Abstract
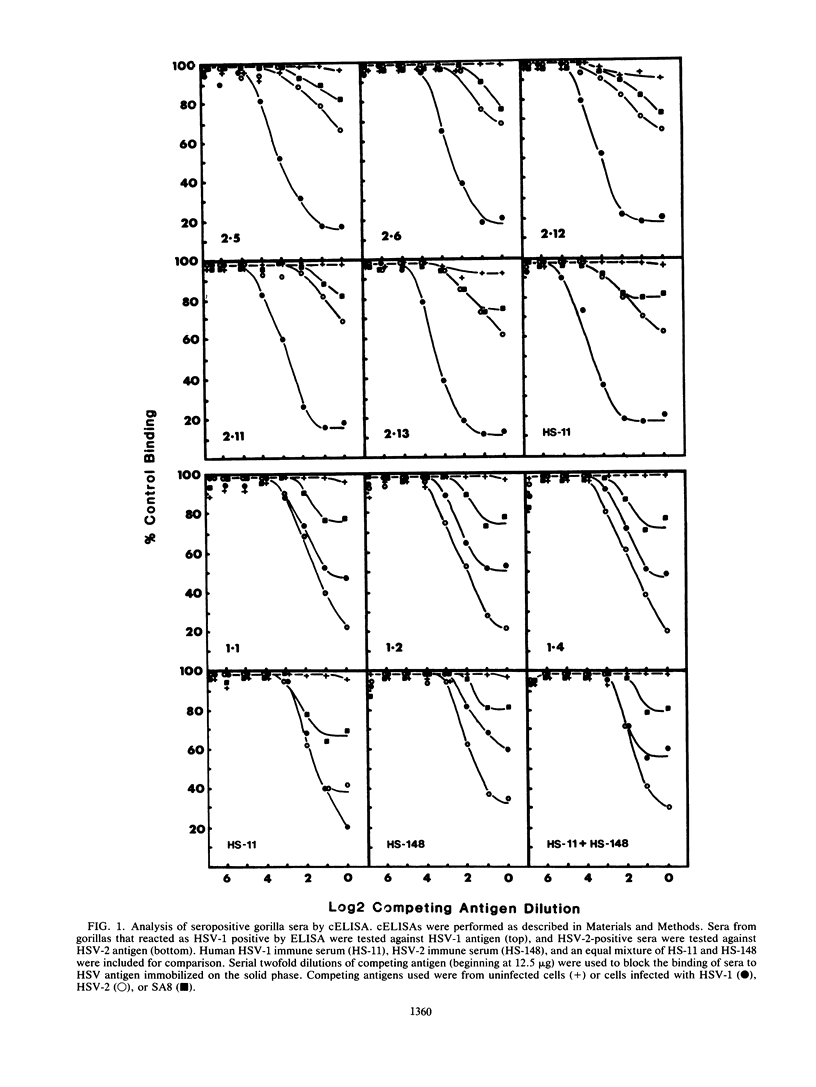
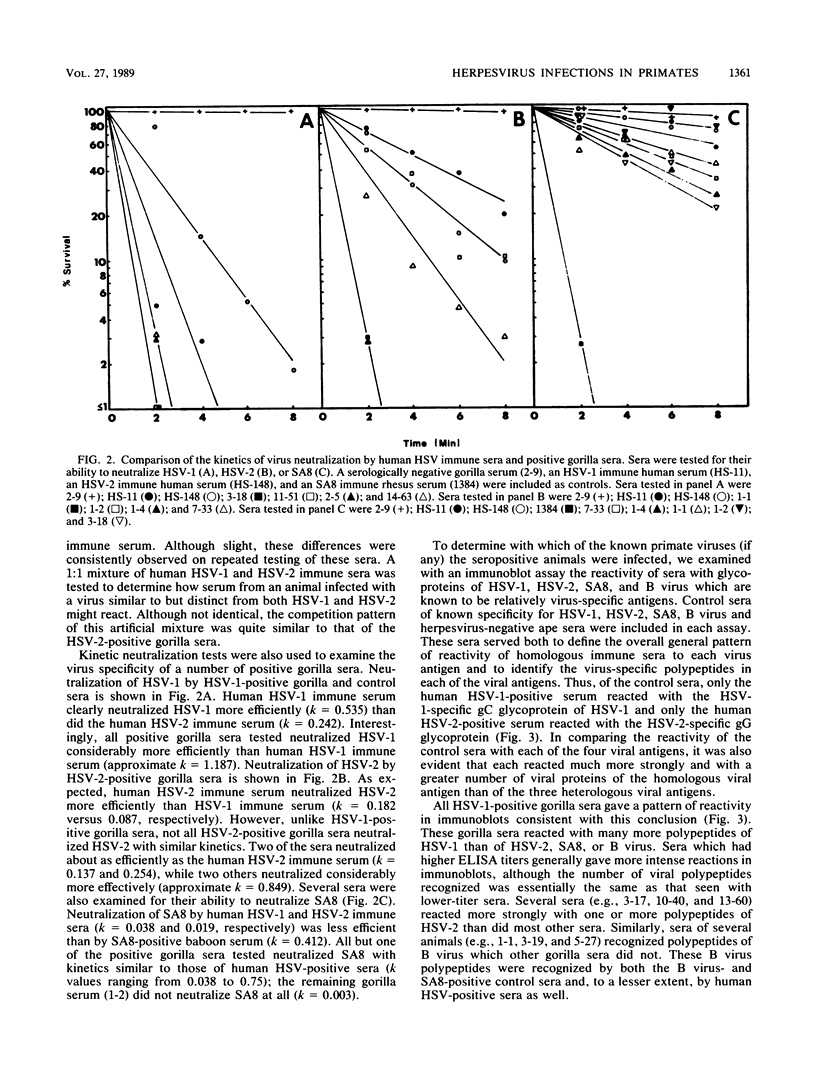
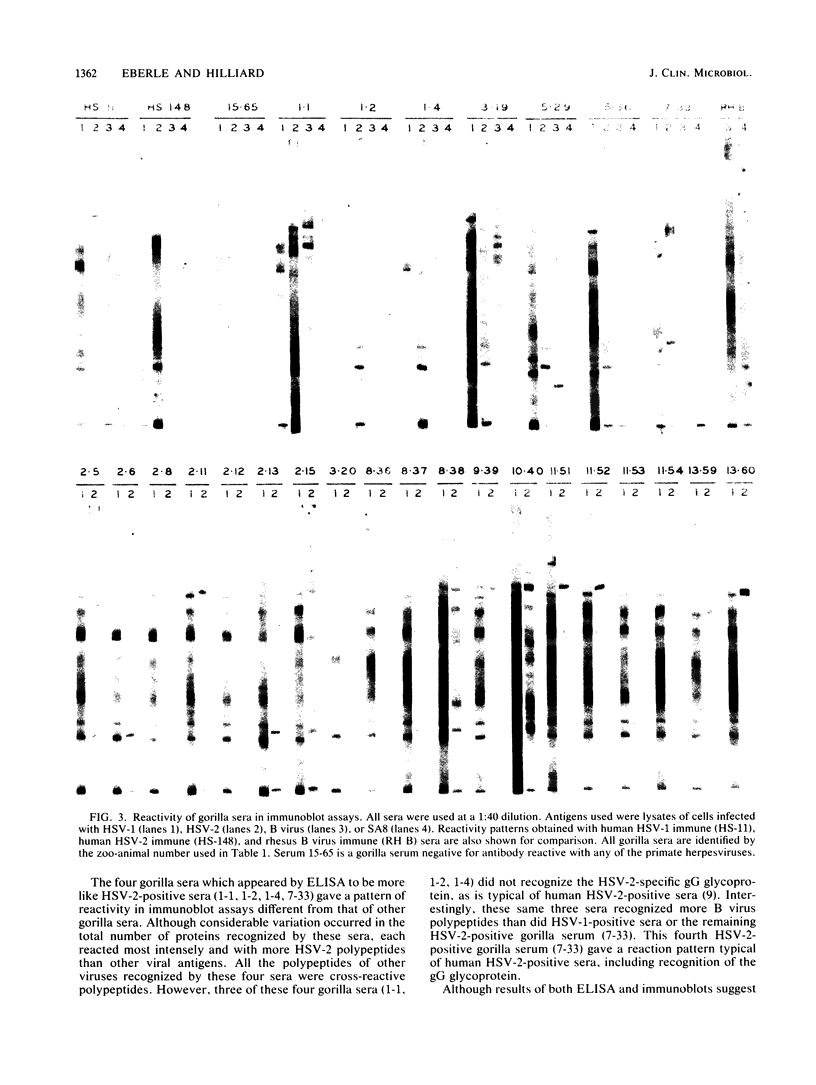
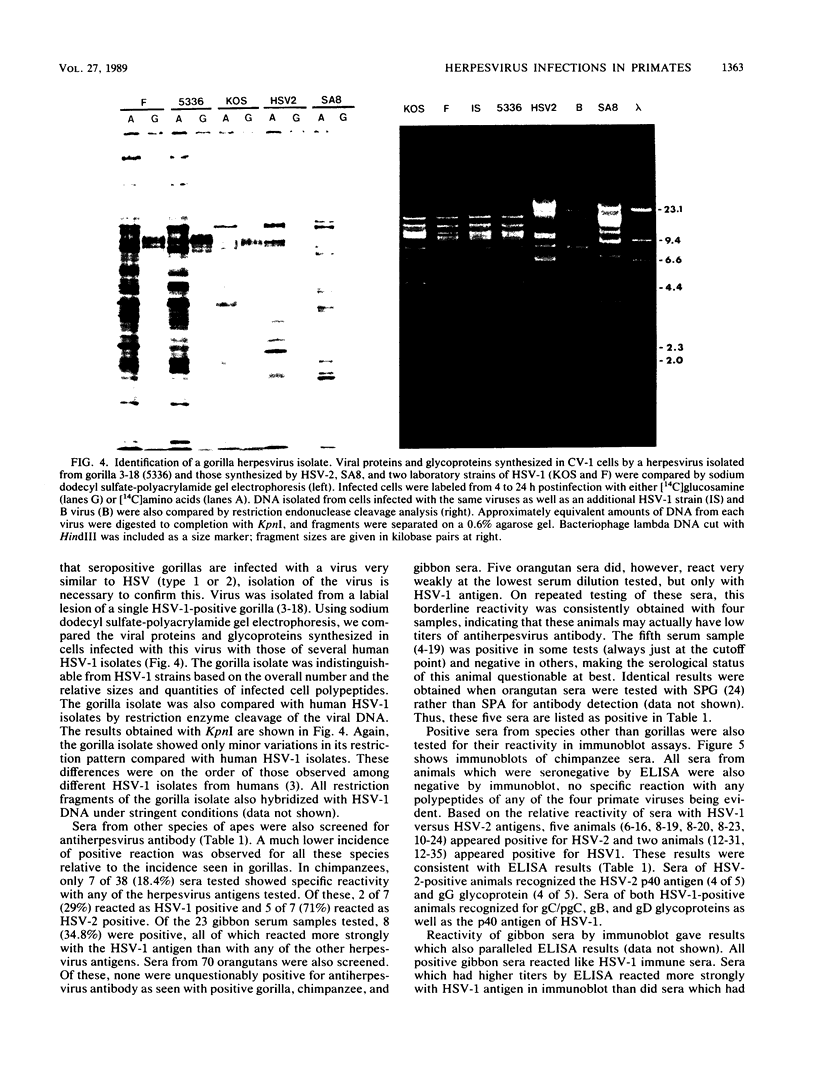
Sera from captive lowland gorillas, chimpanzees, orangutans, and gibbons were screened by enzyme-linked immunosorbent assay (ELISA) for antibody to herpesviruses serologically related to human herpes simplex virus types 1 and 2 (HSV-1, HSV-2), a baboon virus (SA8), and a macaque herpesvirus (B virus). The incidence of herpesvirus antibodies varied considerably among the different species, gorillas having the highest incidence of seropositivity (65.4%) and orangutans the lowest. The virus specificity of positive sera was further analyzed by examining the kinetics of virus neutralization, competition of reactivity in ELISAs, and immunoblotting against HSV-1, HSV-2, SA8, and B virus antigens. Using these assays, the majority of positive gorilla sera (49 of 53, 92%) were determined to react in a manner identical to human HSV-1 immune sera. The remaining four positive gorilla sera reacted as HSV-2-positive sera. In contrast, the majority of positive chimpanzee sera (5 of 7, 71%) reacted as HSV-2 immune rather than HSV-1 immune. All positive sera from gibbon apes reacted as HSV-1 positive. No orangutan sera were identified which gave positive reactions by ELISAs to any of the four primate herpesviruses tested. Although four orangutan sera gave equivocal results against HSV-1 antigen, further analysis by immunoblotting could not confirm any specific reactivity with any of the primate herpesvirus antigens. Varied reactivity among individual animals with both SA8 and B virus proteins was observed, but none of the seropositive primates detected appeared to be infected with either of these simian viruses. Three gorilla sera had antigen recognition patterns slightly different from those of HSV-2-positive human and chimpanzee sera and another HSV-2-positive gorilla serum, raising the possibility that these animals harbor an indigenous virus related to HSV-2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barahona H., Melendez L. V., Melnick J. L. A compendium of herpesviruses isolated from non-human primates. Intervirology. 1974;3(3):175–192. doi: 10.1159/000149754. [DOI] [PubMed] [Google Scholar]
- Boulter E. A. The isolation of monkey B virus (Herpesvirus simiae) from the trigeminal ganglia of a healthy seropositive rhesus monkey. J Biol Stand. 1975;3(3):279–280. doi: 10.1016/0092-1157(75)90031-1. [DOI] [PubMed] [Google Scholar]
- Chaney S. M., Warren K. G., Subak-Sharpe J. H. Variable restriction endonuclease sites of herpes simplex virus type 1 isolates from encephalitic, facial and genital lesions and ganglia. J Gen Virol. 1983 Dec;64(Pt 12):2717–2733. doi: 10.1099/0022-1317-64-12-2717. [DOI] [PubMed] [Google Scholar]
- DAVIDSON W. L., HUMMELER K. B virus infection in man. Ann N Y Acad Sci. 1960 May 12;85:970–979. doi: 10.1111/j.1749-6632.1960.tb50017.x. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Hilliard J. K. Replication of simian herpesvirus SA8 and identification of viral polypeptides in infected cells. J Virol. 1984 May;50(2):316–324. doi: 10.1128/jvi.50.2.316-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Mou S. W., Zaia J. A. Polypeptide specificity of the early antibody response following primary and recurrent genital herpes simplex virus type 2 infections. J Gen Virol. 1984 Oct;65(Pt 10):1839–1843. doi: 10.1099/0022-1317-65-10-1839. [DOI] [PubMed] [Google Scholar]
- Eberle R., Mou S. W., Zaia J. A. The immune response to herpes simplex virus: comparison of the specificity and relative titers of serum antibodies directed against viral polypeptides following primary herpes simplex virus type 1 infections. J Med Virol. 1985 Jun;16(2):147–162. doi: 10.1002/jmv.1890160207. [DOI] [PubMed] [Google Scholar]
- Emmons R. W., Gribble D. H., Lennette E. H. Natural fatal infection of an owl monkey (Aotus trivirgatus) with Herpes T virus. J Infect Dis. 1968 Apr;118(2):153–159. doi: 10.1093/infdis/118.2.153. [DOI] [PubMed] [Google Scholar]
- HEUSCHELE W. P. Varicella (chicken pox) in three young anthropoid apes. J Am Vet Med Assoc. 1960 Mar 15;136:256–257. [PubMed] [Google Scholar]
- HOLMES A. W., CALDWELL R. G., DEDMON R. E., DEINHARDT F. ISOLATION AND CHARACTERIZATION OF A NEW HERPES VIRUS. J Immunol. 1964 Apr;92:602–610. [PubMed] [Google Scholar]
- Holmes A. W., Devine J. A., Nowakowski E., Deinhardt F. The epidemiology of a herpes virus infection of New World monkeys. J Immunol. 1966 Apr;96(4):668–671. [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Hull R. N., Dwyer A. C., Holmes A. W., Nowakowski E., Deinhardt F., Lennette E. H., Emmons R. W. Recovery and characterization of a new simian herpesvirus from a fatally infected spider monkey. J Natl Cancer Inst. 1972 Jul;49(1):225–231. [PubMed] [Google Scholar]
- KEEBLE S. A. B virus infection in monkeys. Ann N Y Acad Sci. 1960 May 12;85:960–969. doi: 10.1111/j.1749-6632.1960.tb50016.x. [DOI] [PubMed] [Google Scholar]
- Kalter S. S., Heberling R. L. Comparative virology of primates. Bacteriol Rev. 1971 Sep;35(3):310–364. doi: 10.1128/br.35.3.310-364.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalter S. S., Ratner J. J., Rodriguez A. R., Heberling R. L., Guilloud N. B. Antibodies to human and simian viruses in the gorilla (Gorilla gorilla). Lab Anim Care. 1969 Feb;19(1):63–66. [PubMed] [Google Scholar]
- Kalter S. S., Ratner J., Kalter G. V., Rodriguez A. R., Kim C. S. A survey of primate sera for antibodies to viruses of human and simian origin. Am J Epidemiol. 1967 Nov;86(3):552–568. doi: 10.1093/oxfordjournals.aje.a120765. [DOI] [PubMed] [Google Scholar]
- Kalter S. S., Weiss S. A., Heberling R. L., Guajardo J. E., Smith G. C., 3rd The isolation of herpesvirus from trigeminal ganglia of normal baboons (Papio cynocephalus). Lab Anim Sci. 1978 Dec;28(6):705–709. [PubMed] [Google Scholar]
- Katz D., Hilliard J. K., Eberle R., Lipper S. L. ELISA for detection of group-common and virus-specific antibodies in human and simian sera induced by herpes simplex and related simian viruses. J Virol Methods. 1986 Sep;14(2):99–109. doi: 10.1016/0166-0934(86)90040-6. [DOI] [PubMed] [Google Scholar]
- Langone J. J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- Loomis M. R., O'Neill T., Bush M., Montali R. J. Fatal herpesvirus infection in patas monkeys and a black and white colobus monkey. J Am Vet Med Assoc. 1981 Dec 1;179(11):1236–1239. [PubMed] [Google Scholar]
- MELNICK J. L., BANKER D. D. Isolation of B virus (herpes group) from the central nervous system of a rhesus monkey. J Exp Med. 1954 Aug 1;100(2):181–194. doi: 10.1084/jem.100.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELNICK J. L., MIDULLA M., WIMBERLY I., BARRERA-ORO J. G., LEVY B. M. A NEW MEMBER OF THE HERPESVIRUS GROUP ISOLATED FROM SOUTH AMERICAN MARMOSETS. J Immunol. 1964 Apr;92:596–601. [PubMed] [Google Scholar]
- Malherbe H., Strickland-Cholmley M. Simian herpesvirus SA8 from a baboon. Lancet. 1969 Dec 27;2(7635):1427–1427. doi: 10.1016/s0140-6736(69)90972-6. [DOI] [PubMed] [Google Scholar]
- McClure H. M., Keeling M. E. Viral diseases noted in the Yerkes Primate Center colony. Lab Anim Sci. 1971 Dec;21(6):1002–1010. [PubMed] [Google Scholar]
- McClure H. M., Swenson R. B., Kalter S. S., Lester T. L. Natural genital herpesvirus hominis infection in chimpanzees (Pan troglodytes and Pan paniscus). Lab Anim Sci. 1980 Oct;30(5):895–901. [PubMed] [Google Scholar]
- Myers M. G., Kramer L. W., Stanberry L. R. Varicella in a gorilla. J Med Virol. 1987 Dec;23(4):317–322. doi: 10.1002/jmv.1890230403. [DOI] [PubMed] [Google Scholar]
- Ramsay E., Stair E. L., Castro A. E., Marks M. I. Fatal Herpesvirus hominis encephalitis in a white-handed gibbon. J Am Vet Med Assoc. 1982 Dec 1;181(11):1429–1430. [PubMed] [Google Scholar]
- Roizman B., Carmichael L. E., Deinhardt F., de-The G., Nahmias A. J., Plowright W., Rapp F., Sheldrick P., Takahashi M., Wolf K. Herpesviridae. Definition, provisional nomenclature, and taxonomy. The Herpesvirus Study Group, the International Committee on Taxonomy of Viruses. Intervirology. 1981;16(4):201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Zwartouw H. T., Boulter E. A. Excretion of B virus in monkeys and evidence of genital infection. Lab Anim. 1984 Jan;18(1):65–70. doi: 10.1258/002367784780864929. [DOI] [PubMed] [Google Scholar]
- Zwartouw H. T., MacArthur J. A., Boulter E. A., Seamer J. H., Marston J. H., Chamove A. S. Transmission of B virus infection between monkeys especially in relation to breeding colonies. Lab Anim. 1984 Apr;18(2):125–130. doi: 10.1258/002367784780891352. [DOI] [PubMed] [Google Scholar]