Abstract
Detailed characterization of estrogen dynamics during the transition to menopause is an important step toward understanding its potential implications for reproductive cancers developing in the transition years. We conducted a 5-year prospective study of endogenous levels of total and unopposed estrogen. Participants (n=108, aged 25–58 years) collected daily urine specimens for six months in each of five consecutive years. Specimens were assayed for estrone-3-glucuronide (E1G) and pregnanediol-3-glucuronide (PDG). Linear mixed-effects models were used to estimate exposure to total and unopposed estrogen by age and reproductive stage. Reproductive stage was estimated using menstrual cycle length variance. E1G mean area under the curve (AUC), and mean E1G 5th and 95th percentiles represented total estrogen exposure. An algorithm identifying days of above-baseline E1G that coincided with the days of baseline PDG was used to identify days of unopposed estrogen. Mean E1G AUC increased with age in the pre- and early transition and decreased in the late transition. Ninety-fifth percentile E1G levels did not decline until after menopause whereas 5th percentile levels declined from the early transition to the post menopause. The number of days of unopposed estrogen was significantly higher during the transition compared with the pre-transition. Given the length of time women spend in the transition, they are exposed to more total and unopposed estrogen than has been previously appreciated. Coupled with epidemiological evidence on lifetime exposure to estrogen, these results suggest that variation in the amount of time spent in the transition may be an important risk factor for reproductive cancers.
Keywords: estrogen, estrone glucuronide, pregnanediol, urinary hormones, menopause, perimenopause, reproductive aging, reproductive cancer, reproductive stage, STRAW
INTRODUCTION
Recent data show that among both white and black women, age-specific incidence rates of malignant cancers of the breast, ovaries, and endometrium rise through the perimenopausal years up to at least the age of 60 (1). Substantial evidence supports an association of endogenous reproductive hormone exposure with increased risk of reproductive cancers (2–4). Greater estrogen exposure, assessed via indirect indicators such as number of years spent having menstrual cycles (e.g. (5)), or direct indicators such as hormone measures (e.g. (6)), is associated with increased risk for cancers of the breast and ovary (3, 7, 8). Similarly, exposure to estrogen unopposed by progesterone is a risk factor for endometrial and ovarian cancer (8). The precise mechanism by which estrogen contributes to reproductive cancers is not known, but etiologic theories highlight estrogen’s role in cell proliferation in the endometrium and breast, and epithelial repair of the ovary following ovulation (3, 7–10).
Erratic estrogen secretion is characteristic of the perimenopausal years (11) and may be an important source of risk for reproductive cancers. However, the perimenopause, which may last several years (12), has not been explicitly considered in studies of hormones and reproductive cancers. Hale and colleagues (13) recently emphasized Pike’s suggestion (14) that the perimenopausal years constitute an important ‘window of risk’ for endometrial cancer. These years may also be an important window of risk for other estrogen-related cancers, such as breast and ovarian cancer (15), as well as for the increased likelihood of uterine fibroids, endometrial hyperplasia, dysfunctional uterine bleeding and progression of endometriosis symptoms observed in the perimenopause (16). Thus, detailed characterization of estrogen dynamics during the perimenopause is important for understanding its potential implications for reproductive cancers and other health outcomes.
Menstrual cycle length changes as women make the transition to menopause. On average, cycles become longer and more variable with increasing proximity to menopause (17). Compared with the hormonal patterns of the prime reproductive years the transition to menopause is associated with increased variability in estrogen (E) and progesterone (P) patterns (18–20). Previous studies have documented higher (21–25), lower (26–31), or unchanged (32–36) estrogen levels in older or perimenopausal women compared with younger or pre-menopausal women. In recent work we found that individual-level urinary E1G increased from 25–45 years and then declined in the late 40’s for most women (37). These disparate results arise because of: 1) differences among studies in the timing of sampling, sample selection or sample size, and study design; 2) erratic fluctuations in hormone levels confounding comparisons during the perimenopause; and 3) the use of age as an anchor for comparisons among women: aggregate estrogen patterns can mask hormonal trajectories that individual women actually experience as they age (37).
Thus, it remains unclear whether women are exposed to more or less overall estrogen during the perimenopause compared with the prime reproductive years. Here we examine estrogen trajectories for individual women across reproductive stages (38). We estimate total and unopposed estrogen exposure by stage, while controlling for age and BMI.
MATERIALS AND METHODS
Participants
Data were collected as part of the Biodemographic Models of Reproductive Aging (BIMORA) project (37). Participants were recruited from the Tremin Research Program on Women’s Health (TREMIN) (39). Participants included women between 25 and 60 years of age, not using prescription reproductive hormones, and who had at least one intact ovary. Pregnant or breastfeeding women, and women receiving cancer treatment, were not eligible. Monetary compensation was provided for participation. All subjects provided written informed consent, and all procedures were approved by the institutional review boards of the University of Utah, the Pennsylvania State University, Georgetown University, and the University of Washington.
Data Collection
First morning urine specimens and information on menstrual bleeding were collected daily from January 15 to July 14 of each year from 1998 to 2002. Daily information was collected on major medical conditions and treatments, and over-the-counter and prescription medication. Most participants continued to record menstrual bleed data on calendar cards for TREMIN. We combined the TREMIN bleed data for July 15 to January 14 with the BIMORA data for January 15 to July 14 for each project year from 1998 to 2001; for 2002 we have only BIMORA bleed data.
Height and weight for body mass index (BMI) come from a 2000 self-administered health survey, at the midpoint of the study. BMI was available for the year 2000 for 90 of the 108 women included in the analyses. For the remaining 18 women we used all available BMI data from previous years for each woman in a linear mixed effects model of BMI by year, and used the estimated model fits to impute a 2000 value. The mean (SD) and median BMI for the sample of 108 women was 24.2 (5.1) and 23.2 kg/m2 respectively. Seventy percent of the BMI’s were below 25 (normal), 18% fell between 25–29 (overweight) and 12% were 30 kg/m2 or greater (obese).
Laboratory methods
Urine specimens were assayed with enzyme immunoassays (EIAs) for estrone-3-glucuronide (E1G), a metabolite of estradiol, and pregnanediol-3-glucuronide (PDG), a metabolite of progesterone. Inter- and intra-assay CV's were 9.2% and 10.3% for the PDG EIA, and 4% and 3.6% for the E1G EIA (40, 41). The metabolites and significant cross-reactants closely parallel the serum levels of estradiol and progesterone (40, 41).
Hormone concentrations were estimated from optical density (Biolinx 1.0 software. Dynex Laboratories, Inc., Chantilly, VA). Urinary hormone concentrations, assayed in duplicate, were adjusted by specimen specific gravity, using a population mean specific gravity of 1.020 (42). E1G concentrations were statistically corrected for slight assay non-parallelism, using a 1:5 dilution as the standard to which all values were corrected (41).
Reproductive Stage
A reproductive stage was assigned to each menstrual cycle in the study, using a four-category scale (Table 1) derived from the Staging Reproductive Aging Workshop (STRAW) recommendations (38). Based on the criteria of variability in cycle length described in STRAW, we used the CV of menstrual cycle length to assign stage.
Table 1.
Criteria for assigning reproductive stage.
| Stage Number | Description | Defining Criteria |
|---|---|---|
| Stage−3 | Pre-Menopausal | CV of length of current cycle + previous 5 (or fewer) cycles < 20% |
| Stage−2 | Early Stage of Menopausal Transition | CV of length of current cycle + previous 5 (or fewer) cycles = 20–40% |
| Stage−1 | Late Stage of Menopausal Transition | CV of length of current cycle + previous 5 (or fewer) cycles = >40% OR Presence of a cycle length ≥ 60 days. |
| Stage +1 | Post-menopausal | No menstruation for the previous 12 months. |
Cycle length was calculated as the number of days from the first day of a menstrual bleed to the last day before the next bleed. A menstrual bleed was defined as a segment with at least 2 days of bleeding in 6 consecutive days, which had to be preceded by at least 5 consecutive days of no bleeding.
A rolling cycle-length CV (the standard deviation of cycle length divided by the mean cycle length) was calculated for each cycle using the length of the current cycle and the five previous cycles; this CV was used to assign stage to each cycle. When fewer than five previous cycles were available, all cycle lengths observed before the current cycle were used.
Cutoff values for the rolling cycle-length CV were chosen to represent reproductive stages as close as possible to the STRAW system (38) (Table 1). Because CV values of less than 20% represent fewer than 7 days deviation from a 34 day cycle, we used it to indicate pre-menopausal cycling (stage −3). A twenty to forty percent CV was chosen to represent the STRAW criterion of cycle length variance greater than 7 days (stage −2). A CV greater than forty percent or the presence of a cycle greater than 60 days in length was used for the STRAW criterion of 2 or more skipped cycles (stage −1). Postmenopause (stage +1) was defined as one year with no menstrual bleeding.
Cycles that were right or left censored at fewer than 60 days within a 6 month interval were assigned a stage using the predominant stage (the stage occurring ≥ 60% of the time within the six-month segment) in the interval. Cycles censored at 60 or more days in length were assigned either stage −1 or +1; in some censored cycles, it was not possible to reliably differentiate between stages −1 and +1, but this occurred in only 11 of 3303 menstrual segments. Intervals where the most prevalent stage occurred less than 60% of the time were coded as mixed stage. This designation was assigned to 39 of the 359 sixmonth intervals. Forty-four percent of the 39 cases included stages −3, −2 and −1 within an interval, eight percent were cases of one reproductive stage and a substantial censored segment, and the remainder were other combinations of the stages −3, −2, −1 and censored segments.
Quantification of Total and Unopposed Estrogen
To quantify total estrogen levels we examined mean E1G area-under the curve (AUC). We also examined the 5th and 95th percentiles of E1G, as indicators of baseline and peak levels of E1G, respectively. We assessed how these varied by age and stage, while controlling for BMI.
To quantify unopposed estrogen we used the total number of days per cycle where PDG was at a per-cycle baseline level and E1G was above a per-cycle baseline level. A daily running 5-day PDG average was used to create a daily PDG ratio (PR): the PDG of the current day divided by the average PDG. A PR below 3.0 was considered baseline PDG; when the PR exceeded 3.0 for at least 3 days in a 5 day sequence (the criterion used in ovulation detection algorithms to identify a sustained rise in PDG— (e.g. (43)), then all days from the first day the PR exceeded 3.0 to the end of the cycle were scored as having sufficient progesterone to oppose estrogen. To identify days where E1G was above baseline level, we began with a day of estrogen take-off (44) that was previously estimated for a separate project (Ferrell et al, nd). All subsequent days were scored as above E1G baseline, until the end of the cycle or a clear decline in E1G occurred. E1G data for menstrual cycles were first smoothed using the function: smoothed E1G = (previous day’s E1G value) (0.25)+(current day’s E1G value)(0.50)+(next day’s E1G value)(0.25). Inactive (baseline estrogen) periods were identified by one of us (RJF) from graphs showing menstrual bleeds and smoothed E1G data. The end of an inactive period was indicated by a sharp and sustained rise in E1G and was scored as the first day of E1G above baseline.
All days between the day of estrogen take-off and the day of progesterone take-off were designated as unopposed; all other days in a cycle were designated as opposed. Additional criteria were needed in about 20% of cycles. In such cases, we used a steroid ratio (SR = E1G/PDG) to indicate days when estrogen was elevated while progesterone was at baseline (SR≥ 20). We developed a hierarchical set of rules to accommodate all possible cases and applied these to all cycles; the rules are presented in the appendix. To test implementation of the rules, a sample of 70 six-month segments of data were scored independently by two investigators (KAO and RJF). Inter-rater agreement based on the kappa statistic was 95% for 12,030 observations (kappa = 0.8533, SD=0.0091). The discrepancies between raters were small, and not systematic. One investigator then assigned days of unopposed estrogen to all data.
Statistical Analyses of Total and Unopposed Estrogen by Age and Reproductive Stage
The unit of analysis was a six-month interval. Total estrogen was calculated as the area under the curve (AUC) for daily E1G over the interval from the 15th of January to the 14th of July of each year. If more than 30 days were missing data, the interval was excluded. If fewer than seven consecutive days were missing, AUC was interpolated. Where more than seven consecutive days were missing or there were missing data at either end, AUC was computed without that segment, and the final result was weighted upward to represent the full 181 day span. We also used daily E1G data to estimate baseline (5th percentile) and peak (95th percentile) E1G levels for the six-month intervals. The total number of days of unopposed estrogen for each interval was estimated by summing the individual days of unopposed E1G across the interval. For intervals with fewer than 181 days of complete data, the sums were weighted upward to represent counts over 181 days.
We used linear mixed effects models to assess whether AUC, baseline, peak, and unopposed estrogen differed by stage. Models had two levels: individual, and within-individual. Individual was considered a random effect; fixed effects comprised stage, age at study entry and within-subject longitudinal aging (difference between age at study entry and age at beginning of 6 month interval). Analyses were performed on logged values of E1G.
RESULTS
One hundred fifty-six women ranging in age from 26 to 58 years participated in BIMORA. Fifty-three women participated for the full 30 months (five 6-month study intervals); the average length of participation was 21 months (37). For this paper, data were excluded for 1) participants with no uterus; 2) three months following exogenous hormone use, pregnancy, breastfeeding, miscarriage, major surgery, chemotherapy, or use of any medications known to affect reproductive hormone or menstrual bleed patterns; and 3) ambiguous bleed or cycle day information. The resulting sample included 108 women, 64,671 woman-days of observation and 359 six-month intervals.
Reproductive Stage
The CV method for assigning stage is very similar to the standard deviation method used by Lisabeth et al (45) for classifying women into stages; comparison of the two methods using our data yielded 92% agreement in assigning the same discrete stages based on cycle length variation.
Although mean age increased across stages, each stage had a broad and similar range of ages represented (Table 2).
Table 2.
Age and E1G (mean ± SD) for 6month intervals by stage
| Stage –3 | Stage-2 | Mixed Stage | Stage –1 | Stage +1 | |
|---|---|---|---|---|---|
| (n=103)1 | (n=17) | (n=34) | (n=71) | (n=134) | |
| Age at beginning of interval | 41.8 ± 6.5 | 47.5 ± 4.2 | 47.9 ± 6.7 | 51 ± 4.2 | 56.5 ± 3 |
| E1G AUC ((pg/L)*# days) | 6495 ± 1846 | 7332 ± 2413 | 5376 ± 1713 | 4362 ± 2274 | 1479 ± 680 |
| 5th percentile (pg/L) | 13.7 ± 4.6 | 13.9 ± 6.4 | 9.5 ± 4.8 | 7.0 ± 3.4 | 4.6 ± 1.7 |
| 95th percentile (pg/L) | 71.2 ± 22.9 | 83 ± 28.3 | 64.2 ± 21.4 | 58.6 ± 32.2 | 14.2 ± 10.3 |
n= number of six-month intervals in the stage
Total, Baseline and Peak Estrogen
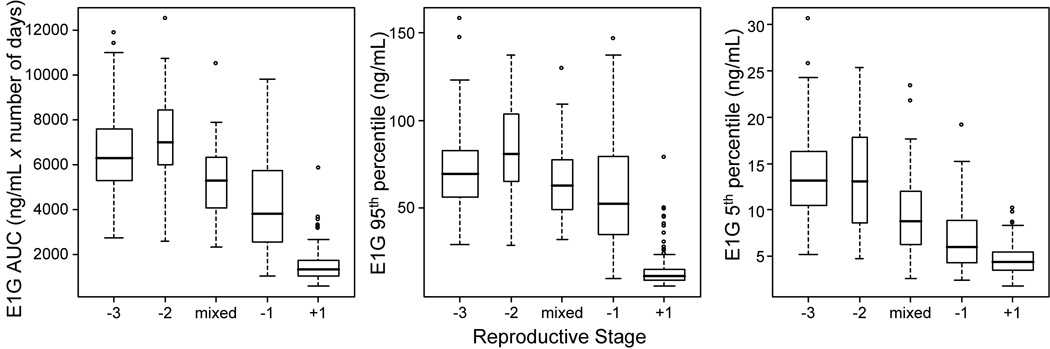
Participant age, and mean (±SD) values for E1G AUC, 5th and 95th percentiles are shown for each stage in Table 2. Total (AUC), baseline (5th percentile), and peak (95th percentile) E1G varied by stage, adjusting for age (p <.0001 for all 3 models) (Figure 1). E1G AUC was higher for stage −3 than for stages −1 (p=.0013) and +1 (p<.0001); there were no differences in AUC among −3, −2 and mixed stages (Figure 1). There were differences (p<.0001) in 5th and 95th E1G percentiles by stage while adjusting for cross-sectional (age at beginning of interval) and longitudinal age (time in study). Mean peak E1G was higher in stage −3 than stage +1 (p<.0001), and mean baseline E1G was higher in stage −3 than the −1 and +1 stages (p<.0001 for each). There were no significant differences in mean peak or total E1G between stage −3 and the transition stages combined (−2, mixed, −1). Baseline E1G was higher in stage −3 than the transition stages combined (p<.001) (Figure 1).
Figure 1.
Boxplot of 6 month E1G AUC (left panel), E1G 95th percentile (middle panel) and E1G 5th percentile (right panel) by stage. Box width is proportional to the number of intervals in a stage.
There was a significant interaction between subject age and stage for total and peak E1G (p<.0001), with a much weaker result for baseline E1G (p=.032). In general, even after adjusting for age at study entry, E1G tended to increase with age in stage −3 but decrease with age in stage −1 (Figure 2).
Figure 2.
Trellis plots of 6 month means of E1G AUC by age for stages −3, −2, −1, +1 and mixed. Each line represents a single participant.
The above findings, that average EIG AUC and average baseline E1G did not decline until late in the transition (stage −3), while average peak E1G did not decline until the postmenopause (stage +1), were unchanged with the addition of BMI to the models.
Unopposed Estrogen
Figure 3 shows typical examples of six months of E1G, PDG, and menses for participants in stage −3 (panel A), −2 (panel B), −1 (panel C), and +1 (panel D). In pre-menopausal women the days of unopposed estrogen cluster tightly in the mid to late follicular phase. Anovulatory cycles and prolonged periods of follicular development contribute to days of unopposed estrogen in stage −2 women. In stage −1 prolonged follicular growth and estrogen secretion, with much of it unopposed by progesterone, is typical. Panel D is from a recently (within the two previous years) menopausal participant with low E1G and PDG but a clear period of follicular development not followed by ovulation or a menses.
Figure 3.
Illustrative examples of six months of E1G, PDG and menses for four participants in reproductive stages −3 (panel A; 45 yrs old), −2 (panel B; 47 years old), −1 (panel C; 51 years old), and +1 (panel D; 53 years old).
Two of the 359 intervals were excluded because more than 30 days of data could not be scored for unopposed estrogen. Eighty two percent (110) of intervals in stage +1 had no days of unopposed estrogen, while other stages always had days of unopposed estrogen. We therefore focused analysis on the other stages, comprising 223 intervals from 78 women. We found no differences in mean total days of unopposed estrogen (TDUE) among these stages (p=.10). However, mean TDUE for stage −2 and the mixed stage were higher than mean TDUE for stage −3 (Figure 4). There was no significant difference between mean TDUE for stages −3 and −1, which may be partially a result of the large between- and within-subject variability of TDUE in stage −1 (Figure 5). When the transitional stages (−2, mixed, −1) were combined into a single category, TDUE was higher in the transition compared to stage −3 (p=.027) (Figure 4). In each of these models there was significant interaction between stage and longitudinal aging (p<.0001), with TDUE tending to increase with age in stage−3, and decrease with age in stages −2 and −1 (Figure 5). The results were unchanged by the addition of BMI to the models.
Figure 4.
Boxplot of 6 month number of days of unopposed estrogen by stage. Box width is proportional to the number of intervals in each stage.
Figure 5.
Trellis plot of total days of unopposed estrogen by age for stages −3, −2, −1, and mixed. Each line represents a single participant.
DISCUSSION
Our results show that reproductive stage, as assessed by a running CV of menstrual cycle length, is an important correlate of a woman’s total E1G: women of the same age can have quite different E1G profiles if they are in different stages. In previous work, we showed that E1G increased with age until the mid- to late-40’s (37); here we show that this trend is characteristic of stage −3, and that E1G decline with age is typical in stage −1 (Figure 2). Total E1G levels as assessed by AUC were similar and at their highest in −3, −2 and mixed stages; they did not decline until the late transition, stage −1 (Figure 1). The low E1G levels in stage −1 are largely attributable to long periods of no ovarian activity in the longer cycles characteristic of this stage (44). Peak E1G levels did not decline until the post menopause (stage +1); baseline E1G levels declined across each stage from −3 or −2 to the mixed stage, to the −1 stage and still further to stage +1 (Figure 1).
Other studies have examined estrogen indicators by reproductive stage, but they used different stage definitions or hormone measures, rendering comparisons difficult. Overall, the results of our study and others (25) suggest that total and peak estrogen levels do not increase across the transition to menopause, when the transition is defined by cycle-length variability, and do not begin to decline until late in the menopausal transition. Thus, one would predict that cancer risk related to total or peak estrogen exposure would not change with the timing of onset of, or be directly related to the duration of, the menopausal transition. Hormone levels clearly vary by reproductive stage, and cannot be predicted using age alone, so this relationship must be regarded as an important area for future research. Indeed, this was one of the reasons the STRAW system was developed (38).
Total level of estrogen is a risk factor for breast cancer in premenopausal (6) and postmenopausal (46, 47) women, with higher levels associated with higher risk. Similarly, long term exposure to elevated estrogen is a risk factor for ovarian cancer (7, 48). Lifetime exposure to estrogen has long been of epidemiologic interest, and late menopause is considered a major risk factor for endometrial, ovarian and breast cancer (48). The mechanism by which elevated or prolonged exposure to estrogens contribute to each of these cancers are not clear, and may vary depending upon the target organ (48).
In contrast to total and peak E1G, the number of days of unopposed estrogen increased during the transition to menopause, and remained high even when total and peak E1G began to decline late in the transition (Figure 1, Figure 4 and Figure 5). The number of days of unopposed estrogen exposure did not decline until the postmenopause (stage +1). Exposure to relatively high levels of unopposed estrogen even occurs, albeit at a greatly reduced frequency, in the postmenopausal years (Figure 4 and Figure 5). This evidence of follicular development occurred in seven participants who were tentatively classified as post-menopausal within the previous 1–2 years, and seven other participants whose last bleeds were known to have been 2–8 years prior. Metcalf and colleagues reported similar evidence for post-menopausal follicular development (49).
One other study (50) has characterized unopposed estrogen exposure in premenopausal (defined as regularly cycling and younger than age 40) and perimenopausal (defined as having a spontaneous break in cycle regularity and older than age 40) women. That study used a ratio of estrogen to progesterone to estimate unopposed estrogen exposure (50). The percentage of time spent at high levels of urinary E1G (>50nmol or 70nmol/24hr) with reduced urinary PDG exposure was significantly higher in perimenopausal than premenopausal women. Unusually long cycles (greater than 50 days) were common in perimenopausal women and were associated with prolonged episodes of unopposed estrogen secretion. The differences between the Metcalf measure of unopposed estrogen and ours allows only a rough comparison but the findings are similar: both reveal, first, that there is significant exposure to unopposed estrogen during the perimenopause and second, that both longer and shorter cycles include long periods of unopposed estrogen (Figure 3, panels B and C). To obtain more comparable results, we also used a simple estrogen-to-progesterone ratio. Our results did not change significantly (data not shown). We have important concerns, however, about the ratio as applied to our data: inter-individual variation in PDG and E1G levels (37) confounded the variation associated with stage and age. For some women, the ratio did not capture unopposed estrogen at all, because compared with other women, their PDG tended to be high relative to their E1G (data not shown). An advantage of our approach is that it is independent of absolute hormone levels.
The link between unopposed estrogen and endometrial cancer is well established (13, 51). Relatively low levels of either exogenous or endogenous estrogen trigger endometrial proliferation (51), with proliferation beginning as early as the second day of the cycle and continuing at significant levels until mitotic proliferation begins to decline shortly after ovulation (13). It is not clear at what point there is sufficient progesterone secretion to counteract estrogen’s proliferative effects; but the level and duration of elevated progesterone could be factors influencing endometrial cancer (52). High levels of progesterone may be needed to effectively oppose high levels of estrogens (13). Duration of progesterone exposure may also be important; a minimum of 12–16 days of progestins in oral contraceptives is required to prevent hyperplastic endometrial response (13). Our study did not make a distinction between high and low levels of E1G or PDG, nor did it measure duration of PDG elevation. Consequently, our approach may underestimate the number of days of unopposed estrogen. If this bias is important, and if ovulatory cycles also declined in frequency across the transition, we could be missing an overall decline in number of days of unopposed estrogen from stage −3 to transitional stages. However, even if we corrected for this possible bias, the number of days of unopposed E1G would remain high across the transition, especially late in the transition when total E1G declines.
Our results suggest that the perimenopausal years are an important period of exposure to both unopposed and high estrogen levels. This result, coupled with the epidemiological evidence on lifetime exposure to estrogen, suggests that variation in the amount of time spent in the transition to menopause may be an important correlate or risk factor for ovarian, breast and endometrial cancers. To the best of our knowledge, only one study has reported statistical information on the length of the perimenopause: McKinlay and colleagues give a median of 3.8 years, based on a large prospective study (12). Given the findings presented here, quantifying the length of the perimenopause and how this may vary among women is an important area for future research.
Our results may not be applicable to all women. Our sample is biased toward 1) women who chose not to use either hormone replacement therapy as they went through the menopausal transition or oral contraceptives to prevent pregnancy, and 2) white, middle-class, and college educated women (37). Of particular concern is whether the women who did not participate in all 30 months of the study were in any way different from those for whom there is no missing data. The most common reason for withdrawal from our study was use of HRT, but there were no significant differences in mean age between women who withdrew from the study (for HRT or other reasons) and those who did not (37). There is evidence indicating that breast cancer type and risk may differ between HRT users and neverusers (53); this suggests that cycle length and/or estrogen characteristics may differ between women who did and did not choose to go on HRT. Perimenopausal symptoms leading to HRT use have been associated with reduced estrogen secretion (54), which our and other data (44) show tends to be associated with elongated cycles. Our analyses here may thus be overestimating estrogen exposure across the transition to menopause for some women.
A limitation of our study is that we have only one measure of BMI, taken at the midpoint of the study, and these data were available for only 90 of the 108 women in our analyses. We were able to impute BMI for the year 2000 using data from previous years for the remaining 18 women. BMI is associated with estrogen levels, and it is this link that is believed to explain the association between body weight and cancer risk (8, 55). Studies that have examined BMI change across the transition to menopause report an average annual increase in BMI of 0.2 to 0.4 kg/m2 per year (56, 57). Thus, although adding BMI did not alter the results of our analyses, our midpoint estimate may not be an accurate representation of BMI across the study period.
The cycle length variance we chose as cutoffs for each stage could have been determined in a different manner. Our goal was to operationalize the STRAW system for use with a large collection of cycle data. The advantage of a CV-based approach is that it is sensitive to the mean. Like the method used by Lisabeth and colleagues (45), our approach replicates the spirit of the original STRAW system, designed to be used by women, clinicians, and researchers alike. That reproductive stage is broadly predictive of estrogen exposure is promising for future work examining how other, more nuanced, serial indicators of menstrual cycle length may be associated with hormone levels. This study focused on variability in menstrual cycle length as an indicator of reproductive status. This can be expanded to include an assessment of menstrual cycle length regularity, using an indicator of serial irregularity in time-series data—approximate entropy (ApEn). In previous work using ApEn with TREMIN data on cycles from women older than 40 years of age, we found that both increased variability and greater serial regularity were significant predictors of the onset of menopause (17). A woman’s lifetime history of menstrual cycle variability and stability may also be an important predictor of hormone status across the transition to menopause; in recent work on a cohort of TREMIN women we identified five categories of women’s menstrual histories based on variability and stability, and found that taxonomic category was associated with age at menarche, number of births and age at menopause (58). For the BIMORA women we have up to 30 years of reproductive histories which we are linking with the hormone data to explore how hormone levels across the transition may be associated with a woman’s lifetime history of more stable or more erratic patterns of menstrual cycle lengths.
CONCLUSIONS
We found that first, E1G levels increased with age in stage −3 women up until the mid to late 40’s; second, E1G AUC did not decline significantly until later in the menopausal transition (stage −1); third, while baseline (5th percentile) E1G levels declined across the transition, peak (95th percentile) levels did not decline until the post menopause; and fourth, the total number of days of unopposed estrogen exposure were higher in the transition than before it, and remained high until the post menopause. Given the length of time women spend in the transition to menopause, they are exposed to more total and unopposed estrogen than has been previously appreciated.
ACKNOWLEDGMENTS
This research was supported by NIH (NIA RO1 AG15141, NICHD HD34159, NIA 5 T32 AG00208, NICHD 2 P30 HD28263 and NICHD F32 HD 07994-02), the Population Research Institute of Pennsylvania State University, the Center for Studies in Demography and Ecology of the University of Washington, and the Center for Population Health, Georgetown University. Thanks to SH Barsom, PK Mansfield, JW Wood and B Trumble for their contributions to this work. Profound gratitude is extended to the BIMORA participants for their dedicated contributions.
APPENDIX
Scoring Cycles for Days of Unopposed Estrogen
Definitions
FD=follicular development, SR=steroid ratio, PR=PDG ratio, C=close of inactive phase (start of E1G secretion)
Rules
Summary: mark all days of SR≥ 20 as unopposed EXCEPT the standard case, where we go strictly from (C + 1) to (PR≥3.0-1). The standard case constitutes much of the data, and the other captures most of the exceptions.
-
Look at profile
-
if yes menopausal (no bleeds)
if no evidence of FD (FD≥5 days of increase and decline in E1G), mark all days as opposed.
-
If yes evidence of FD
if no evidence of ovulation, mark all days as unopposed w/in period of FD with SR≥20.
if yes evidence of ovulation then mark all days as unopposed from first day within period of FD where SR ≥ 20 up to the day before the first PR ≥3.0 where ovulation is occurring.
if not menopausal proceed to #2
-
-
If not menopausal, find first day of PR ≥ 3.0
-
if yes PR ≥ 3.0
if first day PR≥3.0 is within the first 5 days of the cycle, ignore and find next time PR≥ 3.0.
-
If there is run of PR≥3.0 (at least 3 days within a five day period) and it is not within the first 5 days of the cycle, and it is not within a very long cycle (long ≥40 days), this is ovulation. Mark the first day where PR ≥ 3.0 and Go to Rule #3.
If a run of PR≥ 3.0 occurs within a very long cycle, consult profile to identify if these are anomalous, anovulatory or ovulatory. Mark all days to end of cycle with SR≥ 20 as unopposed.
If there are multiple days scattered through cycle where PR≥ 3.0 (statistical artifact of long cycles with inactive phases), visually assess where ovulation is occurring and identify the closest PR≥ 3.0 to close the period of unopposed estrogen. If cycle is anovulatory (ovul2=−1) mark all days up to end of cycle with SR≥20 as unopposed.
-
if no PR ≥ 3.0
-
If there is only one or no days of PR ≥ 3.0 outside of the first five days of the cycle, & cycle is anovulatory (ovul2=−1), & SR is ≥20, mark all days from (C +1) on as unopposed as long as SR≥20.
If no C use all days where SR>20.
For right censored cases, mark to end of cycle if SR ≥ 20.
If ovulation is present in graph but PR doesn’t pick it up, use all days where SR ≥20 up to day before ovulation on graph.
-
-
-
If yes C has a value, mark all days unopposed from (C+1) to (PR marked day-1), regardless of SR values.
If C is a negative number, assign first cycle day as beginning of unopposed estrogen.
C cannot come after PR>− 3.0. Visually assess and use alternative criteria (SR).
-
Check graph re C→
Left censored cycles are a place where this commonly occurs. If SR≥ 20, record as unopposed E.
This will catch unopposed estrogen that is from a previous cycle but is not linked to the current cycle’s C. If SR≥ 20, record as unopposed E.
If no C value mark all days SR≥20 as unopposed up to day before PR≥3.0. If no PR≥3.0 see 2b.
Ignore C if no hormone data for a day; don’t score cycle--it probably has no unopposed E days.
If cycle is missing more than 10 days of data, with most missing days consecutive, do not score.
Right and left censored cycles can be scored using above rules in conjunction with viewing the profile.
Check graphs when scoring cycles. For cases that do not fit into above, score visually and/or use SR criteria.
References Cited
- 1.Surveillance, Epidemiology and End Results (SEER) Program (www.seer.cancer.gov), SEER*Stat Database: Incidence-SEER 17 Regs Limited-Use, Nov 2006 Sub (2000–2004), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released 2007, based on the November 2006 submission.
- 2.Henderson BE, Ross RK, Pike MC, Casagrande JT. Endogenous hormones as a major factor in human cancer. Cancer Res. 1982;42:3232–3239. [PubMed] [Google Scholar]
- 3.Key TJ. Hormones and cancer in humans. Mutat Res. 1995;333:59–67. doi: 10.1016/0027-5107(95)00132-8. [DOI] [PubMed] [Google Scholar]
- 4.Ursin G, Wilson M, Henderson BE, et al. Do urinary estrogen metabolites reflect the differences in breast cancer risk between Singapore Chinese and United States African-American and white women? Cancer Res. 2001;61:3326–3329. [PubMed] [Google Scholar]
- 5.Pike MC, Pearce CL, Peters R, Cozen W, Wan PC, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertil Steril. 2004;82:186–195. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 7.Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomarkers Prev. 2005;14:98–107. [PubMed] [Google Scholar]
- 8.Persson I. Estrogens in the causation of breast, endometrial and ovarian cancers - evidence and hypotheses from epidemiological findings. J Steroid Biochem Mol Biol. 2000;74:357–364. doi: 10.1016/s0960-0760(00)00113-8. [DOI] [PubMed] [Google Scholar]
- 9.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 10.Henderson BE, Ross R, Bernstein L. Estrogens as a cause of human cancer: the Richard and Hinda Rosenthal Foundation award lecture. Cancer Res. 1988;48:246–253. [PubMed] [Google Scholar]
- 11.Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 12.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Am J Hum Biol. 1992;4:37–46. doi: 10.1002/ajhb.1310040107. [DOI] [PubMed] [Google Scholar]
- 13.Hale GE, Hughes CL, Cline JM. Endometrial cancer: hormonal factors, the perimenopausal “window of risk,” and isoflavones. J Clin Endocrinol Metab. 2002;87:3–15. doi: 10.1210/jcem.87.1.8132. [DOI] [PubMed] [Google Scholar]
- 14.Pike MC. Age-related factors in cancers of the breast, ovary, and endometrium. J Chronic Dis. 1987;40(Suppl 2) doi: 10.1016/s0021-9681(87)80009-7. 59S-69S. [DOI] [PubMed] [Google Scholar]
- 15.Sherman BM, Koreman SG. Inadequate corpus luteum function: a pathophysiological interpretation of human breast cancer epidemiology. Cancer. 1974;33:1306–1312. doi: 10.1002/1097-0142(197405)33:5<1306::aid-cncr2820330515>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Park SJ, Goldsmith LT, Weiss G. Age-related changes in the regulation of luteinizing hormone secretion by estrogen in women. Exp Biol Med. 2002;227:455–464. doi: 10.1177/153537020222700709. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein M, Gorrindo T, Riley A, et al. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol. 2003;158:782–791. doi: 10.1093/aje/kwg223. [DOI] [PubMed] [Google Scholar]
- 18.Prior JC. Perimenopause: The complex endocrinology of the menopausal transition. Endocrine Reviews. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 19.Klein NA, Soules MR. Endocrine changes of the perimenopause. Clin Obstet Gynecol. 1998;41:912–920. doi: 10.1097/00003081-199812000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Santoro N. The menopausal transition. Am J Med. 2005;118(Suppl 12B):8–13. doi: 10.1016/j.amjmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Shideler SE, DeVane GW, Kaira PS, Benirschke K, Lasley BL. Ovarian-pituitary hormone interactions during the perimenopause. Maturitas. 1989;11:331–339. doi: 10.1016/0378-5122(89)90029-7. [DOI] [PubMed] [Google Scholar]
- 22.Santoro N, Adel T, Skurnick JH. Decreased inhibin tone and increased activin A secretion characterize reproductive aging in women. Fertil Steril. 1999;71:658–662. doi: 10.1016/s0015-0282(98)00529-9. [DOI] [PubMed] [Google Scholar]
- 23.Santoro N, Brown JR, Adel T. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 24.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105–111. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- 25.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92:3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 26.Sherman BM, West JH, Korenman SG. The menopausal transition: analysis of LH, FSH, estradiol, and progesterone concentrations during menstrual cycles of older women. J Clin Endocrinol Metab. 1976;42:629–636. doi: 10.1210/jcem-42-4-629. [DOI] [PubMed] [Google Scholar]
- 27.Burger HG, Dudley E, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84:4025–4030. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 28.Burger HG, Dudley E, Hopper JL, et al. The endocrinology of the menopausal transition: a cross-sectional study of population-based sample. J Clin Endocrinol Metab. 1995;80:3537–3545. doi: 10.1210/jcem.80.12.8530596. [DOI] [PubMed] [Google Scholar]
- 29.Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–1561. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 30.Overlie I, Moen MH, Morkrid L, Skjaeraasen JS, Holte A. The endocrine transition around menopause--a five years prospective study with profiles of gonadotropines, estrogens, androgens and SHBG among healthy women. Acta Obstet Gynecol Scand. 1999;78:642–647. [PubMed] [Google Scholar]
- 31.Hall Moran V, Leathard HL, Coley J. Urinary hormone levels during the natural menstrual cycle: the effect of age. J Endocrinol. 2001;170:157–164. doi: 10.1677/joe.0.1700157. [DOI] [PubMed] [Google Scholar]
- 32.Reyes FI, Winter JS, Faiman C. Pituitary-ovarian relationships preceding the menopause. I. A cross-sectional study of serum follice-stimulating hormone, luteinizing hormone, prolactin, estradiol, and progesterone levels. Am J Obstet Gynecol. 1977;129:557–564. [PubMed] [Google Scholar]
- 33.Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88:5502–5509. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 34.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81:1038–1045. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 35.Reame NE, Wyman TL, Phillips DJ, de Kretser DM, Padmanabhan V. Net increase in stimulatory input resulting from a decrease in inhibin B and an increase in activin A may contribute in part to the rise in follicular phase follicle-stimulating hormone of aging cycling women. J Clin Endocrinol Metab. 1998;83:3302–3307. doi: 10.1210/jcem.83.9.5130. [DOI] [PubMed] [Google Scholar]
- 36.Bjornerem A, Straume B, Midtby M, et al. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromso Study. J Clin Endocrinol Metab. 2004;89:6039–6047. doi: 10.1210/jc.2004-0735. [DOI] [PubMed] [Google Scholar]
- 37.Ferrell RJ, O'Connor KA, Rodríguez G, et al. Monitoring Reproductive Aging in a Five Year Prospective Study: Aggregate and Individual Changes in Steroid Hormones and Menstrual Cycle Lengths with Age. Menopause. 2005;12:567–577. doi: 10.1097/01.gme.0000172265.40196.86. [DOI] [PubMed] [Google Scholar]
- 38.Soules MR, Sherman S, Parrott E, et al. Executive Summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 39.Mansfield PK, Bracken SL. TREMIN: A History of the World's Oldest Ongoing Study of Menstruation and Women's Health. Lemont, PA: East Rim Publishers; 2003. [Google Scholar]
- 40.O'Connor KA, Brindle E, Holman DJ, et al. Urinary estrone conjugate and pregnanediol-3-glucuronide enzyme immunoassays for population research. Clin Chem. 2003;49:1139–1148. doi: 10.1373/49.7.1139. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor KA, Brindle E, Shofer JB, et al. Statistical correction for non-parallelism in a urinary enzyme immunoassay. J Immunoassay Immunochem. 2004;25:259–278. doi: 10.1081/ias-200028078. [DOI] [PubMed] [Google Scholar]
- 42.Miller RC, Brindle E, Holman DJ, et al. Comparison of specific gravity and creatinine methods for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50:924–932. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor KA, Brindle E, Miller RC, et al. Ovulation detection methods for urinary hormones: precision, daily and intermittent sampling and a combined hierarchical method. Hum Reprod. 2006;21:1442–1452. doi: 10.1093/humrep/dei497. [DOI] [PubMed] [Google Scholar]
- 44.Miro F, Parker SW, Aspinall LJ, Coley J, Perry PW, Ellis JE. Origins and consequences of the elongation of the human menstrual cycle during the menopausal transition: The FREEDOM study. J Clin Endocrinol Metab. 2004;89:4910–4915. doi: 10.1210/jc.2003-031731. [DOI] [PubMed] [Google Scholar]
- 45.Lisabeth L, Harlow S, Qaqish B. A new statistical approach demonstrated menstrual patterns during the menopausal transition did not vary by age at menopause. J Clin Epidemiol. 2004;57:484–496. doi: 10.1016/j.jclinepi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 47.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 48.Henderson BE, Bernstein L, Ross R. Etiology of cancer: hormonal factors. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott-Raven; 1997. pp. 219–229. [Google Scholar]
- 49.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function before, during, and after the menopause: a longitudinal study. Clin Endocrinol. 1982;17:489–494. doi: 10.1111/j.1365-2265.1982.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 50.Metcalf MG, Mackenzie JA. Menstrual cycle and exposure to oestrogens unopposed by progesterone. J Endocrinol. 1985;104:137–141. doi: 10.1677/joe.0.1040137. [DOI] [PubMed] [Google Scholar]
- 51.Key TJA, Pike MC. The dose-effect relationship between “unopposed” oestrogens and endometrial mitotic rate. Br J Cancer. 1988;57:205–212. doi: 10.1038/bjc.1988.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pike MC, Ross RK. Progestins and menopause: epidemiological studies of risks of endometrial and breast cancer. Steroids. 2000;65:659–664. doi: 10.1016/s0039-128x(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 53.Brewster AM, Do KA, Thompson PA, et al. Relationship between epidemiologic risk factors and breast cancer recurrence. J Clin Oncol. 2007;25:4438–4444. doi: 10.1200/JCO.2007.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss G, Skurnick JH, Goldsmith LT, Santoro N, Park SJ. Menopause and Hypothalamic-Pituitary Sensitivity to Estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 55.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 56.Wing RR, Matthews KA, Kuller LH, Meilahn EN, Plantinga PL. Weight gain at the time of menopause. Arch Intern Med. 1991;151:97–102. [PubMed] [Google Scholar]
- 57.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895–901. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gorrindo T, Lu Y, Pincus S, et al. Lifelong menstrual histories are typically erratic and trending: a taxonomy. Menopause. 2007;14:74–88. doi: 10.1097/01.gme.0000227853.19979.7f. [DOI] [PubMed] [Google Scholar]