We present a microfluidic method that uses control of interfacial chemistry in nanoliter droplets to enable miniaturized in vitro measurements of protein aggregation. Measuring aggregation of proteins experimentally is fundamental to understanding the biophysics of amyloidosis[1, 2] and for developing methods for detection and treatment of amyloid diseases.[3] In traditional in vitro aggregation experiments, adsorption of amyloid peptides at the air/water[4] and solid/water[5, 6] interfaces accelerates peptide aggregation by enhancing nucleation. Intrinsic stochasticity of nucleation phenomena[7] implies that obtaining statistically significant data often requires multiple experiments performed in parallel, and this makes miniaturization of these experiments attractive. The interfacial effects, however, become even more pronounced on miniaturization of aggregation experiments, as miniaturization increases the surface-to-volume ratio. Miniaturization of aggregation experiments is especially desirable for samples available only in small volumes, such as cerebrospinal fluid (CSF) from mice. The CSF is known to contain components that inhibit formation of amyloid aggregates, and the balance of inhibitory and pro-aggregation activities changes with aging and progression of disease.[8–10] Therefore, CSF and interstitial brain fluid[11] are increasingly interesting for biomarker discovery and monitoring effects of drugs in animal models of Alzheimer’s diseases. Such panels of experiments are not easily done with standard well-plate in vitro assays that require tens of microliters of sample, given that the volume of CSF from a single mouse is only a few microliters. Here we show how such experiments can be performed by using a microfluidic system.
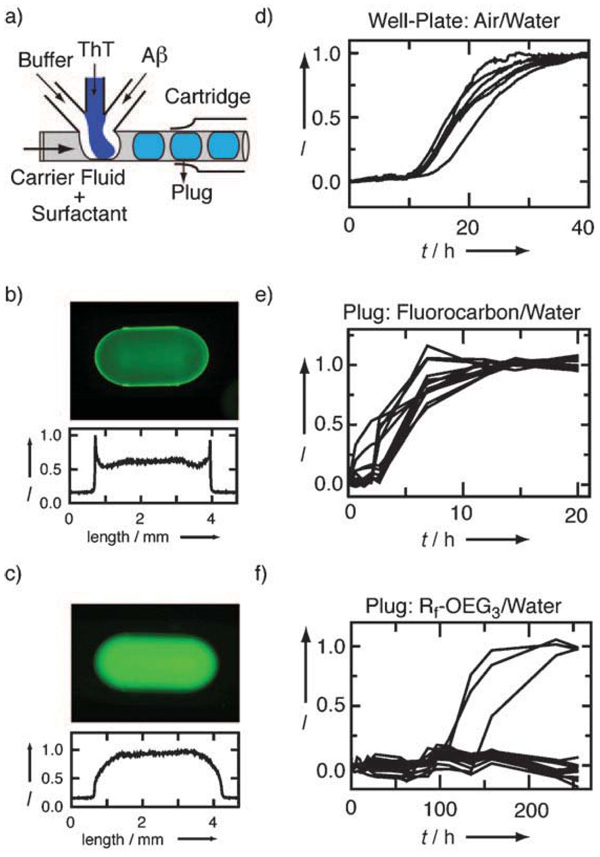
To miniaturize aggregation experiments while controlling the interfacial chemistry, we used a plug-based microfluidic approach[12] in polydimethylsiloxane (PDMS)[13] microfluidic devices modified with teflon tubing.[14] Plugs are nanoliter fluorocarbon-surrounded aqueous droplets formed in the flow of immiscible fluids inside microfluidic channels. To test this approach, we used an exceptionally well characterized amyloid beta peptide, namely, Aβ40. Once Aβ40 is encapsulated inside a plug, it is protected from the surfaces of microchannels by a layer of fluorocarbon, and the surface chemistry of the aqueous/fluorous interface, rather than aqueous/channel interface, becomes important. To test whether adsorption of Aβ40 at the aqueous/fluorous interface can be minimized, we compared the behavior of Aβ40 labeled with HiLyte-488 at the N terminus at two liquid/liquid interfaces: 1) aqueous peptide/fluorocarbon interface and 2) aqueous peptide/Rf-OEG3-protected fluorocarbon interface, where Rf-OEG3 is an amphiphilic fluorinated surfactant (see Supporting Information) that is added to the carrier fluid, assembles spontaneously at the aqueous/fluorous interface, presents triethylene glycol groups to the aqueous phase, and thereby prevents protein adsorption.[15] In plugs with the fluorocarbon/water interface, an increase in the fluorescence signal of the labeled Aβ40 peptide at the plug edges indicated adsorption of the peptide at the interface 2 h after encapsulation (Figure 1 b). In contrast, in plugs with an Rf-OEG3/water interface, the fluorescence signal of the labeled peptide was evenly distributed (Figure 1c), that is, Rf-OEG3 prevents Aβ40 adsorption at the interface. The effectiveness of Rf-OEG3 is a function of its concentration in the carrier fluid (see Supporting Information). Because prior to encapsulation of Aβ40 in plugs, the peptide is exposed to the walls of the channels, we inserted teflon tubing[14] into the Aβ inlet. To confirm that Aβ40 was not lost during the encapsulation process, we measured the fluorescence intensity of a series of plugs protected with Rf-OEG3 containing between 5 and 40 µm fluorescein-labeled Aβ40 after encapsulation. We found a linear increase in intensity that coincided with the increase of intensity of fluorescein (combined R2 = 0.99), a compound that is not significantly adsorbed on surfaces in our devices (Figure S1, Supporting Information).
Figure 1.
Encapsulation and aggregation of Aβ40 in plugs with controlled interfaces compared with plugs without interfacial control and with a well plate. a) Schematic of the microfluidic device. Fluorescence images of 22 µm HiLyte-488-labeled Aβ40 in plugs with b) fluorocarbon/water and c) Rf-OEG3/water interface. Corresponding line scans of the plugs are given below each image. Aggregation kinetics of 50 µm Aβ40 in 50 µm Tris buffer at 37°C measured by ThT fluorescence in d) a well plate with an air/water interface, e) plugs with a fluorocarbon/water interface, and f) plugs with an Rf-OEG3/water interface. Note the difference in timescales.
Having established the inertness of the plug/fluorocarbon interface to peptide adsorption, we monitored the differences in the aggregation kinetics of Aβ40 in plugs with and without Rf-OEG3 by using Thioflavin T (ThT), a dye widely used to monitor aggregation, and observing the increase in the fluorescence of ThT on binding to amyloid aggregates.[16] The aggregation kinetics of Aβ40 depends on nucleation and is typically described by a sigmoidal curve.[1] First, we measured aggregation kinetics of 50 µm Aβ40 in volumes of 100 µL in well plates, where we observed a lag period of about 10 h before ThT fluorescence indicated Aβ40 aggregation (Figure 1d). In plugs with the fluorocarbon/water interface, the lag time of the aggregation reaction shortened to about 2 h (Figure 1e), correlated with the adsorption of the peptide to the fluorocarbon/water interface and also with the increased surface-to-volume ratio in 10 nL volumes. In contrast, in plugs with an Rf-OEG3/water interface the lag time of aggregation increased to many days (Figure 1 f). After 250 h, only 3 plugs out of 15 showed evidence of Aβ40 aggregation. Even after 2 months, only 8 plugs out of 15 showed evidence of Aβ40 aggregation (data not shown). Thus, control of the interfacial chemistry decreases the aggregation kinetics of Aβ40 by at least an order of magnitude.
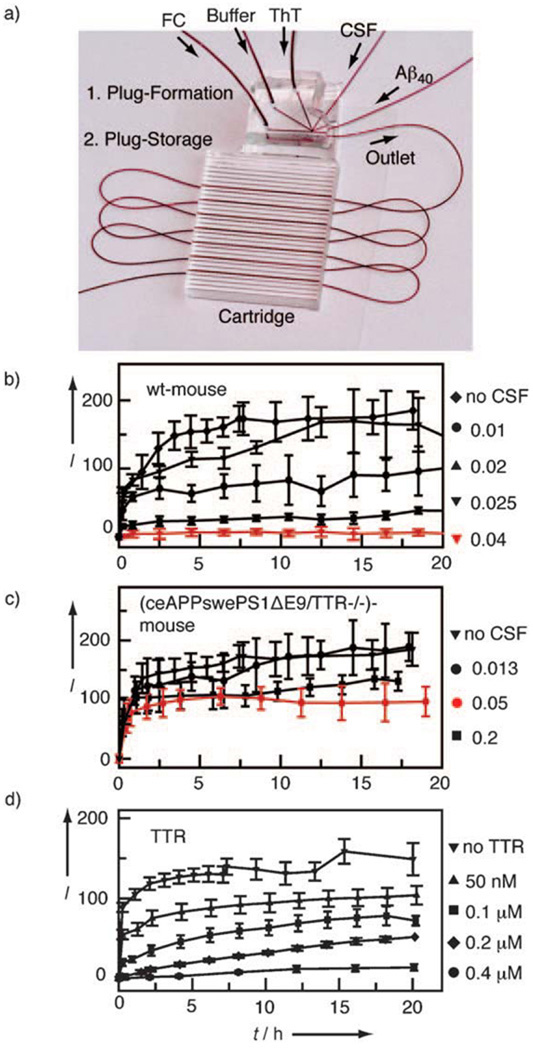
Next we tested whether this system could reliably handle aggregation experiments with small volumes by using CSF from a single mouse for a set of experiments. We compared the ability to inhibit Aβ40 aggregation for CSF (5 µL) from two mouse strains. In this comparison, we used a nontransgenic (wild-type, wt) mouse and a ceAPPswePS1ΔE9/TTR-/- transgenic mouse that exhibits amyloid deposition throughout the cortex and hippocampus.[17] The ceAPPswePS1ΔE9/TTR-/- mice lack the gene encoding transthyretin (TTR), a molecule that is enriched in CSF[18] and known to associate with Aβ40 in both in vitro and in vivo settings.[19, 20] The inhibitory potency of CSF for each mouse was tested by generating plugs containing buffer, ThT, Aβ40, and different volumes of CSF. We described the amount of CSF in each plug in terms of “volume fraction” (the ratio of the volume of CSF in the plug to the total aqueous volume of the plug). Titrations were performed by changing the relative flow rates of all inlet streams[21] to generate plugs containing constant concentrations of ions and buffer, ThT, and Aβ40, but different volume fractions of CSF (see Supporting Information for the titration algorithm). For each of the 15 different concentrations of CSF, 50 plugs were generated, giving 750 experiments for each sample of CSF. Aggregation of Aβ40 was then monitored by the increase in ThT fluorescence. In contrast to the experiments with low ionic strength (Figure 1), the ionic strength of the buffer solution in CSF experiments was adjusted to accelerate aggregation and to resemble the ion composition of mouse CSF, which includes a high concentration of Ca2+ and Mg2+ ions. In accord with previous reports, the higher ionic strength led to a decrease in the lag time of aggregation of Aβ40[22] (see also Supporting Information).
Cerebrospinal fluid from the wild-type mouse was able to inhibit Aβ40 aggregation when added to plugs at volume fractions higher than 0.04 (Figure 2b). On the other hand, CSF from the ceAPPswePS1ΔE9/TTR-/- mouse did not inhibit Aβ40 aggregation at volume fractions as high as 0.2 (Figure 2c). These results agree with the difference in the inhibitory potency of human CSF from patients with and without Alzheimer’s disease.[23] A qualitatively similar inhibitory effect on Aβ40 aggregation was observed with recombinant TTR alone in plugs (Figure 2d), although this effect was substantially weaker. Concentrations of TTR of about 0.2–0.4 µm, close to the physiological concentration of TTR in CSF,[9] were sufficient to inhibit aggregation. On the other hand, CSF from a wild-type mouse could be diluted 25-fold (to a water fraction of 0.04) without detectable loss of inhibitory activity. A dilution of TTR alone by less than 10- fold (to 50 µm) caused a substantial decrease in its ability to inhibit aggregation. This result implies that mouse TTR made, and possibly further modified, in vivo is considerably better at inhibiting aggregation than heterologously expressed human TTR, or it suggests that other factors in CSF can inhibit aggregation. The microfluidic system described here is attractive for performing further experiments to identify these putative factors in human CSF.
Figure 2.
CSF titration experiments. a) Microfluidic device designed for performing 250 aggregation trials of Aβ40 with different volume fractions of CSF or concentrations of protein. Aggregation kinetics of 50 µm Aβ40 on addition of various volume fractions of CSF b) from wild-type mouse and c) from a mouse which is a model for Alzheimer’s disease (ceAPPswePS1ΔE9/TTR-/-). The volume fraction is defined as the ratio of volume of CSF to total aqueous plug volume and is shown to the right of the graphs. d) Aggregation kinetics of 50 µm Aβ40 on addition of various concentrations of TTR. Aggregation was measured by ThT fluorescence, where I is the fluorescence intensity of ThT normalized to the fluorescence intensity at t = 0. Each data point is averaged over 15 plugs, and the error bars are the standard deviations.
In conclusion, we used plug-based microfluidics and control of surface chemistry to miniaturize peptide aggregation experiments to nanoliter volumes. Although here we used the ThT assay to analyze aggregation in the micromolar range, plug-based microfluidics is compatible with other analytical methods potentially applicable to analysis in the nanomolar range, for example, mass spectrometry[24] or fluorescence correlation spectroscopy.[25] This approach could further advance in vitro aggregation biophysics, for example, time-controlled aging and nucleation–growth experiments[26] on amyloid peptides. Reducing “extrinsic” aggregation nucleated and driven by interfaces is attractive for understanding intrinsic aggregation mechanisms, and for finding molecules added in solution or attached to the interfaces[27] that can either accelerate or inhibit aggregation. Using plug-based microfluidics, we demonstrated that in aggregation assays the inhibitory activity of 5 µL of CSF from a single mouse can be evaluated by setting up hundreds of experiments. In the area of Alzheimer’s research, we are especially interested in the additional opportunities for evaluating potential diagnostic methods and for monitoring potential treatments. This approach may enable repeated analysis of CSF or brain interstitial fluid[11] from the same live animal, for example, by using the chemistrode.[25]
Supplementary Material
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200805225.
Footnotes
This research was supported by the National Institutes of Health NIBIB EB000557 and the NIH Director’s Pioneer Award DP1 OD003584 to R.F.I. and by Cure Alzheimer’s Fund and NIH AG027854 to S.S.S. R.F.I. is a Camille Dreyfus Teacher-Scholar. M.M. was supported by the Alexander von Humboldt Society and J.K.-D. by the NIH Roadmap training program T90 DK070076. We thank Dr. A. M. Seddon and Dr. E. Chan for preliminary experiments and E. W. Haney for editing this manuscript.
Contributor Information
Dr. Matthias Meier, Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA)
Julia Kennedy-Darling, Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA).
Se Hoon Choi, The Center for Molecular Neurobiology, University of Chicago, 947 E 58th Street, Chicago, IL 60637 (USA).
Dr. Eric M. Norstrom, The Center for Molecular Neurobiology, University of Chicago, 947 E 58th Street, Chicago, IL 60637 (USA)
Prof. Dr. Sangram S. Sisodia, The Center for Molecular Neurobiology, University of Chicago, 947 E 58th Street, Chicago, IL 60637 (USA)
Prof. Dr. Rustem F. Ismagilov, Department of Chemistry, University of Chicago, 929 East 57th Street, Chicago, IL 60637 (USA)
References
- 1.Harper JD, Lansbury PT. Annu. Rev. Biochem. 1997;66:385. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg D, Nelson R, Sawaya MR, Balbirnie M, Sambashivan S, Ivanova MI, Madsen AØ, Riekel C. Acc. Chem. Res. 2006;39:568. doi: 10.1021/ar0500618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joachim CL, Mori H, Selkoe DJ. Nature. 1989;341:226. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- 4.Schladitz C, Vieira EP, Hermel H, Möhwald H. Biophys. J. 1999;77:3305. doi: 10.1016/S0006-3495(99)77161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacomelli CE, Norde W. Macromol. Biosci. 2005;5:401. doi: 10.1002/mabi.200400189. [DOI] [PubMed] [Google Scholar]
- 6.Terzi E, Holzemann G, Seelig J. J. Mol. Biol. 1995;252:633. doi: 10.1006/jmbi.1995.0525. [DOI] [PubMed] [Google Scholar]
- 7.Hofrichter J, Ross PD, Eaton WA. Proc. Natl. Acad. Sci. USA. 1974;71:4864. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanekiyo T, Ban T, Aritake K, Huang ZL, Qu WM, Okazaki I, Mohri I, Murayama S, Ozono K, Taniike M, Goto Y, Urade Y. Proc. Natl. Acad. Sci. USA. 2007;104:6412. doi: 10.1073/pnas.0701585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serot JM, Christmann D, Dubost T, Couturier M. J. Neurol. Neurosurg. Psychiatry. 1997;63:506. doi: 10.1136/jnnp.63.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Yee A, Brewer HB, Das S, Potter H. Nature. 1994;372:92. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 11.Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, Zipfel GJ, Holtzman DM. Science. 2008;321:1221. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song H, Chen DL, Ismagilov RF. Angew. Chem. 2006;118:7494. doi: 10.1002/anie.200601554. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:7336. [Google Scholar]
- 13.McDonald JC, Whitesides GM. Acc. Chem. Res. 2002;35:491. doi: 10.1021/ar010110q. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Mustafi D, Fu Q, Tereshko V, Chen DL, Tice JD, Ismagilov RF. Proc. Natl. Acad. Sci. 2006;103:19243. doi: 10.1073/pnas.0607502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roach LS, Song H, Ismagilov RF. Anal. Chem. 2005;77:785. doi: 10.1021/ac049061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LeVine H. Methods Enzymol. 1999;309:274. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 17.Choi S, Leight S, Lee V, Li T, Wong P, Johnson J, Saraiva M, Sisodia S. J. Neurosci. 2007;27:7006. doi: 10.1523/JNEUROSCI.1919-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbert J, Wilcox JN, Pham KT, Fremeau RT, Jr, Zeviani M, Dwork A, Soprano DR, Makover A, Goodman DS, Zimmerman EA, Roberts JL, Schon EA. Neurology. 1986;36:900. doi: 10.1212/wnl.36.7.900. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzman AL, Gregori L, Vitek MP, Lyubski S, Strittmatter WJ, Enghilde JJ, Bhasin R, Silverman J, Weisgraber KH, Coyle PK. Proc. Natl. Acad. Sci. USA. 1994;91:8368. doi: 10.1073/pnas.91.18.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Link CD. Proc. Natl. Acad. Sci. USA. 1995;92:9368. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song H, Ismagilov RF. J. Am. Chem. Soc. 2003;125:14613. doi: 10.1021/ja0354566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sengupta P, Garai K, Sahoo B, Shi Y, Callaway DJ, Maiti S. Biochemistry. 2003;42:10506. doi: 10.1021/bi0341410. [DOI] [PubMed] [Google Scholar]
- 23.Ono K, Noguchi M, Matsumoto Y, Yanase D, Iwasa K, Naiki H, Yamada M. Neurobiol. Dis. 2005;20:233. doi: 10.1016/j.nbd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Hatakeyama T, Chen DL, Ismagilov RF. J. Am. Chem. Soc. 2006;128:2518. doi: 10.1021/ja057720w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen D, Du W, Liu Y, Liu W, Kuznetsov A, Mendez FE, Philipson LH, Ismagilov RF. Proc. Natl. Acad. Sci. USA. 2008;105:16843. doi: 10.1073/pnas.0807916105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerdts CJ, Tereshko V, Yadav MK, Dementieva I, Collart F, Joachimiak A, Stevens RC, Kuhn P, Kossiakoff A, Ismagilov RF. Angew. Chem. 2006;118:8340. doi: 10.1002/anie.200602946. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:8156. [Google Scholar]
- 27.Kreutz JE, Li L, Roach LS, Hatakeyama T, Ismagilov RF. J. Am. Chem. Soc. doi: 10.1021/ja808697e. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.200805225.