Abstract
Non-alcoholic fatty liver disease (NAFLD) is a burgeoning problem. We have previously shown that Hispanics were at greater risk for NAFLD than African-Americans despite a similar prevalence of risk factors between these groups. We have performed the largest, population-based study to date (n=2,170) utilizing proton magnetic resonance (MR) spectroscopy, dual-energy x-ray absorptiometry, and multi-slice abdominal MR imaging to determine the contribution of body fat distribution to the differing prevalence of hepatic steatosis in the three major U.S. ethnic groups (African-American, Hispanic, Caucasian). Despite controlling for age and total adiposity, African-Americans had less intraperitoneal (IP) fat and more lower extremity (LE) fat than their Hispanic and Caucasian counterparts. The differences in hepatic triglyceride content (HTGC) between these groups remained after controlling for total, abdominal subcutaneous, and LE adiposity; however, controlling for IP fat nearly abolished the differences in HTGC, indicating a close association between IP and liver fat regardless of ethnicity. Despite the lower levels of IP and liver fat in African-Americans, their prevalence of insulin resistance was similar to Hispanics, who had the highest levels of IP and liver fat. Furthermore, insulin levels and HOMAIR values were highest and serum triglyceride levels were lowest among African-Americans after controlling for IP fat. In conclusion, IP fat is linked to HTGC, irrespective of ethnicity. The differing prevalence of hepatic steatosis between these groups was associated with similar differences in visceral adiposity. The metabolic response to obesity and insulin resistance differs in African-Americans when compared to either Hispanics or Caucasians: African-Americans appear to be more resistant to both the accretion of triglyceride in the abdominal visceral compartment (adipose tissue and liver) and hypertriglyceridemia associated with insulin resistance.
Keywords: fatty liver, ethnic groups, African-Americans, Hispanic Americans, obesity, body fat distribution, abdominal fat, intra-abdominal fat, abdominal subcutaneous fat, adiposity, insulin resistance, dyslipidemia, hypertriglyceridemia, metabolic syndrome X
BACKGROUND
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of disorders defined by abnormal accumulation of triglyceride in liver (1). It is estimated that ~71 million individuals in the U.S. over the age of 18 have hepatic steatosis (Census 2000)(2), with 20% of all office visits for newly diagnosed chronic liver disease being related to NAFLD (3). These data indicate the increasing importance of this disease with regard to morbidity and healthcare expenditures in the US. Previously, our group (2,5) and others (4) reported a significant difference in the prevalence of NAFLD between ethnicities (4, 5). Using proton magnetic resonance spectroscopy (1H-MRS), we measured hepatic triglyceride content (HTGC) in a large, multiethnic, population-based study, the Dallas Heart Study (DHS) (2). We found that Hispanics had the highest prevalence of hepatic steatosis followed by Caucasians and then African-Americans and that these differences were not attributable to differences in body mass index, insulin sensitivity, or ethanol ingestion (2). These findings have been replicated and expanded in other studies, suggesting that African-Americans tend not to develop progressive disease (steatohepatitis) even when NAFLD is present (3, 6, 7). Why African-Americans are less prone to develop hepatic steatosis and its sequelae is currently unknown but may be important in delineating individuals who are at risk for development and progression of NAFLD.
The pathogenesis of NAFLD is a complex process thought to be linked to insulin resistance/metabolic syndrome (8, 9). As with hepatic steatosis, the frequency of this syndrome and its components (abdominal obesity, atherogenic dyslipidemia, increased blood pressure, and a proinflammatory state (10)), also demonstrate significant ethnic variability. In particular, African-Americans are less likely to develop visceral adiposity and atherogenic dyslipidemia (high triglycerides, low HDL-C) than Hispanics or Caucasians and also have the lowest age-specific prevalence of metabolic syndrome of these groups (11–13). However, the frequency of insulin resistance among African-Americans is similar to that of Hispanics and greater than that of Caucasians (2). The degree to which this phenotypic variation in metabolic syndrome accounts for the variation in frequency of hepatic steatosis among these groups is currently not known.
Here we examine the relationship between HTGC and body fat distribution in African-American, Hispanic, and Caucasian DHS participants (14). This report represents the first population-based study (n=2,170) to use a combination of 1H-MRS, dual energy x-ray absorptiometry (DEXA), and multi-slice abdominal magnetic resonance imaging (MRI) to simultaneously determine HTGC and body composition/distribution in a multiethnic population (1,058 African-Americans, 393 Hispanics, and 719 Caucasians). Our goal was to determine if ethnic differences in the relationship between these and additional metabolic factors contribute to the differing prevalence of hepatic steatosis in the three major U.S. ethnic groups.
METHODS
Subjects
The DHS is a multi-ethnic, probability-based sample designed to examine ethnic differences in cardiovascular risk (14). Sampling weights were used in the DHS such that African-Americans were oversampled (~50% of DHS population) while maintaining the ability of the study population to reflect the characteristics of Dallas County, TX (14). The DHS participants included in this study were those who underwent 1H-MRS (for HTGC measurement), DEXA (for body fat quantitation), and multi-slice abdominal MRI (for abdominal subcutaneous and visceral fat quantitation) (n=2,971). Each participant in the DHS completed a 60-min structured questionnaire that provided detailed information regarding the demographics, medications, and ethanol intake of each subject. Alcohol consumption (g/d) was determined from responses to previously validated questions (15). The study was approved by the institutional review board (UT Southwestern) and all subjects provided written informed consent prior to participation.
A total of 2,971 participants from the DHS completed the final clinic visit and constituted the entire DHS cohort. Of these subjects, 2,349 underwent 1H-MRS and MRI. Some DHS study participants failed to complete the MR studies for the following reasons: claustrophobia (n=191), medical contraindications (n=49), equipment failure (n=19), refusal (n=74), and scheduling conflicts (n=289). Of the 2,349 1H-MRS measures obtained, 2,287 subjects had spectra of sufficient quality (i.e., without significant motion artifact) to determine HTGC. Of these subjects, 2,270 also had multi-slice abdominal MRI of sufficient quality to determine abdominal fat mass. Finally, of the 2,270 subjects with suitable MR data for analysis, 2,228 also underwent body composition analysis by DEXA, constituting the final study population. Due to the weight limitations of the Gyroscan Intera table, the prevalence of obesity was lower in the study group (43%) than in the DHS sample at the final clinic visit (47%), reflected by a slightly lower mean body mass index (BMI) and waist circumference in the study group. All other demographic and clinical variables were similar between the two groups.
Blood Samples
All study participants underwent phlebotomy after an overnight fast. A total of 40 mL of blood was collected in tubes containing a serum separator or citrate-EDTA and maintained at 4°C for <4 h prior to processing. The tubes were centrifuged (1,000 g for 15 min at 4°C), and plasma was isolated. Serum chemistries were performed within 24 hours. Fasting serum insulin levels were measured by Linco Research Incorporated (St. Charles, MO). The homeostasis model assessment (HOMAIR) was calculated from fasting values of insulin and glucose (16). Insulin resistance was defined as the top quartile of HOMAIR in non-diabetic, normoglycemic DHS subjects (17).
Spectroscopic and Imaging Studies
Proton-MRS
Localized 1H-NMR spectra of the liver were acquired with subjects in the supine position using a 1.5T Gyroscan Intera MR system (Philips Medical Systems, The Netherlands), as previously described (18). Sagittal, coronal and axial slices through the right lobe of the liver were acquired and a 27 cm3 volume of interest was positioned, avoiding major blood vessels, intra-hepatic bile ducts, and the lateral margins of the liver. After the system was tuned and shimmed, spectra were collected using a Q-body coil for radio frequency transmission. The HTGC was calculated as a ratio of methylene and combined methylene and water signals corrected for spin-spin relaxation and is expressed as weight percent (g triglyceride per 100 g wet liver tissue) via methods previously validated in humans and animals (19, 20). Hepatic steatosis was defined as the 95th percentile of liver triglyceride content in a group of low-risk DHS participants (>5.5%) (21).
Dual Energy X-ray Absorptiometry (DEXA)
Fat mass (kg), fat-free mass (kg), and bone mineral mass (kg) in the total body, trunk, lower body, and upper extremities were measured using DEXA scanning, as previously described (22).
Multi-Slice Abdominal Magnetic Resonance Imaging (MRI)
The clinical magnet used to obtain 1H-NMR spectra was also used for the multi-slice MRI studies. The abdominal region (diaphragm to pelvis) was scanned using contiguous axial 10-mm slices, as previously described (23). Within each slice, the volume of fat as well as the locality of fat (subcutaneous, intraperitoneal, retroperitoneal) was measured. The calculated volume of each abdominal fat region was then converted to a weight (kg) based upon the known composition and density of adipose tissue.
Statistics
Continuous demographic characteristics are presented as medians with interquartile range and dichotomous characteristics are presented as relative frequencies. Comparisons between ethnicities and genders were done using the Kruskal-Wallis test and subsequent pair-wise comparisons used Dunn's test. For dichotomous characteristics, Fischer's exact test was used for comparisons. Analysis of Covariance (ANCOVA) was employed to compare continuous variables between ethnicities while controlling for biologically important covariates. In these analyses, log-transformations were used for variables that were skewed in order to meet assumptions. The aptness of ANCOVA was further established by non-significant (P>0.05) interactions between independent variables, and the results are reported as adjusted geometric means ± SEM. Subsequent multiple comparisons of means were computed using Tukey's test. In cases of significant interactions, the slopes between regression lines were compared using Tukey's test. Spearman partial correlation coefficients were used to assess the relationship between continuous variables while simultaneously controlling for covariates. Statistical significance was taken at P < 0.05. Statistical analysis was carried out using SAS 9.1.3 software (SAS Institute Inc., Cary, NC, USA.)
RESULTS
Study Population
A total of 490 Hispanics, 1500 African-Americans, and 923 Caucasians participated in the third and final visit of the DHS in which imaging and spectroscopic studies were performed. At this visit, 1,058 African-Americans (474 men; 584 women), 393 Hispanics (169 men; 224 women) and 719 Caucasians (364 men; 355 women) underwent body composition assessment by DEXA scan, abdominal compartment fat quantitation by multi-slice MRI, and hepatic triglyceride determination by 1H-MRS. The characteristics of this population are similar to those reported previously and are presented in Table 1 (2, 21, 22). Participants from other ethnicities (n=58) were not included in the analysis.
TABLE 1.
Clinical characteristics of the study population after stratification by gender and race.
| Men (n=1,007) | Women (n=1,163) | |||||
|---|---|---|---|---|---|---|
| African-American (n=474) |
Hispanic (n=169) |
Caucasian (n=364) |
African-American (n=584) |
Hispanic (n=224) |
Caucasian (n=355) |
|
| Age (yr) | 45 (38–54) | 40 (33–47)* | 44 (38–52) | 46 (37–54) | 38 (34–48)* | 48 (38–55) |
| Height (cm) | 175 (170–180) | 168 (164–173)* | 178 (173–182) | 162 (158–168) | 154 (151–159)* | 163 (158–168) |
| Weight (kg) | 85 (74–101) | 82 (73–93)* | 89 (79–98) | 83 (71–97)* | 71 (62–82) | 71 (63–86) |
| HTGC (%) | 3.2 (2.0–5.2)* | 4.6 (2.7–11.8) | 4.3 (2.4–8.4) | 3.3 (2.0–5.3) | 4.6 (2.6–9.9)* | 3.0 (1.8–5.2) |
| BMI (kg/m2) | 28 (24–32) | 29 (26–32) | 28 (25–31) | 31 (27–37)* | 30 (26–34)* | 27 (23–32)* |
| Glucose (mg/dL) | 93 (85–106) | 97 (90–103)* | 93 (86–100) | 91 (84–102) | 94 (87–104)* | 90 (83–97) |
| Insulin (uU/mL) | 11 (6–20) | 12 (8–19) | 10 (6–17) | 15 (9–22) | 14 (8–20) | 10 (6–15)* |
| HOMAIR | 2.8 (1.5–5.0) | 3.3 (1.8–4.9) † | 2.3 (1.4–4.2) | 3.5 (2.0–5.4) | 3.3 (1.7–5.5) | 2.1 (1.3–3.5)* |
| CRP | 2.2 (1.1–5.9)† | 1.9 (1.1–3.4) | 1.7 (0.8–3.3) | 4.2 (1.7–9.0) | 4.0 (1.9–8.1) | 2.9 (1.3–6.7)* |
| Triglycerides (mg/dL) | 91 (64–133)* | 130 (84–211) | 122 (81–188) | 81 (60–113)* | 111 (79–158) | 98 (69–144) |
| Lean Mass (kg) | 65.2 (59.1–72.8) | 60.2 (55.3–64.9)* | 63.8 (59.3–70.1) | 49.6 (44.3–55.1) * | 42.4 (38.5–46.7)* | 44.2 (39.8–49.3)* |
| Fat Mass (kg) | 19.2 (13.1–26.6) | 20.3 (16.0–24.6) | 22.0 (17.3–28.1)* | 31.9 (24.5–41.3)* | 27.9 (22.6–33.6) | 26.6 (20.9–36.6) |
| Abdominal (kg) | 5.4 (3.7–7.5)* | 5.9 (4.9–7.4) | 6.4 (4.9–8.0) | 7.5 (5.4–10.2) | 7.1 (5.6–9.0) | 6.0 (4.4–8.5)* |
| Subcutaneous (kg) | 3.2 (2.0–4.6) | 3.2 (2.5–4.0) | 3.5 (2.6–4.4) | 5.7 (3.9–7.9)* | 5.1 (3.8–6.9)* | 4.3 (2.9–6.3)* |
| Intraperitoneal (kg) | 1.3 (.89–1.8)* | 1.7 (1.3–2.1) | 1.7 (1.2–2.2) | 1.1 (.763–1.5) | 1.2 (.907–1.6)* | 1.1 (.700–1.5) |
| Lower Extremity (kg) | 6.4 (4.3–9.0) | 5.8 (4.4–7.2)* | 6.7 (5.2–8.8) | 11.9 (9.4–15.6)* | 9.4 (7.8–11.8) | 10.2 (8.0–13.1) |
| Body Fat (%) | 22.6 (17.6–27.1)* | 24.9 (22.2–28.5) | 25.9 (22.2–29.6) | 38.9 (34.7–43.3) | 40.3 (35.7–42.7) | 38.6 (33.9–42.8) |
| Abdominal (%) | 29.0 (26.2–31.6) | 30.2 (28.2–32.2)* | 28.7 (26.3–31.1) | 23.6 (20.7–26.4)* | 25.7 (23.1–28.2)* | 22.5 (19.9–25.2)* |
| Subcutaneous (%) | 17.2 (14.7–19.6)* | 16.2 (14.5–18.3) | 16.1 (14.1–18.1) | 17.7 (15.4–20.4) | 18.2 (16.2–21.1) | 15.8 (13.6–18.5)* |
| Intraperitoneal (%) | 6.8 (5.4–8.3)* | 8.3 (7.1–10.1)* | 7.4 (5.9–9.0)* | 3.4 (2.6–4.3)* | 4.4 (3.5–5.1)* | 3.8 (3.1–4.7)* |
| Lower Extremity (%) | 33.9 (30.3–37.1)* | 28.4 (26.4–31.2)* | 30.7 (27.4–34.3)* | 39.0 (34.8–43.2) | 34.6 (31.7–39.0)* | 39.1 (34.8–43.3) |
| Obesity (%) ‡ | ||||||
| BMI | 36 | 46* | 33 | 56 | 50 | 32* |
| % Body Fat | 36* | 49 | 56 | 91 | 91 | 86* |
| Hepatic Steatosis (%) ‡ | 22* | 46 | 41 | 24 | 45* | 23 |
Note: values are median with interquartile range unless otherwise specified. Obesity was defined either by a BMI ≥ 30 kg/m2 or a percent body fat > 25% for men and >30% for women.
Abbreviations: body mass index (BMI), homeostasis model assessment (HOMAIR), hepatic triglyceride content (HTGC), total percent body fat (%BF).
Conversions: glucose (mg/dL) × 0.05551=mmol/L; triglyceride (mg/dL) × 0.02586=mmol/L.
Indicates values that are significantly different from the others for that gender group (P<0.05)
Indicates that the value is significantly different from Caucasians within the gender group (P<0.05)
Indicates prevalence values (%).
Fasting glucose levels were higher in Hispanic men and women compared to the others. Levels of fasting insulin and insulin resistance (HOMAIR) did not differ between Hispanics and African-Americans regardless of gender. Caucasians tended to be less insulin resistant than the other ethnic groups, though no difference in HOMAIR was apparent between African-American and Caucasian men (Table 1). Despite levels of insulin resistance among African-Americans that were similar to Hispanics, levels of plasma and hepatic triglycerides were significantly lower in African-Americans. Likewise, insulin resistance among African-Americans was greater than (women) or equal to (men) that observed in Caucasians but was associated with equal (women) or lower (men) levels of intrahepatic triglyceride. Though African-Americans generally had the lowest values of HTGC, this was the group with the highest CRP levels. African-American men had a lower percent body fat (%BF) than Hispanic or Caucasian men; however, no ethnic differences in total adiposity were apparent between the women.
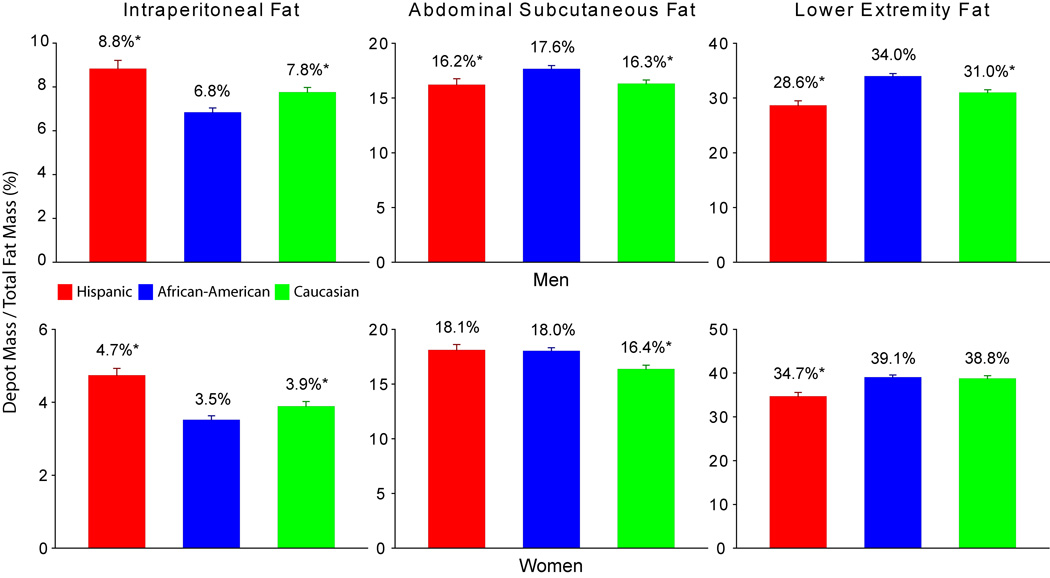
Ethnic and gender specific differences in body fat distribution among the three major fat depots (intraperitoneal, abdominal subcutaneous and lower extremity) were apparent (Table 1) and persisted after adjusting for overall adiposity and age (Figure 1). African-American men tended to have proportionally less intraperitoneal fat and more subcutaneous and lower extremity fat than either Caucasians or Hispanics. African-American women, like their male counterparts, had proportionally less intraperitoneal fat, but amounts of subcutaneous fat that were similar to Hispanics and amounts of lower extremity fat that were similar to the Caucasians. Caucasian women had significantly less abdominal subcutaneous adiposity when compared to the other ethnic groups of similar gender.
Figure 1. Comparisons of the Distribution of Intraperitoneal, Abdominal Subcutaneous, and Lower Extremity Fat Between Hispanics, African-Americans, and Caucasians.
* indicates values are significantly different from African-Americans within the gender group (P<0.01).
The prevalence of hepatic steatosis was similar between Hispanic and Caucasian men and lowest among African-American men, as previously reported (2). Conversely, the prevalence of hepatic steatosis among women was similar between African-Americans and Caucasians and highest among Hispanics.
Irrespective of ethnicity, variations in %BF, body fat distribution, and metabolic variables were evident between the gender groups (Table 2). Men had less %BF, subcutaneous and lower extremity fat, and lower insulin resistance (HOMAIR) than women. However, men had proportionally more intraperitoneal fat and higher levels of plasma and hepatic triglycerides.
TABLE 2.
Body fat distribution and metabolic variables by gender.
| Men (n=1,007) | Women (n=1,163) | |
|---|---|---|
| Total Body Fat (%) | 24.4 (20.3–28.4) | 39.0 (34.5–43.0)* |
| Abdominal (%) | 29.1 (26.5–31.6) | 23.6 (20.8–26.6)* |
| Subcutaneous (%) | 16.6 (14.4–18.7) | 17.3 (14.9–20.0)* |
| Intraperitoneal (%) | 7.3 (5.8–8.9) | 3.7 (2.9–4.6)* |
| Lower Extremity (%) | 31.4 (28.2–35.5) | 38.1 (33.8–42.6)* |
| HTGC (%) | 3.7 (2.2–6.8) | 3.4 (2.0–5.9)† |
| Triglycerides (mg/dL) | 105 (72–168) | 92 (66–130)* |
| HOMAIR | 2.7 (1.5–4.6) | 3.0 (1.7–4.9)† |
Note: values are median with interquartile range unless otherwise specified.
Abbreviations: homeostasis model assessment (HOMAIR), hepatic triglyceride content (HTGC).
Indicates values that are significantly different from men (P<0.01)
Indicates values that are significantly different from men (P<0.05)
Hepatic Triglyceride Content and Total Adiposity
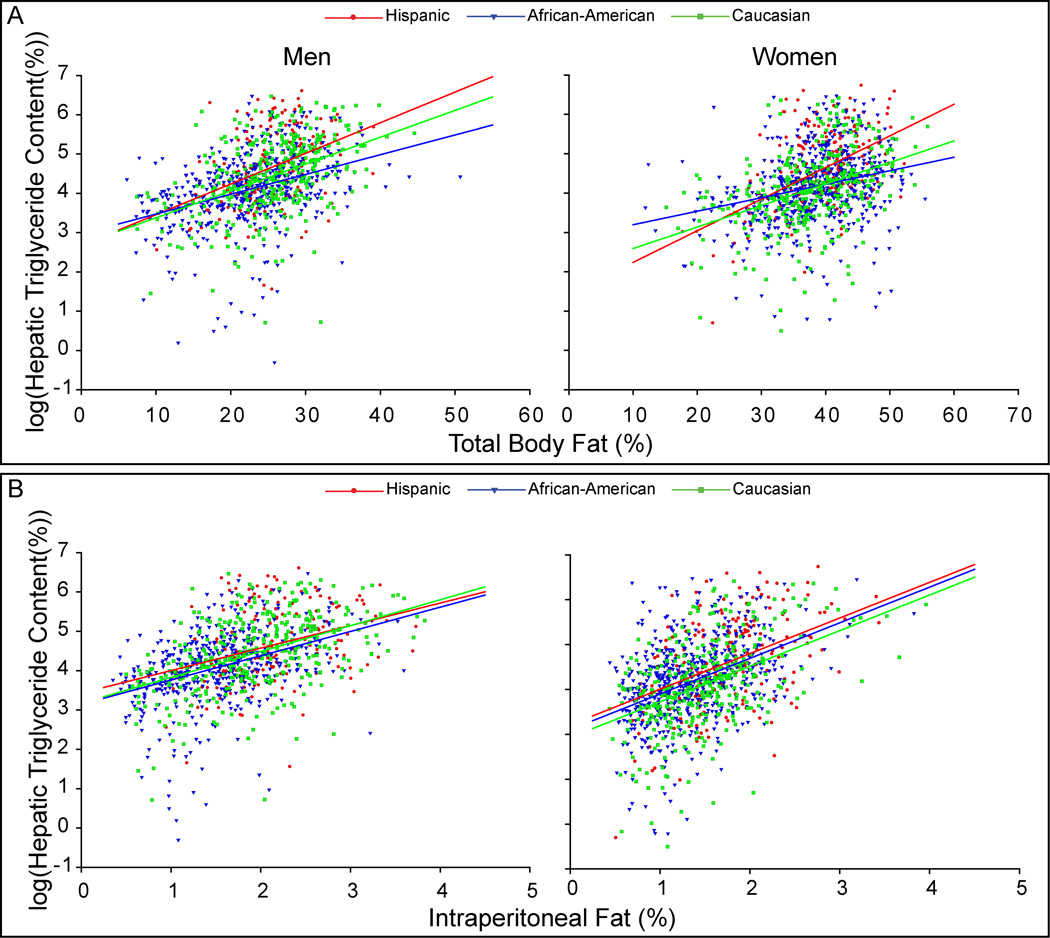
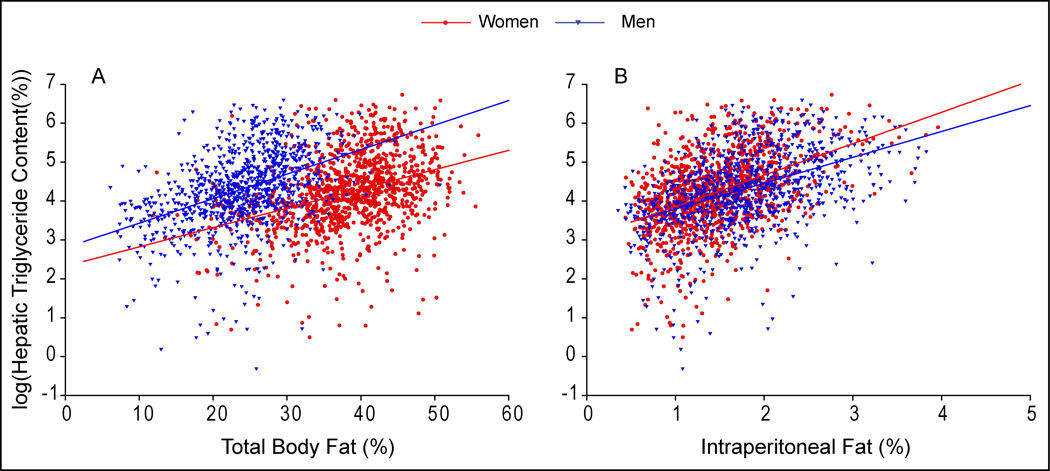
Figure 2A depicts HTGC as a function of percent body fat in men and women. As expected, HTGC increased as a function of adiposity in all groups, but this relationship varied by ethnicity. Despite adjustment for total adiposity, the differences in HTGC observed between the ethnicities remained (Hispanic > Caucasian > African-American). Differences in the relationship between HTGC and total adiposity were most striking among African-American men and women, where liver triglyceride accumulated to a lesser degree with increasing adiposity as compared to Caucasians or Hispanics. Conversely, Hispanics either had higher liver triglyceride levels for any level of %BF (men) or accumulated more HTGC with increasing adiposity (women) than Caucasians. Figure 3A provides the same analysis, but for men and women. Besides the higher levels of HTGC compared to women (Table 2), the slope of the relationship between HTGC and %BF was greater in men indicating an enhanced accumulation of hepatic triglycerides with increasing adiposity.
Figure 2. Relationship Between Hepatic Triglyceride Content and Total/Regional Adiposity.
Hepatic triglyceride content (HTGC) was log transformed to meet assumptions of ANCOVA analysis. As expected, HTGC increased as a function of total and regional adiposity in all groups, but this relationship varied by ethnicity. (A) The plot of HTGC vs. percent body fat (%BF) in men and women. In Hispanic and Caucasian men, the difference in slopes of this relationship was not significant (P=0.539); however, the slope of this relationship trended toward lower values in African-American men compared to the other ethnicities (vs. Hispanic, P=0.067; vs. Caucasian, P=0.054). Hispanics had the highest mean value of HTGC followed by Caucasian then African-American men after adjustment for %BF (P<0.033). Among the women, all ethnicities demonstrated different slopes for this relationship (P<0.025). The greatest slope was observed in Hispanic women followed by Caucasian then African-American women. (B) The plot of HTGC vs. percent intraperitoneal fat (%IP) (intraperitoneal fat mass / total body mass) in men and women. All regression lines had equal slopes and the ethnic-specific adjusted mean values of HTGC were similar among the men (Hispanics 4.2±1.0 vs. Caucasians 3.8±1.0 vs. African-Americans 3.5±1.0 %, P>0.05 for all comparisons). Among the women, all regression lines had equal slopes. The adjusted mean value of HTGC was similar between Hispanic and African-American women once adjusted for %IP (3.9±1.1 vs. 3.5±1.0 %, respectively; P=0.260). However, the mean value of HTGC in Caucasian women was slightly lower than the other ethnicities after adjustment for %IP (3.0±1.1 %, P<0.01). Adjusting HTGC for subcutaneous and lower extremity fat failed to account for the difference in HTGC between these ethnic groups. Indeed, adjustment for subcutaneous fat yielded results similar to adjustment for %BF. Likewise, the slope of the relationship between HTGC and lower extremity fat either approached zero (men) or was not significantly different from zero (women).
Figure 3. Relationship Between Hepatic Triglyceride Content and Total/Regional Adiposity by Gender.
Hepatic triglyceride content (HTGC) was log transformed to meet assumptions of ANCOVA analysis. As expected, HTGC increased as a function of total and regional adiposity in both sexes. (A) The plot of HTGC vs. percent body fat (%BF) in men and women. The slopes of this relationship were significantly different (Slope Men 0.06 vs. Slope women 0.05 P=0.019). (B) The plot of HTGC vs. percent intraperitoneal fat (%IP) (intraperitoneal fat mass / total body mass) in men and women. The slopes of this relationship were significantly different (Slope Men 0.66 vs. Slope women 0.81 P=0.022). Adjustment for subcutaneous fat yielded results similar to adjustment for %BF.
Hepatic Triglyceride Content and Regional Adiposity
The above data indicated that ethnic and gender_differences in HTGC were not driven by differences in total adiposity. We, therefore, sought to determine if the differences noted in fat distribution (Table 1 and Figure 1) contributed to the observed differences in HTGC between these groups. As can be seen by the correlation values presented in Table 3, intraperitoneal and lower extremity adiposity were most strongly correlated with HTGC after adjustment for total adiposity in all groups, though in opposing directions. Figure 2B depicts the plot of HTGC vs. percent intraperitoneal fat (%IP) for men and women. Adjustment for %IP almost completely abolished the differences in HTGC between the groups, though Caucasian women maintained slightly lower values than the other female groups over the entire range of %IP. Adjusting HTGC for both abdominal subcutaneous and lower extremity fat failed to eliminate the observed difference in HTGC between the ethnicities _(data not shown). Despite the near abolition of ethnic differences in HTGC within the two gender groups after controlling for %IP fat, differences between the genders were apparent after a similar analysis (Figure 3B). In contrast to the relationship seen between HTGC and %BF (Figure 3A), women tended to have more HTGC at equal levels of %IP compared to men. Furthermore, women had a greater slope than men, indicating that they accumulate hepatic triglycerides to a greater degree than men with increasing visceral adiposity. These data suggest that intraperitoneal adiposity is related to HTGC to a greater degree than total adiposity or the amount of fat in the other depots, regardless of ethnicity. In addition, the relationship between total adiposity, intraperitoneal adiposity, and liver triglycerides is gender dependent.
TABLE 3.
Correlations between body fat distribution and hepatic triglyceride content.
| Men (n=1,007) | Women (n=1,163) | |||||
|---|---|---|---|---|---|---|
| African-American (n=474) |
Hispanic (n = 169) |
Caucasian (n=364) |
African-American (n = 584) |
Hispanic (n = 224) |
Caucasian (n = 355) |
|
| Total Body Fat (%) | 0.43 | 0.40 | 0.47 | 0.31 | 0.43 | 0.45 |
| Abdominal | 0.49 | 0.42 | 0.54 | 0.45 | 0.50 | 0.53 |
| (0.26) | (0.18) | (0.29) | (0.35) | (0.30) | (0.31) | |
| Intraperitoneal | 0.43 | 0.35 | 0.48 | 0.40 | 0.45 | 0.47 |
| (0.26) | (0.24) | (0.33) | (0.35) | (0.37) | (0.33) | |
| Subcutaneous | 0.40 | 0.32 | 0.37 | 0.39 | 0.41 | 0.46 |
| (0.06*) | (0.03*) | (−0.02*) | (0.26) | (0.14) | (0.17) | |
| Lower Extremity | 0.25 | 0.08* | 0.17 | −0.06* | 0.06* | 0.03* |
| (−0.29) | (−0.40) | (−0.40) | (−0.34) | (−0.28) | (−0.41) | |
Note: Partial Correlation Coefficients adjusted for percent total body fat are presented in parenthesis.
Indicates that correlation coefficients were not significantly different from zero (P> 0.05)
Insulin Resistance and Intraperitoneal Adiposity
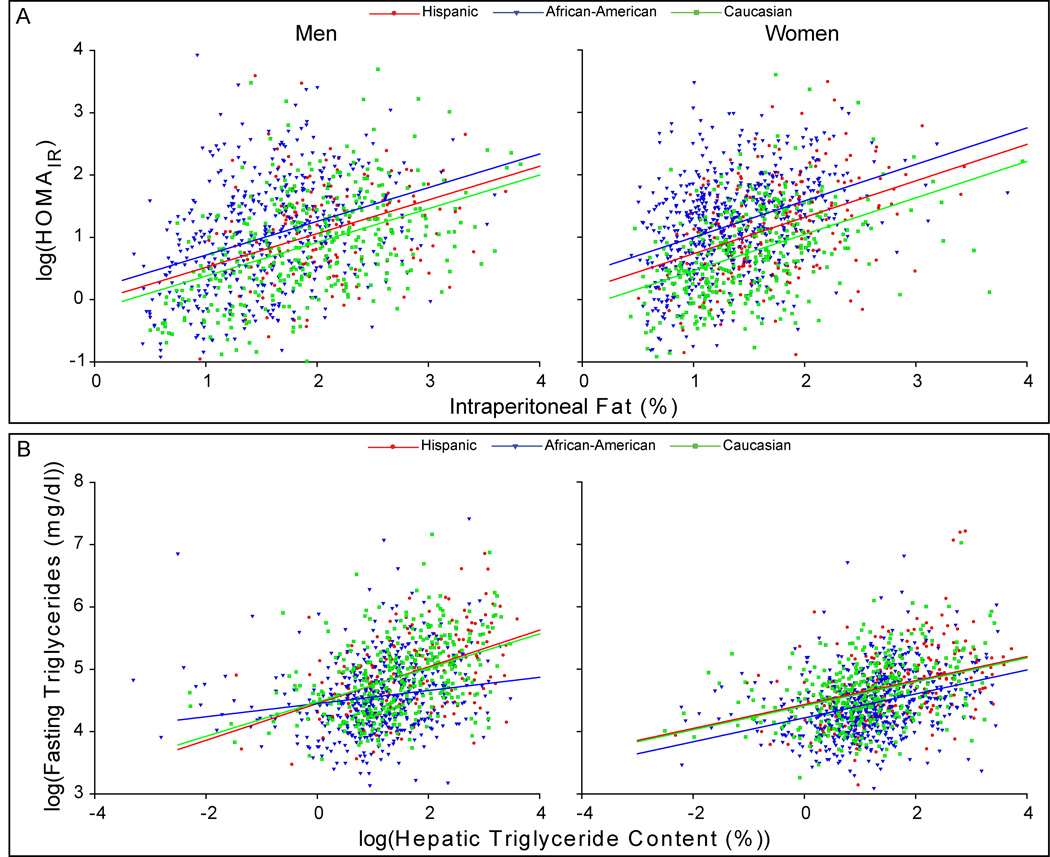
Given that African-Americans had a lower likelihood of accumulating fat in the intraperitoneal compartment or in liver and yet had a high prevalence of insulin resistance, we examined how differences in %IP and HTGC were related to insulin sensitivity in these groups. Adjustment of HOMAIR for %IP revealed that African-Americans were more insulin resistant for any level of visceral adiposity than the other ethnic groups (Figure 4A), even Hispanics, who had identical levels of HTGC after adjusting %IP (Figure 2B). Adjustment of HOMAIR for HTGC yielded similar results (data not shown). For any given level of intraperitoneal adiposity and/or HTGC, African-Americans, regardless of gender, were more insulin resistant than the other ethnic groups in this study.
Figure 4. Relationship Between Insulin Resistance, Intraperitoneal Adiposity, Hepatic Triglyceride Content and Plasma Triglyceride Levels.
(A) The plot of HOMAIR vs. %IP in men and women. Among the men, all regression lines had equal slopes. The mean value of HOMAIR was higher in African-American men after adjustment for %IP (P<0.023), while Hispanic and Caucasian men had similar mean HOMAIR values after this adjustment (P=0.138). Among the women, all regression lines had equal slopes. After adjustment for %IP, African-American women had the highest values of HOMAIR followed by Hispanic then Caucasian women (P<0.001). (B) The plot of serum triglyceride (TG) vs. hepatic triglyceride content (HTGC) in men and women. The slope of the relationship between HTGC and TG in African-American men was significantly lower than the other men (P<0.001). No difference in slope or mean value of TG was apparent between Hispanic and Caucasian men after adjustment for HTGC. Among the women, all regression lines had equal slopes. African-American women had lower mean TG levels after adjustment for HTGC (P<0.001). Mean TG levels remained similar between Hispanic and Caucasian women after adjustment for HTGC (P=0.878).
Hypertriglyceridemia and Hepatic Triglyceride Content
We next examined and compared another phenotype associated with insulin resistance in the three ethnic groups: hypertriglyceridemia. Insulin resistance is associated with hypetriglyceridemia due to increased delivery of free fatty acids from the periphery to liver and to an increase in hepatic triglyceride synthesis (24). African-Americans had both lower HTGC and lower mean plasma levels of triglycerides (TG) than either Hispanics or Caucasians (Table 1). After adjusting for HTGC, African-Americans continued to demonstrate significantly lower TG levels than the other ethnicities (Figure 4B). Adjustment of TG for HOMAIR in these groups yielded similar results (data not shown). These data indicate that lipid metabolism and its relationship to insulin resistance and hepatic steatosis differs substantially in African-Americans.
DISCUSSION
In the present study, the relationship between metabolic factors (total/regional adiposity, insulin resistance, hypertriglyceridemia) and HTGC was examined in a large multiethnic population-based sample (n=2,170) containing the three major U.S. ethnic groups: African-Americans, Hispanics, and Caucasians (2). This is the largest study to date to examine the relationships between HTGC, total adiposity, and body fat distribution, inclusive of the abdominal compartments (intraperitoneal and subcutaneous) in any population. A major finding of this study was that controlling for intraperitoneal fat content almost entirely eliminated ethnic differences in levels of HTGC and prevalence of hepatic steatosis. This was not the case with insulin resistance, total adiposity, or other fat depots. Despite the lower levels of intraperitoneal adipose tissue and liver fat in African-Americans, their prevalence of insulin resistance was similar to Hispanics, the group with the highest levels of intraperitoneal and liver fat. Furthermore, insulin levels and HOMAIR values were higher and serum triglyceride levels were lower among African-Americans, even after adjusting for intraperitoneal adiposity and HTGC. Thus, the metabolic response to obesity and insulin resistance differs in African-Americans when compared to either Hispanics or Caucasians: African-Americans appear to be more resistant to both the accretion of triglyceride in the abdominal visceral compartment (adipose tissue and liver) and hypertriglyceridemia associated with insulin resistance.
Controlling for differences in total body fat, abdominal subcutaneous fat, and lower extremity fat failed to account for the observed variability in HTGC between the ethnic groups. Only after adjusting for intraperitoneal fat content, were the ethnic differences in HTGC between the groups nearly abolished (Figure 2B). Thus, visceral adiposity appears to be more closely linked to HTGC than other parameters of body fat content or distribution. These findings extend previous work in this area (25–27) by suggesting that the factors responsible for reduced visceral adiposity in African-Americans are causally linked to the reduced propensity for this group to deposit triglycerides in hepatocytes. Whether visceral fat is causative in the development of hepatic steatosis or is simply a marker of an underlying metabolic derangement that is contributing to excess liver fat could not be determined by this study.
Visceral fat has been postulated to play a role in the development of hepatic steatosis/insulin resistance via the release of free fatty acids and adipokines directly into the portal circulation. Indeed, the surgical removal of visceral fat, but not subcutaneous abdominal fat, improves peripheral and hepatic insulin sensitivity (28–30). However, only 5–20% of the fatty acids entering the portal circulation originate from visceral adipose tissue (31): the remainder is derived from upper and lower body subcutaneous fat. Furthermore, only ~60% of triglycerides within steatotic livers originate from adipose tissue derived fatty acids (i.e., lipolysis) (24). Taken together, these data suggest that the contribution of fatty acids derived from visceral fat lipolysis to HTGC is relatively small. Though it appears that visceral fat and hepatic fat are metabolically connected, this connection is unlikely to be mediated via fatty acid delivery and uptake alone.
Interestingly, although several studies have indicated a link between visceral/liver fat content and insulin resistance, the relationship between these two variables differs in African-Americans, as compared to Hispanics and Caucasians. Insulin levels and HOMAIR values were significantly higher in African-Americans after adjusting for both intraperitoneal and liver fat. Similar results were found in the Insulin Resistance Atherosclerosis Study, where African-Americans had more insulin resistance than Hispanics or Caucasians after controlling for obesity, body fat distribution, and environmental factors (32). In addition, several studies comparing African-Americans to Caucasian have failed to account for the differences in insulin resistance between these two groups based upon adiposity alone (33–35), with one study indicating that a genetic basis for these differences was likely (36).
The multiple phenotypic changes in carbohydrate and lipid metabolism that occur in association with insulin resistance has been referred to as the metabolic syndrome (37). Hepatic steatosis is also considered by many to be a feature of this syndrome (9, 25–27). Our data, as well as the others (11, 38), is consistent with insulin resistance being phenotypically different in African-Americans. African-Americans have less visceral adiposity, lower levels of plasma triglycerides, higher levels of HDL-c, and lower levels of liver triglyceride than either Hispanics or Caucasians with similar levels of insulin resistance. African-Americans also appear to be protected from the accumulation of intramyocellular triglyceride (13), another feature of insulin resistance (39–41). Taken together, these data imply that the term “insulin resistance” equates to a different, but overlapping, set of metabolic derangements in African-Americans.
Heterogeneity in the contribution of liver, muscle, and adipose tissue to the insulin resistant state may account for the different complexion of the metabolic syndrome in African-Americans. Selective inactivation of the insulin receptor in each of these tissues has dramatically different effects on carbohydrate and lipid metabolism. For example, genetic ablation of the insulin receptor in liver leads to hyperinsulinemia, hyperglycemia, and peripheral insulin resistance without the development of hypertriglyceridemia or hepatic steatosis (42). Conversely, isolated loss of the insulin receptor in muscle leads to increased visceral fat mass, free fatty acids, and serum triglycerides, all of which are characteristics of metabolic syndrome; however, hyperinsulinemia and hyperglycemia develop only after insulin resistance is present in additional organs (43). It is possible that the observed differences in the clinical manifestation of insulin resistance between the ethnic groups may be due to tissue-specific differences in insulin signaling.
The relative “health” of adipose tissue in African-Americans may also be a factor in the observed phenotypic differences in this group. Compared to Hispanics, African-Americans tended to have larger subcutaneous depots of adipose tissue, especially with regard to the lower extremities (Figure 1). This was also the case when comparing the genders: as a group, women had more subcutaneous adiposity and less visceral adiposity than men. Work in an animal model (transgenic ob/ob mice over-expressing adiponectin) has demonstrated that the expansion of subcutaneous, but not visceral, adipose tissue ameliorates hepatic steatosis and insulin resistance (44). Thus, the ability to expand subcutaneous adipose tissue in the setting of excessive caloric intake may prevent the accumulation of fat in the visceral compartment (adipose and liver). This may be an adaptive response to increasing adiposity that has different limits in these ethnic and gender groups. Indeed, women tended to have lower levels of liver triglycerides despite a greater degree adiposity than men (Table 2). Such differences in the ability to expand the subcutaneous fat depot may explain, in part, the observed differences in HTGC between these groups. Further support for this idea can be found in the significant negative correlation between lower extremity fat and liver fat content in all subjects (Table 3).
Dietary carbohydrate intake has been postulated to play a role in the development of hepatic steatosis. Indeed, several observational studies support the notion that diets high in carbohydrates relative to the other macronutrients are associated with higher degrees of hepatic steatosis and inflammation (45, 46). We did not obtain structured dietary information in the current study and therefore, could not examine its association with the ethnic-specific differences observed in hepatic steaotsis. However, prior studies examining such ethnic variation would suggest that environmental factors are not solely responsible for the differences in insulin sensitivity between these groups (32).
In conclusion, HTGC is closely linked to visceral adiposity in African-Americans, Hispanics, and Caucasians. Ethnic differences in the propensity to accumulate visceral fat explains, in part, the observed differences in levels of HTGC and prevalence of hepatic steatosis among these ethnic groups. The relative protection from visceral fat accumulation with increasing adiposity experienced by African-Americans is associated with a similar protection from hepatic steatosis but not insulin resistance. Indeed, many of the derangements in lipid metabolism typically associated with insulin resistance were not present in African-Americans. A possible explanation for these findings is that the insulin resistance phenotype is: 1) a function of the organ contributing primarily to reduced insulin sensitivity; and/or 2) a function of the ability to expand subcutaneous adipose tissue in response to overnutrition. Further study is needed to establish the basis for this insulin resistance paradox.
ACKNOWLEDGEMENTS
Special thanks to Jay Horton for helpful discussions as well as to Beverly Huet and Xian-Jin Xie for aid with statistical analysis. Also, the work of the DHS investigators is greatly appreciated, especially DuWayne Willett for data organization and management, Lidia Szczepaniak for liver triglyceride measurement, and Nicola Abate for abdominal MRI interpretation.
Financial Support
This research was supported by the Donald W. Reynolds Cardiovascular Clinical Research Center at Dallas, NIH T32-DK-07745 (R.G.), and NIH 1K23DK074396 (J.D.B). Jeffrey D. Browning is a Disease Oriented Clinical Scholar for the University of Texas Southwestern Medical Center.
List of Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- 1H-MRS
proton magnetic resonance spectroscopy
- DHS
Dallas Heart Study
- HTGC
hepatic triglyceride content
- HDL-c
high density lipoprotein cholesterol
- DEXA
dual energy x-ray absorptiometry
- MRI
magnetic resonance imaging
- BMI
body mass index
- HOMAIR
homeostasis model assessment
- ANCOVA
Analysis of Covariance
- CRP
C-reactive protein
- %BF
Percent body fat
- %IP
percent intraperitoneal fat
- TG
plasma triglyceride
Contributor Information
Richard Guerrero, Email: richard.guerrero@utsouthwestern.edu.
Gloria L. Vega, Email: gloria.vega@utsouthwestern.edu.
Scott M. Grundy, Email: scott.grundy@utsouthwestern.edu.
REFERENCES
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JB, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 3.Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372–379. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 4.Browning J, Kumar S, Saboorian M, Thiele D. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am. J. Gastroenterol. 2004;99:292–298. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? Am. J. Gastroenterol. 2002;97:1496–1500. doi: 10.1111/j.1572-0241.2002.05795.x. [DOI] [PubMed] [Google Scholar]
- 6.Solga SF, Clark JM, Alkhuraishi AR, Torbenson M, Tabesh A, Schweitzer M, Diehl AM, et al. Race and comorbid factors predict nonalcoholic fatty liver disease histopathology in severely obese patients. Surg. Obes. Relat. Dis. 2005;1:6–11. doi: 10.1016/j.soard.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Giday SA, Ashiny Z, Naab T, Smoot D, Banks A. Frequency of nonalcoholic fatty liver disease and degree of hepatic steatosis in African-American patients. J. Natl. Med. Assoc. 2006;98:1613–1615. [PMC free article] [PubMed] [Google Scholar]
- 8.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 9.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, et al. Nonalcoholic fatty liver disease a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition; Circulation; 2004. pp. 433–438. [DOI] [PubMed] [Google Scholar]
- 11.Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am. J. Clin. Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liska D, Dufour S, Zern TL, Taksali S, Cali AM, Dziura J, Shulman GI, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS ONE. 2007;2:e569. doi: 10.1371/journal.pone.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victor RG, Haley RW, Willett D, Peshock RM, Vaeth PC, Leonard D, Basit M, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am. J. Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 15.Institute for Survey Research. Technical Report for the 1995 National Alcohol Survey. Philadelphia: Temple University; 1996. [Google Scholar]
- 16.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Szczepaniak LS, Leonard D, Browning JD, Nurenberg P, Reingold JS, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. 2004 doi: 10.1152/ajpendo.00064.2004. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 19.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, et al. Measurement of intracellular triglyceride stores by H spectroscopy:validation in vivo. Am. J. Physiol. Endocrinol. Metab. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 20.Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, Croce LS, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J. Magn. Reson. Imaging. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 21.Szczepaniak LS, Leonard D, Browning JD, Nurenberg P, Reingold JS, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am. J. Physiol. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. (Epub 2004) [DOI] [PubMed] [Google Scholar]
- 22.Veg GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J. Clin. Endocrinol. Metab. 2006;91:4459–4466. doi: 10.1210/jc.2006-0814. Epub 2006 Aug 4422. [DOI] [PubMed] [Google Scholar]
- 23.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J. Clin. Invest. 1995;96:88–98. doi: 10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eguchi Y, Eguchi T, Mizuta T, Ide Y, Yasutake T, Iwakiri R, Hisatomi A, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J. Gastroenterol. 2006;41:462–469. doi: 10.1007/s00535-006-1790-5. [DOI] [PubMed] [Google Scholar]
- 26.Koda M, Kawakami M, Murawaki Y, Senda M. The impact of visceral fat in nonalcoholic fatty liver disease: cross-sectional and longitudinal studies. J. Gastroenterol. 2007;42:897–903. doi: 10.1007/s00535-007-2107-z. Epub 2007 Nov 2022. [DOI] [PubMed] [Google Scholar]
- 27.Park BJ, Kim YJ, Kim DH, Kim W, Jung YJ, Yoon JH, Kim CY, et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J. Gastroenterol. Hepatol. 2007 doi: 10.1111/j.1440-1746.2007.05212.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Barzilai N, Gupta G. Revisiting the role of fat mass in the life extension induced by caloric restriction. J. Gerontol. A. Biol. Sci. Med. Sci. 1999;54:B89–B96. doi: 10.1093/gerona/54.3.b89. discussion B97–B98. [DOI] [PubMed] [Google Scholar]
- 29.Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- 30.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–1588. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, Howard G, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 33.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet. Med. 1994;11:755–762. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 34.Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R. Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism. 1996;45:1119–1124. doi: 10.1016/s0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 35.Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am. J. Clin. Nutr. 2005;82:1210–1217. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes. 2003;52:1047–1051. doi: 10.2337/diabetes.52.4.1047. [DOI] [PubMed] [Google Scholar]
- 37.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 38.Anuurad E, Chiem A, Pearson TA, Berglund L. Metabolic syndrome components in african-americans and European-american patients and its relation to coronary artery disease. Am. J. Cardiol. 2007;100:830–834. doi: 10.1016/j.amjcard.2007.04.025. Epub 2007 Jun 2028. [DOI] [PubMed] [Google Scholar]
- 39.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 40.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 41.Shulman GI. Cellular mechanisms of insulin resistance. J. Clin. Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solga S, Alkhuraishe AR, Clark JM, Torbenson M, Greenwald A, Diehl AM, Magnuson T. Dietary composition and nonalcoholic fatty liver disease. Dig. Dis. Sci. 2004;49:1578–1583. doi: 10.1023/b:ddas.0000043367.69470.b7. [DOI] [PubMed] [Google Scholar]
- 46.Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, Foess-Wood L, et al. Metabolic Syndrome Is Associated with Greater Histologic Severity, Higher Carbohydrate, and Lower Fat Diet in Patients with NAFLD. Am J Gastroenterol. 2006;101:2247–2253. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]