A. aeolicus GidA has been crystallized in two different crystal forms: forms I and II. X-ray diffraction data were collected to 3.2 and 2.3 Å resolution, respectively, using a synchrotron-radiation source.
Keywords: GidA, Aquifex aeolicus, tRNA, anticodon, modification
Abstract
The 5-carboxymethylaminomethyl modification of uridine at the first position of the tRNA anticodon is crucial for accurate protein synthesis by stabilizing the correct codon–anticodon pairing on the ribosome. Two conserved enzymes, GidA and MnmE, are involved in this modification process. Aquifex aeolicus GidA was crystallized in two different crystal forms: forms I and II. These crystals diffracted to 3.2 and 2.3 Å resolution, respectively, using synchrotron radiation at the Photon Factory. These crystals belonged to space groups I212121 and P21 with unit-cell parameters a = 101.6, b = 213.3, c = 231.7 Å and a = 119.4, b = 98.0, c = 129.6 Å, β = 90.002°, respectively. The asymmetric units of these crystals are expected to contain two and four molecules, respectively.
1. Introduction
Transfer RNA (tRNA) contains a variety of modified nucleosides which are post-transcriptionally introduced in all organisms (Björk, 1995 ▶). In Escherichia coli, ∼1% of the genome is devoted to encoding tRNA-modification enzymes (Grosjean et al., 1995 ▶). Many of the modified nucleosides in tRNA are observed at the first position of the anticodon (the wobble position) and immediately 3′-adjacent to the anticodon. In particular, modifications at the wobble position are indispensable for precise decoding of the genetic code (Björk, 1995 ▶; Urbonavičius et al., 2001 ▶; Yokoyama & Nishimura, 1995 ▶). In eubacteria, 5-carboxymethylaminomethyluridine (cmnm5U) or 5-methylaminomethyluridine (mnm5U) modifications are commonly found at the wobble position of tRNAGlu UUC, tRNALys UUU, tRNAGln UUG, tRNALeu UAA and tRNAArg UCU and are responsible for deciphering the purine-ending split codon boxes (NNR) (Björk, 1995 ▶; Yokoyama & Nishimura, 1995 ▶). The cmnm5U and mnm5U modifications stabilize the correct codon–anticodon pairing on the ribosome (Yarian et al., 2000 ▶; Urbonavičius et al., 2001 ▶; Murphy et al., 2004 ▶), which enables the tRNAs for Glu, Lys, Gln, Leu and Arg to discriminate purines from pyrimidines in order to prevent the incorrect decoding of the NNY codon for translational fidelity.
In eubacteria, two conserved enzymes, GidA and MnmE, are involved in the incorporation of the cmnm5 group at the 5-position of the wobble uridine (Elseviers et al., 1984 ▶; Brégeon et al., 2001 ▶). In some eubacteria, such as E. coli, the cmnm5 group is then converted into the final mnm5 group by MnmC (Bujnicki et al., 2004 ▶). GidA and MnmE are strictly conserved in eukarya, in which MSS1 and MTO1 have been identified as their respective homologues (Decoster et al., 1993 ▶; Colby et al., 1998 ▶).
Biochemical studies have revealed that GidA interacts with MnmE to form a functional complex, suggesting that these two proteins collaborate to modify the 5-position of the wobble uridine in an interdependent manner (Yim et al., 2006 ▶). GidA contains a consensus dinucleotide-binding motif, GXGXXG, a portion of the Rossmann-fold domain which is present in a large number of flavin adenine dinucleotide (FAD) binding proteins (Dym & Eisenberg, 2001 ▶). In fact, GidA binds FAD, which has been proposed to be involved in the cmnm5U modification process (Yim et al., 2006 ▶). However, the precise function of GidA during the modification process remains elusive. To gain insight into the structure–function relationships of GidA, we cloned and overproduced GidA from Aquifex aeolicus, which is referred to as AaGidA. In the present paper, we report the crystallization and preliminary X-ray diffraction analysis of AaGidA.
2. Materials and methods
2.1. Protein preparation
A DNA fragment encoding AaGidA was produced by PCR amplification and cloned into the expression vector pET28b (Novagen). The recombinant AaGidA protein with a His tag attached to the N-terminus was produced in E. coli strain BL21-CodonPlus (DE3)-RIL and was purified as follows. After sonication of the E. coli cells in 20 mM Tris–HCl buffer pH 8.0 containing 300 mM NaCl, 10 mM imidazole, 7 mM β-mercaptoethanol, 1 mM PMSF and 1 mM benzamidine, the clarified lysate was incubated at 338 K for 20 min. The heat-treated lysate was centrifuged at 8000 rev min−1 for 30 min and the supernatant was loaded onto an Ni–NTA column (Qiagen) equilibrated with buffer A (20 mM Tris–HCl pH 8.0, 300 mM NaCl, 10 mM imidazole and 7 mM β-mercaptoethanol). AaGidA was eluted with buffer B (as buffer A but containing 250 mM imidazole). The eluate from the Ni–NTA column was dialyzed against buffer C (10 mM potassium phosphate pH 6.0, 200 mM NaCl and 7 mM β-mercaptoethanol). The protein solution was then loaded onto a HiTrap Heparin column (16 × 25 mm) previously equilibrated with buffer C. A linear gradient was developed from 200 to 1000 mM NaCl in buffer C. The fractions rich in the target protein were combined and dialyzed against buffer D (20 mM potassium phosphate pH 6.0, 100 mM NaCl and 7 mM β-mercaptoethanol). After the concentration of NaCl in the protein solution had been reduced to 50 mM by mixing with an equal volume of buffer E (20 mM potassium phosphate pH 6.0 and 7 mM β-mercaptoethanol), the protein solution was further purified on a Resource S column (16 × 30 mm) previously equilibrated with buffer F (20 mM potassium phosphate pH 6.0, 50 mM NaCl and 7 mM β-mercaptoethanol). GidA was eluted with a linear gradient of 50–1000 mM NaCl. The fractions enriched in AaGidA were combined and dialyzed against buffer G (20 mM Tris–HCl pH 8.0, 100 mM NaCl and 7 mM β-mercaptoethanol) and the solution was concentrated to 5–10 mg ml−1. Selenomethionine-labelled AaGidA was produced in the methionine auxotroph E. coli strain B834-CodonPlus (DE3)-RIL and was purified using the same procedure as used for the native protein.
2.2. Crystallization
Crystallization conditions were screened by the sitting-drop vapour-diffusion method at 293 K using the following commercial crystallization screening kits: Crystal Screen, Crystal Screen 2, Crystal Screen Lite, Crystal Screen Cryo, Natrix, PEG/Ion, Index and SaltRx (Hampton Research). Sitting drops were prepared by mixing 1 µl reservoir solution with 1 µl His-tagged AaGidA solution (5 mg ml−1) and were equilibrated against 100 µl reservoir solution. AaGidA was crystallized in two different crystal forms: forms I and II. Form I crystals were obtained within 3–4 d using reservoir solution containing 1.0–1.2 M sodium/potassium phosphate pH 6.9–7.2. Form II crystals grew within two weeks in 200 mM ammonium chloride and 10–20% PEG 3350. The form II crystals prepared as described above were polycrystals that were unsuitable for X-ray diffraction experiments. Therefore, we improved the crystallization conditions for the form II crystal by using a pH Buffer kit and an Additive Screen kit (Hampton Research). Finally, large single crystals that were suitable for X-ray diffraction experiments were obtained using a reservoir solution containing 160 mM ammonium chloride, 100 mM HEPES pH 7.2, 10 mM sodium citrate pH 5.6, 200 mM 1,6-hexanediol and 10–20% PEG 3350.
2.3. X-ray data collection
For data collection, the AaGidA form I and II crystals were cryoprotected in reservoir solution containing 20% ethylene glycol and 20% glycerol, respectively. The crystals were mounted in a cryoloop and were flash-cooled in a nitrogen stream at 100 K. X-ray diffraction data were collected on the NW12A and BL-17A beamlines of KEK (Ibaraki, Japan) using ADSC Q210r and ADSC Q270 CCD detectors, respectively. Diffraction data were processed with the program HKL-2000 (Otwinowski & Minor, 1997 ▶). The processing statistics are summarized in Table 1 ▶.
Table 1. Data-collection statistics.
Values in parentheses are for the last shell.
| Form I | Form II | |
|---|---|---|
| Wavelength (Å) | 0.97928 | 1.00 |
| Space group | I212121 | P21 |
| Unit-cell parameters (Å, °) | a = 101.6, b = 213.3, c = 231.7 | a = 119.4, b = 98.0, c = 129.6, β = 90.002 |
| Resolution (Å) | 50–3.20 (3.26–3.20) | 50–2.30 (2.33–2.30) |
| Measured reflections | 333405 (10435) | 436815 (13035) |
| Unique reflections | 41584 (2046) | 123831 (5214) |
| Redundancy | 8.0 (5.1) | 3.7 (2.5) |
| Completeness (%) | 99.5 (99.3) | 92.2 (78.5) |
| 〈I/σ(I)〉 | 11.5 (2.2) | 12.2 (2.0) |
| Rmerge† | 0.126 (0.398) | 0.083 (0.248) |
R
merge = 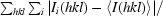
 , where I
i(hkl) is the observed intensity and 〈I(hkl)〉 is the average intensity over symmetry-equivalent measurements.
, where I
i(hkl) is the observed intensity and 〈I(hkl)〉 is the average intensity over symmetry-equivalent measurements.
3. Results and discussion
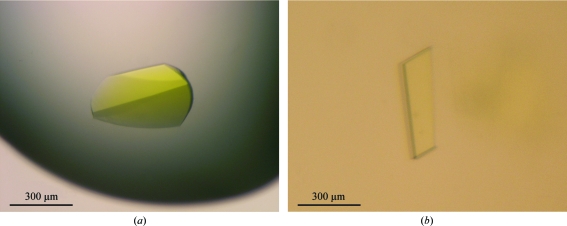
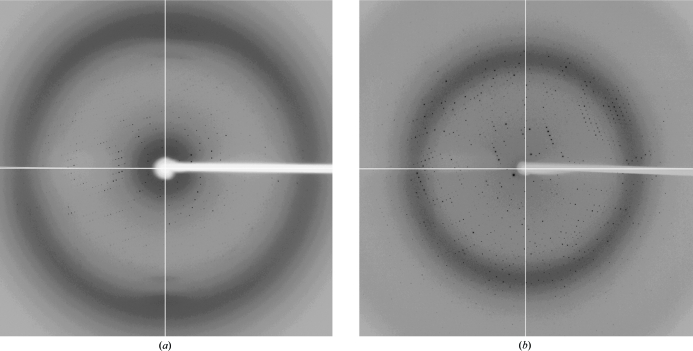
The His-tagged AaGidA protein was overexpessed in E. coli cells using the pET28b expression vector and was purified to homogeneity by several chromatography steps. The purified protein was coloured yellow, indicating that the cofactor FAD was co-purified with AaGidA from the E. coli cell lysate. This is consistent with the presence of the FAD-binding motif GXGXXG (Gly14, Gly16 and Gly19 in AaGidA), which is conserved among the GidAs. The His-tagged AaGidA was crystallized in two different crystal forms: forms I and II (Fig. 1 ▶). The form I crystals, which grew to maximum dimensions of 300 × 150 × 50 µm, diffracted to a resolution of 3.2 Å (Fig. 2 ▶ a) and belonged to the orthorhombic space group I212121, with unit-cell parameters a = 101.6, b = 213.3, c = 231.7 Å. On the basis of the molecular mass of AaGidA (70 kDa), the form I crystals are expected to contain two molecules per asymmetric unit, which corresponds to a solvent content of 71.9% and a Matthews coefficient of 4.37 Å3 Da−1. The form II crystals grew to dimensions of 300 × 200 × 10 µm within two weeks and diffracted to 2.3 Å resolution (Fig. 2 ▶ b). The form II crystals belonged to the monoclinic space group P21, with unit-cell parameters a = 119.4, b = 98.0, c = 129.6 Å, β = 90.002°. The calculated Matthews coefficient was approximately 2.64 Å3 Da−1, assuming the presence of four molecules in the asymmetric unit, with a solvent content of 53.5%. Both AaGidA crystals have a deep yellow colour (Fig. 1 ▶), suggesting that the crystals contain the FAD cofactor. Since FAD binding to GidA is required for the synthesis of cmnm5U (Yim et al., 2006 ▶), structural analysis of AaGidA complexed with FAD could reveal the catalytic site of GidA and provide structural insight into the reaction mechanism of the cmnm5U modification process. Attempts to solve the AaGidA structure complexed with FAD by the SAD method, with selenium as the anomalous scattering atom, are in progress.
Figure 1.
Two crystal forms of A. aeolicus GidA. (a) Form I crystals belonging to space group I212121. (b) Form II crystals belonging to space group P21.
Figure 2.
Diffraction patterns of form I crystals (a) and form II crystals (b) of A. aeolicus GidA.
Acknowledgments
We thank H. Hori for donating the A. aeolicus genomic DNA. We also thank the beamline staff at NW12A, BL-5A and BL-17A of KEK (Ibaraki, Japan) for technical assistance during data collection. This work was supported by a PRESTO Program grant from JST (Japan Science and Technology), a Grant-in-Aid for Young Scientists from JSPS and grants from the Sumitomo Foundation, the Kurata Memorial Hitachi Science and Technology Foundation and the Kato Memorial Bioscience Foundation to TN.
References
- Björk, G. R. (1995). tRNA: Structure, Biosynthesis and Function, edited by D. Söll & U. L. RajBhandary, pp. 165–205. Washington DC: American Society for Microbiology.
- Brégeon, D., Colot, V., Radman, M. & Taddei, F. (2001). Genes Dev.15, 2295–2306. [DOI] [PMC free article] [PubMed]
- Bujnicki, J. M., Oudjama, Y., Roovers, M., Owczarek, S., Caillet, J. & Droogmans, L. (2004). RNA, 10, 1236–1242. [DOI] [PMC free article] [PubMed]
- Colby, G., Wu, M. & Tzagoloff, A. (1998). J. Biol. Chem.273, 27945–27952. [DOI] [PubMed]
- Decoster, E., Vassal, A. & Faye, G. (1993). J. Mol. Biol.232, 79–88. [DOI] [PubMed]
- Dym, O. & Eisenberg, D. (2001). Protein Sci.10, 1712–1728. [DOI] [PMC free article] [PubMed]
- Elseviers, D., Petrullo, L. A. & Gallagher, P. J. (1984). Nucleic Acids Res.12, 3521–3534. [DOI] [PMC free article] [PubMed]
- Grosjean, H., Björk, G. & Maden, B. E. H. (1995). Biochimie, 77, 3–6.
- Murphy, F. V., Ramakrishnan, V., Malkiewicz, A. & Agris, P. F. (2004). Nature Struct. Mol. Biol.11, 1186–1191. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Urbonavičius, J., Qian, Q., Durand, J. M., Hagervall, T. G. & Björk, G. R. (2001). EMBO J.20, 4863–4873. [DOI] [PMC free article] [PubMed]
- Yarian, C., Marszalek, M., Sochacka, E., Malkiewicz, A., Guenther, R., Miskiewicz, A. & Agris, P. F. (2000). Biochemistry, 39, 13390–13395. [DOI] [PubMed]
- Yim, L., Moukadiri, I., Björk, G. R. & Armengod, M. E. (2006). Nucleic Acids Res.34, 5892–5905. [DOI] [PMC free article] [PubMed]
- Yokoyama, S. & Nishimura, S. (1995). tRNA: Structure, Biosynthesis and Function, edited by D. Söll & U. L. RajBhandary, pp. 207–224. Washington DC: American Society for Microbiology.