11.1) | 0.014 | ||
| 4 | Phosphodiesterase 5 (PDE5A) | rs11731756 | Intron 7 | C | 0.39 | 0.24 | 2.11 (1.05–4.22) | 0.036 |
| rs10034450 | Intron 11 | G | 0.39 | 0.24 | 2.11 (1.05–4.22) | 0.036 | ||
| rs1155576 | Intron 11 | C | 0.40 | 0.25 | 2.11 (1.06–4.20) | 0.033 | ||
| rs3775843 | Intron 16 | G | 0.39 | 0.24 | 2.11 (1.05–4.23) | 0.036 | ||
| 8 | Angiopoietin 1 (ANGPT1) | rs4324901 | Intron 1 | A | 0.26 | 0.38 | 0.48 (0.24–0.97) | 0.041 |
| rs4268102 | Intron 6 | C | 0.34 | 0.19 | 2.30 (1.16–4.56) | 0.017 | ||
| 1 | Calcium binding protein A4 (S100A4) | rs743687 | 3′utr | G | 0.18 | 0.07 | 3.82 (1.53–9.53) | 0.004 |
| rs1810765 | 3′utr | G | 0.19 | 0.11 | 2.38 (1.09–5.20) | 0.030 | ||
| 3 | Retinoic acid receptor, beta (RARB) | rs871963 | Intron 2 | T | 0.63 | 0.46 | 1.92 (1.05–3.54) | 0.035 |
| rs1153584 | Intron 3 | A | 0.35 | 0.49 | 0.44 (0.23–0.88) | 0.019 | ||
| 7 | Caveolin 1 (CAV1) | rs926198 | Intron 2 | G | 0.23 | 0.38 | 0.40 (0.19–0.84) | 0.016 |
| 15 | Homolog of Drosophila mothers against dpp 3 (Smad3) | rs12324036 | Intron 1 | A | 0.34 | 0.48 | 0.50 (0.26–0.95) | 0.035 |
| rs4776881 | Intron 1 | G | 0.34 | 0.48 | 0.49 (0.26–0.95) | 0.035 | ||
| 21 | Runt-related transcription factor 1 (RUNX1) | rs2294163 | Intron 1 | A | 0.29 | 0.17 | 1.96 (1.00–3.85) | 0.049 |
| 4 | Recombining binding protein 1 for J-kappa (RBPSUH) | rs2077777 | Intron 2 | G | 0.11 | 0.05 | 3.47 (1.15–10.45) | 0.027 |
| 2 | Xanthine dehydrogenase (XDH) | rs1896846 | Intron 24 | C | 0.39 | 0.23 | 1.96 (1.06–3.70) | 0.031 |
| 6 | Superoxide dismutase 2 (SOD2) | rs5746136 | 3′utr | A | 0.37 | 0.23 | 2.00 (1.02–4.00) | 0.043 |
| 11 | NADPH oxidase 4 (NOX4) | rs3017887 | 5′utr | A | 0.05 | 0.14 | 3.88 (1.05–14.29) | 0.042 |
| 7 | Plasminogen activator inhibitor 1 (SERPINE1) | rs2227714 | 3′utr | A | 0.06 | 0.02 | 7.14 (1.47–33.33) | 0.014 |
| 17 | Nitric oxide synthase 2A (NOS2A) | rs1137933 | D384D | A | 0.13 | 0.28 | 0.39 (0.17–0.91) | 0.030 |
Definition of abbreviations: Chr = chromosome; CI = confidence interval; OR=odds ratio; SNP=single-nucleotide polymorphism; utr=untranslated region.
Four of nine SNPs genotyped in phosphodiesterase 5 (PDE5A) were in tight linkage disequilibrium (r2 = 0.95–1.00) (Table E2) and all were associated with an increased risk of PPHTN (all OR = 2.11; 95%CI, 1.05–4.22; P = 0.03) (Table 3). Two tightly linked SMAD3 intron 1 SNPs (rs4776881 and rs12324036, r2 > 0.97) were associated with a decreased risk of PPHTN (both OR = 0.50; 95%CI, 0.26–0.95; P = 0.035). Two SNPs in each of three genes—calcium binding protein A4 (S100A4), angiopoietin 1 (ANGPT1), and retinoic acid receptor, β (RARB)—were associated with case status and were not in linkage disequilibrium (Tables 3 and E2, Figure E3). Of note, polymorphisms in BMPR2 or genes coding for bone morphogenetic protein receptor Type Ia (BMPR1A), activin A receptor type II-like 1 (ACVRL1), or endoglin (ENG) were not associated with PPHTN.
There were no significant differences in allele frequencies of the 60 null alleles between cases and controls (all P > 0.05), lessening the chance of population stratification. We assessed for potential confounding by liver disease etiology (other than autoimmune hepatitis). Eight SNPs associated with PPHTN were also independently associated with various liver disease etiologies; in all cases, adjustment for liver disease etiology resulted in either no significant change or strengthening of the association between the SNP and case status (Table E3).
There were no significant differences in the results from the main analyses and results of analyses performed in females only (n = 60), self-identified whites (n = 121), and subjects with white genetic ancestry (n = 124) (data not shown), indicating that neither sex nor race (nor genetic ancestry-based) differences accounted for our results.
Plasma Estradiol
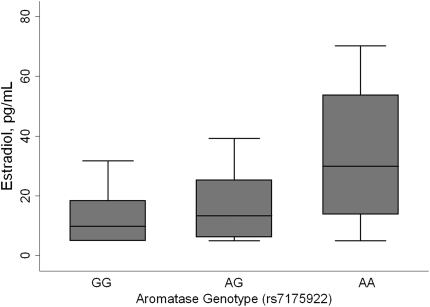
Estradiol levels were measured in 28 cases and 98 controls with available plasma (see online supplement for assay details). Estradiol levels increased in a dose-dependent fashion with the A allele of the aromatase rs7175922 SNP (the allele associated with an increased risk of PPHTN), even after adjustment for sex (Figure 1). Estradiol levels were not associated with genotypes of the other aromatase SNP associated with case status (data not shown).
Figure 1.
Estradiol levels and aromatase genotype adjusted for sex (test for trend, P = 0.03; n = 126). Median, interquartile range (box), and adjacent values (whiskers) are shown. Aromatase genotype distribution: GG (n = 88), AG (n = 34), AA (n = 4).
DISCUSSION
This is the first study to document genetic risk factors for PPHTN. Using a high-throughput candidate gene approach, we found SNPs in a variety of genes that were associated with the development of PAH in patients with advanced liver disease. Pathways with multiple gene “hits” included estrogen signaling, cellular growth/apoptosis, and oxidative stress. Other SNPs associated with case status included those in genes coding for recombination signal-binding protein 1 for J-kappa (RBPSUH), inducible nitric oxide synthase (NOS2A), and plasminogen activator inhibitor-1 (SERPINE1 or PAI-1). A number of the genes and signaling pathways found here have also been implicated in human or experimental PAH, supporting the concept that there may be shared pathogenetic mechanisms. In addition, several novel associations have been shown that may provide important mechanistic and therapeutic insights.
The role of estrogen signaling and increased estradiol levels in the pathogenesis of PAH and PPHTN has not been defined. PPHTN (like idiopathic and familial PAH) affects females more commonly than males (11), an association that may be related to a high estrogen state. However, estrogen has traditionally been believed to play a protective role in the systemic and pulmonary vasculature, modulating proliferative and vasoactive signaling by direct and receptor-mediated mechanisms (23, 24). In animal models of pulmonary hypertension, estrogen increases nitric oxide and prostacyclin production and decreases endothelin-1 (25–27), resulting in beneficial vascular effects. Such data are seemingly difficult to reconcile with studies showing adverse cardiovascular effects of estrogen. For instance, the Women's Health Initiative revealed that (despite many observational studies suggesting otherwise) hormone replacement therapy actually increased the risk for adverse cardiovascular events (28).
These apparent paradoxes may be explained by the complexity of the influence of estrogen on vascular homeostasis, resulting from variable expression of estrogen receptors 1 and 2 (α and β), cell and tissue specificity, and the influential balance between estrogen and other steroid hormones, such as testosterone and progesterone (29–31). We found that genetic variation in both the estrogen receptor 1 and aromatase (the rate-limiting enzyme in the conversion of androgens to estrogens) was associated with the risk of PPHTN, independent of sex. The two aromatase SNPs (rs1902584 and rs7175922) are located in the 93-kb region upstream (5′) of exon 2, where numerous tissue-specific promoters reside and thus could differentially influence aromatase expression in tissues (32). Furthermore, the association between the high-risk aromatase allele (rs7175922) and increased estradiol levels supports a functional effect of this SNP. Together, these findings strongly implicate estrogen signaling in the pathogenesis of PPHTN and define specific putative genetic factors that may contribute.
Several of the genes identified in our study participate in the regulation of cellular growth and apoptosis and have been implicated in human PAH and/or animal models of PAH. For example, we found that variation in PDE5A, which codes for a key enzyme in cyclic guanine monophosphate (cGMP) metabolism, was associated with PPHTN. Phosphodiesterase 5 inhibitors, such as sildenafil, potentiate the antiproliferative and vasodilatory effects of cGMP and improve hemodynamic features in PPHTN and other forms of PAH (33–35). Our finding supports a role for altered cGMP production in causing disease in these patients and introduces PDE5A genotype as a potential pharmacogenomic target.
We also found a relationship between genetic variability in ANGPT1 and risk of PPHTN. ANGPT1 plays a pivotal role in angiogenesis, and enhanced ANGPT1 expression or signaling has been reported to have beneficial effects in several experimental models of PAH (36–38). Although the exact role of ANGPT1 in pulmonary hypertension remains obscure, our findings support a role for this molecule in human disease. Finally, we found an association between PPHTN and genetic variation in a SNP in S100A4, a member of a family of calcium-binding proteins involved in regulation of endothelial proliferation and adhesion (39). S100A4 is expressed in the plexiform lesions of individuals with certain types of PAH (40). In a murine model, overexpression of S100A4 results in increased arteriolar remodeling, plexiform lesions (41), and pulmonary hypertension in response to hypoxia (42). We have now found a potential causal link between S100A4 and human disease.
An additional 10 genes had SNPs associated with PPHTN. Six of these—SERPINE1, RARB (43), caveolin 1 (CAV1) (44), SMAD3, runt-related transcription factor 1 (RUNX), and RBPSUH (45)—play a significant role in angiogenesis. SERPINE1 codes for PAI-1, which modulates the proliferative and migratory properties of pulmonary artery smooth muscle cells (PASMC) and has been shown to be down-regulated in individuals with IPAH (46). We found that genetic variation in NADPH oxidase 4 (NOX4), xanthine dehydrogenase, and superoxide dismutase 2 was associated with PPHTN. Redox signaling has recently been implicated as a potential node of control for pulmonary vascular response (47). NOX4 localizes to the media of small pulmonary arteries and, in a hypoxic mouse model, contributes to PASMC proliferation. Pulmonary arterioles from IPAH patients demonstrate a significantly increased level of NOX4 protein, confirming a potentially important role of NOX4 overexpression in PAH (48). Last, genetic variation in NOS2A may contribute the hypercoagulability and vasoconstriction characteristic of PPHTN. By the nature of their roles in angiogenesis, control of coagulation, and vascular tone pathways, these 10 genes offer plausible candidates for determining the risk of PPHTN.
Disruption in bone morphogenetic protein/transforming growth factor β signaling has been demonstrated in familial and idiopathic forms of PAH (49, 50). Although we cannot rule out the possibility of a rare coding mutation in our subjects, use of regional linkage disequilibrium and haplotype-tagging SNPs makes a contribution of common genetic variation in BMPR2, BMPR1A, ACVRL1, or ENG to portopulmonary hypertension unlikely.
There are several limitations to this study. First, the sample size was small, limiting our ability to find genetic alleles associated with PPHTN that are rare, have small effect sizes, or whose effect depends on gene–gene or gene–environment interaction. However, this is one of the largest epidemiologic studies of PPHTN with very strict case and control phenotypes ever performed and the first in PAH to use high-throughput genotyping.
A fundamental challenge in high-throughput genetic analyses is the control of type I error. Given that we analyzed multiple SNPs for each of more than 90 genes, we can reasonably expect a certain number of statistically significant associations due to chance alone. We attempted to minimize the chance of false positives by using a curated candidate gene list, thus increasing the prior probability that one or more of these genes has mechanistic importance in PPHTN. There are commonly used frequentist methods to adjust for multiple comparisons in high-throughput studies, such as the Bonferroni correction and false discovery rate (51). Both methodologies assume that the association of each individual SNP with case status is entirely independent of those of the other SNPs. We have documented patterns of linkage disequilibrium between genotyped SNPs. Because most accepted methods to account for multiple comparisons do not consider such relatedness, they are overly conservative for this purpose. We have therefore presented the results without adjustment and consider these results to be hypothesis-generating. Although replication would be important, the biologic plausibility of our findings, the multiple gene “hits” in certain pathways (estrogen signaling and oxidative stress), and the demonstration of functionality (aromatase genotype and plasma estradiol levels) are reassuring in terms of the validity of the findings (52).
Our results implicate common genetic variation in the pathogenesis of PPHTN. Future studies should focus on replication in other populations and the mechanisms that explain the associations between the SNPs of interest and PPHTN.
Supplementary Material
Acknowledgments
The authors thank May Huang, John Schlatterer, and John O'Connor, PhD from the Irving Institute for Clinical and Translational Research at Columbia University for their technical assistance.
Additional members of the Pulmonary Vascular Complications of Liver Disease Study Group are: Columbia University College of Physicians and Surgeons: Jeffrey Okun, B.A., Lori Rosenthal, N.P., Sonja Olsen, M.D., Jenna Reinen, B.S., Debbie Rybak, B.S.; Mayo Clinic: Vijay Shah, M.D., Russell Wiesner, M.D., Linda Stadheim, R.N.; University of Alabama: J. Stevenson Bynon, M.D., Devin Eckhoff, M.D., Harpreet Singh, Rajasekhar Tanikella, Keith Wille, M.D., Dorothy Faulk; University of Colorado: Lisa Forman, M.D., Ted Perry; The University of North Carolina at Chapel Hill: Roshan Shrestha, M.D., Carrie Nielsen, R.N.; University of Pennsylvania School of Medicine: Vivek Ahya, M.D., Harold Palevsky, M.D., Rajender Reddy, M.D., Michael Harhay, B.S., Sandra Kaplan, R.N.
Supported by National Institutes of Health grants DK064103, DK065958, RR00645, RR00585, RR00046, RR00032, HL67771, and HL089812.
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200809-1472OC on February 12, 2009
Conflict of Interest Statement: K.E.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.F.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.K. is on a scientific advisory board with Invitrogen, Inc., and received $12,500 in 2008. J.A.K. also holds a patent on the involvement of BMPRII in PAH and congenital heart disease. D.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.L.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.B.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.B.T. receives research support for participation in the REVEAL registry ($175,000, Actelion). E.M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. N.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.K. has received lecture fees, consultancy fees, funding for a CME course, and/or other support from Actelion, Pfizer, Gilead, Encysive, Lilly, INO Therapeutics, United Therapeutics, Gerson Lehrman, and Clinical Advisors. S.M.K. has received a $50,000 grant from Pfizer for an investigator-initiated clinical trial in chronic obstructive pulmonary disease.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–1030. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell PJ, Toye PA, Hutchins GM. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis 1983;127:437–441. [DOI] [PubMed] [Google Scholar]
- 3.Colle IO, Moreau R, Godinho E, Belghiti J, Ettori F, Cohen-Solal A, Mal H, Bernuau J, Marty J, Lebrec D, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology 2003;37:401–409. [DOI] [PubMed] [Google Scholar]
- 4.Kawut SM, Taichman DB, Ahya VN, Kaplan S, Archer-Chicko CL, Kimmel SE, Palevsky HI. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl 2005;11:1107–1111. [DOI] [PubMed] [Google Scholar]
- 5.Krowka MJ, Swanson KL, Frantz RP, McGoon MD, Wiesner RH. Portopulmonary hypertension: results from a 10-year screening algorithm. Hepatology 2006;44:1502–1510. [DOI] [PubMed] [Google Scholar]
- 6.Swanson KL, Wiesner RH, Nyberg SL, Rosen CB, Krowka MJ. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant 2008;8:2445–2453. [DOI] [PubMed] [Google Scholar]
- 7.Le Pavec J, Souza R, Herve P, Lebrec D, Savale L, Tcherakian C, Jais X, Yaici A, Humbert M, Simonneau G, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med 2008;178:637–643. [DOI] [PubMed] [Google Scholar]
- 8.Herve P, Le Pavec J, Sztrymf B, Decante B, Savale L, Sitbon O. Pulmonary vascular abnormalities in cirrhosis. Best Pract Res Clin Gastroenterol 2007;21:141–159. [DOI] [PubMed] [Google Scholar]
- 9.Sussman N, Kaza V, Barshes N, Stribling R, Goss J, O'Mahony C, Zhang E, Vierling J, Frost A. Successful liver transplantation following medical management of portopulmonary hypertension: a single-center series. Am J Transplant 2006;6:2177–2182. [DOI] [PubMed] [Google Scholar]
- 10.Krowka MJ, Mandell MS, Ramsay MA, Kawut SM, Fallon MB, Manzarbeitia C, Pardo M Jr, Marotta P, Uemoto S, Stoffel MP, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl 2004;10:174–182. [DOI] [PubMed] [Google Scholar]
- 11.Kawut SM, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N, et al. Clinical risk factors for portopulmonary hypertension. Hepatology 2008;48:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Roisin R, Krowka MJ, Herve P, Fallon MB. Pulmonary-hepatic vascular disorders (PHD). Eur Respir J 2004;24:861–880. [DOI] [PubMed] [Google Scholar]
- 13.Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 2001;108:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willers ED, Newman JH, Loyd JE, Robbins IM, Wheeler LA, Prince MA, Stanton KC, Cogan JA, Runo JR, Byrne D, et al. Serotonin transporter polymorphisms in familial and idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2006;173:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado RD, Koehler R, Glissmeyer E, Veal C, Suntharalingam J, Kim M, Carlquist J, Town M, Elliott CG, Hoeper M, et al. Genetic association of the serotonin transporter in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006;173:793–797. [DOI] [PubMed] [Google Scholar]
- 16.Roberts KE, Fallon MB, Krowka MJ, Benza RL, Knowles JA, Badesch DB, Brown RS, Taichman DB, Trotter J, Zacks S, et al. Serotonin transporter polymorphisms in patients with portopulmonary hypertension. Chest 2009; Jan 13 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 17.Kawut S, Krowka M, Roberts K, Benza R, Taichman D, Badesch D, Horn E, Rabinowitz D, Trotter J, Forman L, et al. A multicenter case-control study of genetic risk factors for portopulmonary hypertension (abstract E3278). European Respiratory Society Annual Congress, September 15–17, 2007; Stockholm, Sweden.
- 18.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- 19.Seldin MF, Shigeta R, Villoslada P, Selmi C, Tuomilehto J, Silva G, Belmont JW, Klareskog L, Gregersen PK. European population substructure: clustering of northern and southern populations. PLoS Genet 2006;2:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005;21:263–265. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet 1999;65:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gauderman W, Morrison J. 2006. QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies [Internet]. Accessed 2007 May. Available from: http://hydra.usc.edu/gxe.
- 23.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation 2001;103:423–428. [DOI] [PubMed] [Google Scholar]
- 24.Pendaries C, Darblade B, Rochaix P, Krust A, Chambon P, Korach KS, Bayard F, Arnal JF. The AF-1 activation-function of ERalpha may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci USA 2002;99:2205–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol 2002;16:938–946. [DOI] [PubMed] [Google Scholar]
- 26.Earley S, Resta TC. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol 2002;283:L86–L93. [DOI] [PubMed] [Google Scholar]
- 27.Lahm T, Crisostomo PR, Markel TA, Wang M, Wang Y, Weil B, Meldrum DR. Exogenous estrogen rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction. Shock 2008;30:660–667. [DOI] [PubMed]
- 28.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349:523–534. [DOI] [PubMed] [Google Scholar]
- 29.Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension 2007;49:1364–1370. [DOI] [PubMed] [Google Scholar]
- 30.O'Lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol 2007;21:1281–1296. [DOI] [PubMed] [Google Scholar]
- 31.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574. [DOI] [PubMed] [Google Scholar]
- 32.Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol 2003;86:219–224. [DOI] [PubMed] [Google Scholar]
- 33.Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–2157. [DOI] [PubMed] [Google Scholar]
- 34.Reichenberger F, Voswinckel R, Steveling E, Enke B, Kreckel A, Olschewski H, Grimminger F, Seeger W, Ghofrani HA. Sildenafil treatment for portopulmonary hypertension. Eur Respir J 2006;28:563–567. [DOI] [PubMed] [Google Scholar]
- 35.Deibert P, Bremer H, Roessle M, Kurz-Schmieg AK, Kreisel W. PDE-5 inhibitors lower portal and pulmonary pressure in portopulmonary hypertension. Eur Respir J 2007;29:220–221. [DOI] [PubMed] [Google Scholar]
- 36.Kugathasan L, Dutly AE, Zhao YD, Deng Y, Robb MJ, Keshavjee S, Stewart DJ. Role of angiopoietin-1 in experimental and human pulmonary arterial hypertension. Chest 2005;128:633S–642S. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto A, Takahashi H, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of angiopoietin-1 and Tie2 in chronic hypoxic pulmonary hypertension. Respiration 2008;75:328–338. [DOI] [PubMed] [Google Scholar]
- 38.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, Yuan JX, Deutsch R, Jamieson SW, Thistlethwaite PA. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med 2003;348:500–509. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt-Hansen B, Klingelhofer J, Grum-Schwensen B, Christensen A, Andresen S, Kruse C, Hansen T, Ambartsumian N, Lukanidin E, Grigorian M. Functional significance of metastasis-inducing S100A4(Mts1) in tumor-stroma interplay. J Biol Chem 2004;279:24498–24504. [DOI] [PubMed] [Google Scholar]
- 40.Greenway S, van Suylen RJ, Du Marchie Sarvaas G, Kwan E, Ambartsumian N, Lukanidin E, Rabinovitch M. S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol 2004;164:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, et al. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L583–L590. [DOI] [PubMed] [Google Scholar]
- 42.Merklinger SL, Wagner RA, Spiekerkoetter E, Hinek A, Knutsen RH, Kabir MG, Desai K, Hacker S, Wang L, Cann GM, et al. Increased fibulin-5 and elastin in S100A4/Mts1 mice with pulmonary hypertension. Circ Res 2005;97:596–604. [DOI] [PubMed] [Google Scholar]
- 43.Preston IR, Tang G, Tilan JU, Hill NS, Suzuki YJ. Retinoids and pulmonary hypertension. Circulation 2005;111:782–790. [DOI] [PubMed] [Google Scholar]
- 44.Peterson TE, Guicciardi ME, Gulati R, Kleppe LS, Mueske CS, Mookadam M, Sowa G, Gores GJ, Sessa WC, Simari RD. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol 2003;23:1521–1527. [DOI] [PubMed] [Google Scholar]
- 45.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 2007;445:781–784. [DOI] [PubMed] [Google Scholar]
- 46.Kouri FM, Queisser MA, Konigshoff M, Chrobak I, Preissner KT, Seeger W, Eickelberg O. Plasminogen activator inhibitor type 1 inhibits smooth muscle cell proliferation in pulmonary arterial hypertension. Int J Biochem Cell Biol 2008;40:1872–1882. [DOI] [PubMed] [Google Scholar]
- 47.Boueiz A, Damarla M, Hassoun PM. Xanthine oxidoreductase in respiratory and cardiovascular disorders. Am J Physiol Lung Cell Mol Physiol 2008;294:L830–L840. [DOI] [PubMed] [Google Scholar]
- 48.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, et al. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 2007;101:258–267. [DOI] [PubMed] [Google Scholar]
- 49.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat 2006;27:121–132. [DOI] [PubMed] [Google Scholar]
- 50.Harrison RE, Flanagan JA, Sankelo M, Abdalla SA, Rowell J, Machado RD, Elliott CG, Robbins IM, Olschewski H, McLaughlin V, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet 2003;40:865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamini Y, Yekutieli D. Quantitative trait loci analysis using the false discovery rate. Genetics 2005;171:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G, Altshuler D, Bailey-Wilson JE, et al. Replicating genotype-phenotype associations. Nature 2007;447:655–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.